- *Corresponding Author:
- J. Bakht
Department of Botany, University of Hazara, Peshawar, Khyber-Pakhtunkhwa-29120, Pakistan
E-mail: jehanbakht@yahoo.co.uk
| Date of Submission | 30 August 2016 |
| Date of Revision | 17 December 2016 |
| Date of Acceptance | 13 March 2017 |
| Indian J Pharm Sci 2017;79(2):257-266 |
Abstract
The current research work was carried out to investigate the antimicrobial, antinociceptive and antipyretic activities of different solvent extracts of Forsskaolea tenacissima. This investigation revealed that ethyl acetate extract exerted maximum inhibition (56%) of the growth of Providencia mirabilis and 48% of Aspergillus fumigatus. Penecillium was the most resistant fungi and was unaffected by any extracts. The analgesic activity of these extracts at a dose of 150 mg/kg increased the reaction time after 60, 90, 120 and 180 min compared to the initial latency as well as that of control group in the hot plate method. The number of writhes recorded after 150 mg/kg extract were comparatively lower (56±3.74) than that of normal saline group (76±4.15) in acetic acid-induced writhing test. The antipyretic effect of the plant extracts at 300 mg/kg was comparable with normal saline.
Keywords
Antimicrobial, analgesic, antipyretic, disc diffusion assay
Both human beings and herbs are closely connected to each other since the inception. Human beings are using herbs both as food and as medicines for the time immemorial. As the time passed on, human beings made improvements in this domain and currently more research is being done in this regard. Although allopathic medicines are in use in parallel, people are reluctant to use these due to their side effects. Public prefer to use herbal medicines since these are aromatic with flavors, perceived to be quite safe to the user as well as the environment, while low on side effects. Currently 75% of the world population use different herbs and their extracts for various aspects of health care and more than 30% of herbs are used for medicinal purposes [1].
Since many centuries herbs are being utilized for the remedial objectives. At the rudimentary phase, these herbs were used in it pure natural form like teas, cataplasm, pulverize and other plant products [2,3]. Developing countries have been using herbal medicines for a continued span of time and the same trend has now been followed by the developed countries [4-7]. Medicinal plants contained some active compounds that could be used against microbes [8-21]. Secondary metabolites in most of the healthy plants are present in their bioactive shape, while some come as inactive forerunners, which are activated metabolically by the pathogen or by the host [22]. Currently most of the pharmaceutical products are being derived from both the wild or cultivated herbs [23].
Forsskaolea tenacissima, commonly known as Nettle Desert, belongs to the family Urticaceae (nettle family) and is a member of the non-stinging nettles genus Forsskaolea. Forsskaolea is comprised of six species, which are found in different regions of the world from Canary Islands and south eastward Spain to Pakistan and India [24-26]. F. tenacissima grows in those areas where other plants cannot survive like road sides, stony valleys and rock gaps. F. tenacissima is used as local therapy for wound healing and hemostatic in many countries. Its leaves are also used as tisane (Herbal tea) for treating rheumatoid arthritis and for the removal of bile stone from gall bladder [27]. The plants also have significant antioxidant potential and larvicidal activity [28,29]. In Pakistan, F. tenacissima L. is being used in folk medicine as antiinflammatory, antispasmodic, antidiabetic and antipyretic [30]. Keeping in view the medicinal importance of F. tenacissima, the present research work was carried out with the objectives to investigate antimicrobial, analgesic and antipyretic activities of different extracts. The present research project was approved by the Institutional Ethics Committee.
Materials and Methods
Fully mature plants of F. tenacissima were collected from the Mountains of Mohmand Agency, Khyber Pakhtunkhwa, Pakistan during June. The plant specimen was identified in the Department of Botany, University of Peshawar, Pakistan. After thorough washing with running tap water, the plant materials were chopped, shade dried and ground in an electric grinder.
Crude extract preparation:
One kilogram of the powdered material was soaked in five litres of methanol, kept at 25° in the dark for one week and agitated three times a day. The mixture was filtered through Whatman filter paper No.1. The residue was mixed with 2500 ml fresh methanol and the whole procedures were repeated thrice. All fractions of the filtered methanol solution were dried at 45° under vacuum pressure using rotary evaporator. The crude extract was divided into two portions, one to be used as crude extract and the other part was fractionated with different solvents.
Fractionation of crude extract:
