- *Corresponding Author:
- Vaishnavi Verma
Department of Pharmacology, KIET School of Pharmacy, KIET Group of Institutions, Ghaziabad, Uttar Pradesh 201206, India
E-mail: vaishnaviverma123.vv@gmail.com
| Date of Received | 21 September 2023 |
| Date of Revision | 19 March 2023 |
| Date of Acceptance | 15 July 2024 |
| Indian J Pharm Sci 2024;86(4):1324-1330 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Hemorrhoids are the most common anorectal disorder associated with the abnormal swelling and dilatation of the venous canal, leading to rectal bleeding and the prevalent figure of hemorrhoids is around 37 % worldwide. The major symptoms include prolapsing of mass, constipation, bleeding, and swelling in the anorectal region. A sedentary lifestyle, obesity, reduced fiber diets and low physical activity are some of the major causes of hemorrhoids. However, the rising demand for synthetic chemicals has several unintended consequences that have a substantial impact on the emergence of various illnesses. The current study investigated the antihemorrhoidal ability of the ethanolic extract of Laurus nobilis in high and low doses against the croton oil caused hemorrhoids model. Himalayan pilex pills (an ayurvedic treatment) were utilized as the benchmark drug for comparing all of the results and findings. The phytochemical screening revealed the presence of alkaloids, glycosides, flavonoids, tannins, saponins, phenols, volatile oils and steroids. The anti-hemorrhoidal potential was measured by evaluating blood parameters, inflammatory parameters, recto-anal coefficient and histological parameters was assessed. It was found that the hydroalcoholic leaf extract of Laurus nobilis demonstrated potential anti-hemorrhoidal activity with recto-anal coefficient 1.86±0.04 at the dose of 300 mg/ kg and 1.94±0.05 at the dose of 150 mg/kg. The extract also demonstrated an increase in the mean hemoglobin level in the low-dose extract (150 mg/kg) group and the high-dose extract (300 mg/kg) group with values of 7.61±0.81 and 8.99±0.65 (g/dl), respectively. The extract also decreased the levels of tumor necrosis factor-α and interleukin-6. Tumor necrosis factor-alpha decreased to 442.47±71.22 at a low dose (150 mg/kg), whereas for a high dose (300 mg/kg) the tumor necrosis factor-α value was found to be 335.85±82.43. The groups treated with the low dose extract showed the interleukin-6 value of 84.72±3.02 and the group treated with high dose extract depicted the mean value of interleukin-6 at 67.85±1.38. It was also found that a high dose (300 mg/kg) of Laurus nobilis extract exhibited a better therapeutic effect than a low dose (150 mg/kg) of Laurus nobilis extract. Pilex, the standard drug, demonstrated better results than the leaf extract.
Keywords
Anti-hemorrhoidal, hemorrhoids, Laurus nobilis, tumor necrosis factor-alpha
Hemorrhoids, commonly known as piles, are a very common anorectal disorder that affects millions. Approximately 37 % of men and women worldwide are affected by this condition. The prevalence of the disease increases with age. The term 'hemorrhoids' is used by both doctors and patients to characterize a multitude of anal problems, and about 90 % of the population experiences hemorrhoids at some point in their lives[1]. It occurs due to severe pressure on the pelvic veins and anorectal region, which triggers abnormal swelling and dilation of the venous canal, leading to rectal bleeding. The term 'hemorrhoids' comes from the Greek words 'heama,' meaning blood, and 'roohs,' meaning flow. Hence, the disease is characterized by swollen and enlarged veins in the anus and rectum. Hemorrhoids can also be explained as the unusual downward shifting and enlargement of the vascular anal cushions, which causes bright red bleeding in the stool, the most prevalent symptom of the disease. Other major symptoms of hemorrhoids include swelling, pain, itching, constipation, protrusion, obstructed defecation, and prolapsed piles or large fungating masses[2,3].
Herbal remedies have proven to be a successful and all-natural method for treating hemorrhoids and piles. As per the studies the plants such as horse chestnut (Aesculus hippocastanum), onion (Allium cepa), turnip (Bergenia ligulate), witch hazel, mango (Mangifera indica), bitter melon (Momordica chiranta), Asian rice (Orya sativa), Bergenia ciliata, Hamamelis virginiana, Ruscus aculeatus, Bergenia stracheyi, European blueberry (Vaccinium myrtillus) and ginger (Zingiber officinale) and Terminalia chebula, are effective in treating piles[4,5].
Laurus nobilis Linn. (L. nobilis) this is commonly known as the “bay leaf” that belongs to the family of laurel or “Lauraceae”. Laurel is a perennial shrub that can reach up to 8 m in height. It is widely spread across the Mediterranean area, Europe, the subtropical and tropical regions of Eastern Asia, North and South America, and Asia minor. L. nobilis is known by different names around the globe. In English, it is called bay laurel or sweet bay. In Hindi, it is known as teejpatta. In Greek, it is called dafni, and in Arabic, it is referred to as waraq ghaar. Morphologically, the bay laurel has smooth, brown, and thin bark, with a height of up to 8 meters and a diameter of up to 15 centimeters. Bay leaves range in size from 4.6-5.0 cm in length and about 1.6-2.0 cm in width. The leaves of bay laurel are simple, alternate, lanceolate, and bipinnate, and they release an aroma when rubbed. The flowers are small, yellow or white, scented, and clustered[6]. Bay leaves have been extensively studied for their chemical constituents. Approximately 55 compounds have been identified so far, accounting for about 91.6 % of the total phytochemicals. The major components are 1,8-cineole at 31.9 %, followed by sabinene at 12.2 %, and linalool at 10.2 %. Other phytoconstituents include Alpha (α)-terpinyl acetate, α-pinene, terpineole, Gamma (γ)-terpinene, Beta (β)-pinene, etc.[7] Eugenol, elemicin, and methyl eugenol (benzene compounds) are present in less quantities ranging from 1 %-12 %. They provide the spicy aroma to bay laurel leaves and are important constituents responsible for their sensory quality[8]. Laurel has been shown to possess pharmacological activities such as antibacterial, antifungal, and antimicrobial properties[7], which contribute to its effectiveness in wound healing due to their synergistic effects[9]. Some studies have also shown that laurel also has antiepileptic and anticonvulsant activity against experimental seizures[10]. Bay leaves exhibit a range of pharmacological and biological activities, including antioxidant activity[11,12], antibacterial activity[13], antiviral activity[14], immunestimulant activity, anticholinergic activity[15], antifungal activity[16], insect repellant activity[17], antimutagenic activity[18], and analgesic and anti- inflammatory activity[19].
Materials and Methods
Plants materials:
Bay leaf (L. nobilis) was purchased from Organic India. The leaves of bay laurel were authenticated by the Phytopharmaceutical division, Indian Pharmacopoeia Commission, Ghaziabad under the supervision of Dr. Manoj K. Pandey (senior scientific officer), with authentication no: IPC/ PHYT/PAC/2022/005.
Extraction and Isolation
Preparation of L. nobilis Extract (LNE): The extract was prepared by the Soxhlet extraction method. The dried leaves of L. nobilis were obtained and coarsely powdered using a mixer grinder. The coarse powder was then sieved to remove fine particles to prevent clogging of the siphon tubes during Soxhlet extraction. The mass of dried bay leaves used for Soxhlation was 25 gm in 200 ml of solvent in each process. The dried bay leaves powder was then defatted using petroleum ether and thoroughly weighed and carefully poured into the thimble. The solvent was prepared by mixing ethanol and distilled water in a ratio of 8:2. For the process, 160 ml of ethanol was mixed with 40 ml of distilled water to prepare the solvent. This 200 ml solvent was then poured into the Soxhlet apparatus and heated to a temperature of 45°. The system was run until a clear, colorless solvent was obtained in the apparatus, after which the solvent was collected. The excess solvent was then evaporated with the help of a water bath at a temperature not exceeding 50° to obtain a semi-solid extract for further use. This process was repeated several times to obtain the required amount of extract[20].
Experimental animals:
Ethical permission from the Institutional Animal Ethics Committee (IAEC) was obtained before commencing the research project. The approved IAEC number for conducting the study is IAEC/ KSOP/2021/14. A total of 40 male Wistar rats, each weighing between 150-200 g, were divided into 5 groups, with 8 animals in each group.
Selection of dose:
The acute toxicity studies of L. nobilis extract have already been done in previous studies as per the Organisation for Economic Co-operation and Development (OECD) 420 guidelines, in which they found that the L. nobilis extract is safe up to the dose of 3000 mg/kg. Hence, we selected the lower dose of 150 mg/kg and the higher dose of 300 mg/kg for the present experimental study[21].
Preliminary phytoconstituents screening:
To determine the phytoconstituents present in L. nobilis, various solvent systems were used, including n-hexane, petroleum ether, acetone, ethyl acetate, methanol, ethanol, water, n-butanol acetic acid-H2O (BAW), and ethanol:water (8:2). The leaves of bay laurel were grounded and coarse powder was obtained. 1 g of this coarsely ground leaf powder was added to 10 ml of each solvent in separate volumetric flasks and left at room temperature for 48 h. After 48 h, it was filtered and the filtrate was used for phytochemical screening by performing the chemical tests for each secondary metabolite[22].
Thin Layer Chromatography (TLC) of L. nobilis extract:
Precoated silica gel-G plates were used for the TLC analysis of L. nobilis extract. The solvent system used for the detection was chloroform and methanol in a ratio of 90:10. The ethanolic extract was spotted at the bottom of the plate. The sample-loaded TLC plate was then placed into the solvent mixture for sample movement. When the sample reached the top marking section, ninhydrin solution was sprayed on it to identify spots, which were then detected under Ultraviolet (UV) light at 365 nm. The Retardation factor (Rf) value was determined through measurement and recording, and it was then computed[13].
Standard drug (STD):
The standard drug used was pilex tablet (Himalayan herbals) was obtained from the local pharmacy, Murad nagar, Ghaziabad, to conduct the experimental study. One container contains of total 60 tablets. The chemical and purity estimation of the pilex tablet is not part of the project.
Preparation of croton oil mixture:
The croton oil mixture consists of deionized water, pyridine, diethyl ether, and 6 % croton oil (in diethyl ether) in a ratio of 1:4:5:10[21]. All the solvents were measured accurately and mixed. As croton oil preparation is very important in the induction of hemorrhoids, therefore, the preparation was stored in a clean and sterilized flask at room temperature. Later on, hemorrhoids were induced in all the groups except the normal control group[23].
Induction of hemorrhoids:
The croton oil mixture was used to induce hemorrhoids in all groups except group 1 (normal control). All animals were subjected to overnight fasting, and on the following day, 100 µl of the croton oil mixture was administered intrarectally using a syringe for 7 consecutive days. After 7 d, a linear change in the rectum was observed[24].
Body Weight (BW) estimation and collection of blood samples:
BW and blood samples of all the animals were recorded twice during the study. Once at the end of the induction period on the 7th d, and again at the end of the treatment period on the 21st d. Retro- orbital sinus technique was used to collect the blood samples. Inhalational isoflurane was used to anesthetize the animal before blood collection. The serum was prepared after the collection of the blood samples in the Non-Heparin Tubes (NHT). The NHT was centrifuged using a Remi cooling centrifuge instrument at 3000 rpm for 15 min. Eppendorf tubes were used to collect the serum and were later used to determine the pre- inflammatory parameters such as Tumor Necrosis Factor (TNF)-α and Interleukin-6 (IL-6).
Determination of recto anal coefficient:
After the treatment period of 21 d, the animals were sacrificed by an overdose of isoflurane anesthesia, and the anorectal part of 20 mm in length was dissected and weighed to determine the Recto-Anal Coefficient (RAC). It was calculated using the following formula[23].
RAC=weight of recto-anal tissue in mg/BW of animal in grams
Histology of anorectal tissue:
After the calculation of RAC, a small portion of the anorectal part was dissected and fixed in a 10 % formalin solution. It was then processed by conventional methods, and 5 μm-thick sections were taken and stained with hematoxylin and eosin. Slides were prepared and examined for pathological changes under a microscope[24].
Blood parameters determination:
All the blood parameters were evaluated by using the quick cell plus automatic cell analyzer. The blood collected in NHT was further used to collect the serum to evaluate the various inflammatory parameters such as TNF-α and IL-6. The blood collected in heparin tubes ware used to determine the various hematological parameters such as Red Blood Cell (RBC) count, Hemoglobin (Hb) count, platelet count, lymphocyte %, and neutrophil %.
Statistical investigation:
Readings were presented as the average±Standard Error of the Mean (SEM). The statistical data has been investigated by GraphPad PRISM software version 9.4.0. The data were compared using one- way Analysis of Variance (ANOVA) followed by the Tukey test. The p values ≤0.0001 were considered statistically significant.
Results and Discussion
Different chemical screening tests were performed on L. nobilis extract to determine the presence of phytochemicals present in it which indicated the presence of alkaloids, glycosides, flavonoids, tannins, saponins, phenols, volatile oils, and steroids. The TLC confirmed the presence of various phytochemicals in the L. nobilis extract such as terpinol (Rf value=0.27), terpenoids (Rf value=0.45), 1,8- cineole (Rf value=0.54), linalool (Rf value=0.61), flavonoids (Rf value=0.86) and α-terpinyl acetate (Rf value=0.72). Hemorrhoids are a clinical disorder characterized by excessive vascular permeability in the recto-anal area, resulting in inflammation of the surrounding cells and tissues. This leads to secondary issues such as fluid draining into interstitial spaces, increased mobility of inflammatory cells, and increased capillary permeability. The inflammation induced by croton oil activates eicosanoids, leukotrienes, bradykinin, nitric oxide, and other pro-inflammatory mediators, including TNF-α and IL-6. All of these factors, whether acting together or separately, regulate the signaling of effector cells such as phagocytes, labrocytes, fibroblasts, and endothelial cells. They also influence novel inflammatory molecules like leukocytes, neutrophils, eosinophils, and lymphocytes, contributing to the induction of inflammation.
In the current investigation, rats administered croton oil for the induction of hemorrhoids had a lower recto-anal coefficient, consistent with past results. The results of this study demonstrated that the croton oil-induced hemorrhoids model caused significant alterations in blood parameters and pro-inflammatory marker levels. There was a significant reduction ( ap<0.0001) in RBC count and Hb levels in group 2 Positive Control (PC) compared to group 1 Normal Control (NC). The pilex treated group exhibited a notable increase ( bp<0.0001) in the mean Hb and RBC level. The L. nobilis extract-treated group exhibited a significant increase ( bp<0.0001) in the RBC count and Hb level when compared to the group 3 (standard group). Comparison of the PC with the NC revealed that the mean PC, NC, and lymphocyte count were significantly increased in the positive group ( ap<0.0001). Hemorrhoids- induced rats when treated with the standard drug, a high dose of 300 mg/kg and low dose extract 150 mg/kg) of L. nobilis extract exhibited a significant decline in the mean PC, NC, and lymphocyte count when compared to the PC. Table 1, contains all the observational findings.
| Group | Treatment | RBC (million/μl) | Platelets (thousand/μl) | Hemoglobin (g/dl) | Neutrophils (%) | Lymphocytes (%) |
|---|---|---|---|---|---|---|
| Group 1 | NC | 6.51±0.34 | 497.5±40.66 | 12.13±0.59 | 48.37±1.51 | 48.71±1.34 |
| Group 2 | PC (croton-induced hemorrhoids ) | 2.29±0.27a | 975.5±37.93a | 5.57±0.55a | 62.1±2.72a | 65.51±1.09a |
| Group 3 | Standard group (STD) (pilex) 200 mg/kg | 5.80±0.31b | 641.8±30.22b | 10.77±0.63b | 43.67±1.99b | 52.2±2.67b |
| Group 4 | Test group 1-low dose extract 150 mg/kg | 2.96±0.44b | 762.8±75.28b | 7.61±0.81 | 51.27±4.63 | 59.97±1.38 |
| Group 5 | Test group 2-high dose extract 300 mg/kg | 3.35±0.37b | 633.5±57.07 | 8.99±0.65 | 41.13±1.93b | 54.48±2.53 |
Note: Each value is demonstrated as mean±SEM, ap<0.0001 vs. group 1 and bp<0.0001 vs. group 2
Table 1: Effect of Treatment on Blood Parameters
There was a rise in the mean BW of the rats after the induction of hemorrhoids with croton oil mixture as compared to the group 1 (normal control). Following oral administration of the standard drug (pilex 200 mg/kg), low dose of L. nobilis extract (150 mg/kg), and high dose of the L. nobilis (300 mg/kg) for 21 d, there was a decrease in the BW of the Wistar rats. A significant rise ( ap<0.0001) in the mean recto-anal weights and RAC of the rats was observed in the group 2 in contrast to the group 1 (normal control). However, the mean recto-anal weights and RAC value decreased significantly ( bp<0.0001) when the animals were treated with the standard drug pilex, low dose extract, and high dose extract of L. nobilis on comparison with the group 2. All the findings are depicted in Table 2.
| Group | Treatment | BW (g) on 7th d | BW (g) on 21st d | Recto-anal weight (mg) | Recto-anal coefficient |
|---|---|---|---|---|---|
| Group 1 | NC | 183.33±6.09 | 187±6.15 | 173.33±2.52 | 0.95±0.02 |
| Group 2 | PC (croton-induced hemorrhoids) | 191±7.24 | 190±6.96 | 481.5±2,44a | 2.54±0.08a |
| Group 3 | Standard group (STD) (pilex) 200 mg/kg | 184.33±6.46 | 181.67±5.11 | 278±1.91b | 1.53±0.04b |
| Group 4 | Test group 1-low dose extract 150 mg/kg | 185.67±6.51 | 179±5.53 | 353.33±2.15b | 1.94±0,05b |
| Group 5 | Test group 2- high dose extract 300 mg/kg | 182.83±5.21 | 175.67±5.01 | 329±1.63b | 1.86±0.04b |
Note: Each value is demonstrated as mean±SEM, ap<0.0001 vs. group 1 and bp<0.0001 vs. group 2
Table 2: Effect of Treatment on BW, Recto-anal weight, and RAC
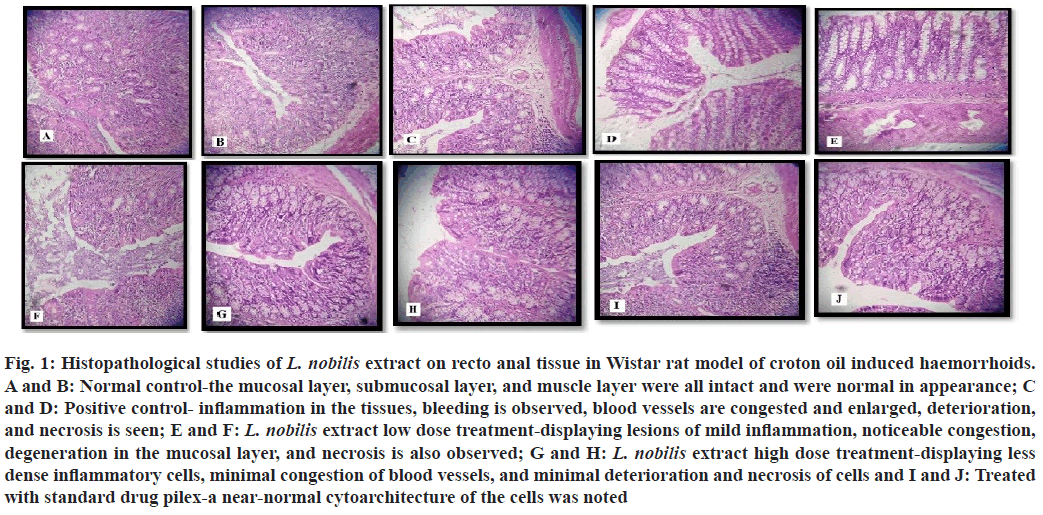
There was a notable increase ( ap<0.0001) observed in the IL-6 and TNF-α values of the group 2 on comparison with the group 1 (normal control). A remarked decline in the values was seen in the groups treated with the standard drug and L. nobilis extract orally for 21 d. All the treated groups caused a significant decrease ( bp<0.0001) in the level of IL-6 and TNF-α when compared to the roup 2 (positive control). All the readings are depicted in Table 3. The recto anal coefficient significantly decreased in the group that received treatment with a high dose of L. nobilis extract (300 mg/kg) and the standard drug Himalaya pilex. The recto anal coefficient also decreased in the low-dose treatment with L. nobilis extract. Histopathological observations of the anorectal tissue in the normal control group showed that the mucosal layer, submucosal layer, and muscle layer were all intact and appeared normal, as depicted in fig. 1A and fig. 1B. The positive control group, which included the untreated hemorrhoid-induced group, exhibited significant inflammation in the tissues, bleeding, congested and enlarged blood vessels, as well as deterioration and necrosis is seen in fig. 1C and fig. 1D. The group that was treated with a low dose of L. nobilis extract (150 mg/kg), displaying lesions of mild inflammation, noticeable congestion, degeneration in the mucosal layer, and necrosis is also observed in fig. 1E and fig. 1F. The group treated with a high dose of L. nobilis extract (300 mg/kg) showed fewer inflammatory cells, minimal congestion of blood vessels, and reduced deterioration and necrosis, as depicted in fig. 1G and fig. 1H. A near-normal cytoarchitecture of the cells was noted in the group that was treated with the standard drug Himalaya pilex at a dose of 200 mg/kg as seen in fig. 1I and fig. 1J.
| Group | Treatment | IL-6 (pg/ml) | TNF-α (pg/ml) |
|---|---|---|---|
| Group 1 | NC | 45.71±3.95 | 352.52± 91.7 |
| Group 2 | PC (croton-induced hemorrhoids) | 107.93±3.40a | 938.42±93.219a |
| Group 3 | Standard group (STD) (pilex) 200 mg/kg | 64.65 ±2.83b | 306.81±90.208b |
| Group 4 | Test group I-low dose extract 150 mg/kg | 84.72±3.02b | 442.47±71.22b |
| Group 5 | Test group II-high dose extract 300 mg/kg | 84.72±3.02b | 335.85±82.43b |
Note: Each value is demonstrated as mean±SEM, ap<0.0001 vs. group 1 and bp<0.0001 vs. group 2
Table 3: Effect of Treatment on Inflammatory Mediators IL-6 and TNF-α
Fig. 1: Histopathological studies of L. nobilis extract on recto anal tissue in Wistar rat model of croton oil induced haemorrhoids. A and B: Normal control-the mucosal layer, submucosal layer, and muscle layer were all intact and were normal in appearance; C and D: Positive control- inflammation in the tissues, bleeding is observed, blood vessels are congested and enlarged, deterioration, and necrosis is seen; E and F: L. nobilis extract low dose treatment-displaying lesions of mild inflammation, noticeable congestion, degeneration in the mucosal layer, and necrosis is also observed; G and H: L. nobilis extract high dose treatment-displaying less dense inflammatory cells, minimal congestion of blood vessels, and minimal deterioration and necrosis of cells and I and J: Treated with standard drug pilex-a near-normal cytoarchitecture of the cells was noted
Based on the research findings related to histopathological parameters, blood parameters, RAC, IL-6, and TNF-α, we concluded that L. nobilis, at both high (300 mg/kg) and low (150 mg/kg) doses, demonstrated potential anti- hemorrhoidal activity against the croton oil- induced hemorrhoid model. It was also found that a high dose (300 mg/kg) of L. nobilis extract exhibited a better therapeutic effect compared to the low dose (150 mg/kg) of L. nobilis extract. Pilex, the standard drug, showed better results compared to the leaf extract. This study leads to the conclusion that L. nobilis extract has anti- hemorrhoidal potential and warrants further exploration. It might be necessary to do additional studies to isolate diverse molecules, which could pave the way for recently created novel compounds to act as pharmacological and chemical agents for various illnesses.
Acknowledgements:
The author remains highly grateful and expresses their profound gratitude to Director Dr. (Col) A. Garg and Joint Director Dr. Manoj Goel, for providing all the essential facilities required for the successful completion of this research work.
Conflict of interests:
The authors declared no conflict of interests.
References
- Poiglase L. Clinical Practice Haemorrhoids: A clinical update.
- Lohsiriwat V. Approach to hemorrhoids. Curr Gastroenterol Rep 2013;15:1-4.
[Crossref] [Google Scholar] [PubMed]
- Malekuti J, Mirghafourvand M, Samadi K, Abbasalizadeh F, Khodaei L. Comparison of the effect of Myrtus communis herbal and anti-hemorrhoid ointments on the hemorrhoid symptoms and quality of life in postpartum women with grade I and II internal hemorrhoid: A triple-blinded randomized controlled clinical trial. J Complement Integr Med 2019;16(4):20180147.
[Crossref] [Google Scholar] [PubMed]
- Chauhan R, Ruby KM, Dwivedi J. Golden herbs used in piles treatment: A concise report. Int J Drug Dev Res 2012;4(4):50-68.
- Misra MC, Imlitemsu. Drug treatment of haemorrhoids. Drugs 2005;65:1481-91.
[Crossref] [Google Scholar] [PubMed]
- Jha SK, Karki R. Evaluation of antiulcer activity of famotidine-loaded microemulsion on experimental animals. J Chronotherapy Drug Deliv 2014;5(1):1-8.
- Caputo L, Nazzaro F, Souza LF, Aliberti L, de Martino L, Fratianni F, et al. Laurus nobilis: Composition of essential oil and its biological activities. Molecules 2017;22(6):930.
[Crossref] [Google Scholar] [PubMed]
- Kaurinovic B, Popovic M, Vlaisavljevic S. In vitro and in vivo effects of Laurus nobilis L. leaf extracts. Molecules 2010;15(5):3378-90.
[Crossref] [Google Scholar] [PubMed]
- Nayak S, Nalabothu P, Sandiford S, Bhogadi V, Adogwa A. Evaluation of wound healing activity of Allamanda cathartica. L. and Laurus nobilis. L. extracts on rats. BMC Complement Altern Med 2006;6:1-6.
[Crossref] [Google Scholar] [PubMed]
- Sayyah M, Valizadeh J, Kamalinejad M. Anticonvulsant activity of the leaf essential oil of Laurus nobilis against pentylenetetrazole-and maximal electroshock-induced seizures. Phytomedicine. 2002;9(3):212-6.
[Crossref] [Google Scholar] [PubMed]
- Elmasta? M, Gulçin I, I?ildak O, Küfrevioglu OI, Ibaoglu K, Aboul-Enein HY. Radical scavenging activity and antioxidant capacity of bay leaf extracts. J Iran Chem Soc 2006;3:258-66.
- Algabri SO, Doro BM, Abadi AM, Shiba MA, Salem AH. Bay leaves have antimicrobial and antioxidant activities. J Pathog 2018;1(1):3.
- Pugazhenthi M, Suganthi R. Screening of phytochemicals and antibacterial poteninal of Laurus nobilis. Glob J Mod Biol Technol 2013;3(2).
- Loizzo MR, Saab AM, Tundis R, Statti GA, Menichini F, Lampronti I, et al. Phytochemical analysis and in vitro antiviral activities of the essential oils of seven Lebanon species. Chem Biodivers 2008;5(3):461-70.
[Crossref] [Google Scholar] [PubMed]
- Brinza I, Boiangiu RS, Hancianu M, Cioanca O, Erdogan I, Hritcu L. Bay leaf (Laurus nobilis L.) incense improved scopolamine-induced amnesic rats by restoring cholinergic dysfunction and brain antioxidant status. Antioxidants 2021;10(2):259.
[Crossref] [Google Scholar] [PubMed]
- Acciarri N, Rotino GL, Sabatini E, Voltattorni S, Cariaci T, Valentino D, et al. Identification of resistant traits to Fusarium and Verticillium wilt in Italian tomato landraces. Plant Pathol J 2007;89(27):257.
- Erler FE, Ulug I, Yalcinkaya B. Repellent activity of five essential oils against Culex pipiens. Fitoterapia 2006;77(7-8):491-4.
[Crossref] [Google Scholar] [PubMed]
- Samejima K, Kanazawa K, Ashida H, Danno GI. Bay laurel contains antimutagenic kaempferyl coumarate acting against the dietary carcinogen 3-amino-1-methyl-5 H-pyrido [4, 3-b] indole (Trp-P-2). J Agric Food Chem 1998;46(12):4864-8.
- Sayyah M, Saroukhani G, Peirovi A, Kamalinejad M. Analgesic and anti?in?ammatory activity of the leaf essential oil of Laurus nobilis Linn. Phytother Res 2003;17(7):733-6.
[Crossref] [Google Scholar] [PubMed]
- Rincon E, Balu AM, Luque R, Serrano L. Mechanochemical extraction of antioxidant phenolic compounds from Mediterranean and medicinal Laurus nobilis: A comparative study with other traditional and green novel techniques. Ind Crop Prod 2019;141:111805.
- Samanta KC. Evaluation of antiulcer activity of leaf extracts of Laurus nobilis Linn. 2012.
- Krishna R. Phytochemical studies.
- Dey YN, Wanjari MM, Kumar D, Lomash V, Jadhav AD. Curative effect of Amorphophallus paeoniifolius tuber on experimental hemorrhoids in rats. J Ethnopharmacol 2016;192:183-91.
[Crossref] [Google Scholar] [PubMed]
- Azeemuddin M, Viswanatha GL, Rafiq M, Thippeswamy AH, Baig MR, Kavya KJ, et al. An improved experimental model of hemorrhoids in rats: Evaluation of antihemorrhoidal activity of an herbal formulation. ISRN Pharmacol 2014;(1):530931.
[Crossref] [Google Scholar] [PubMed]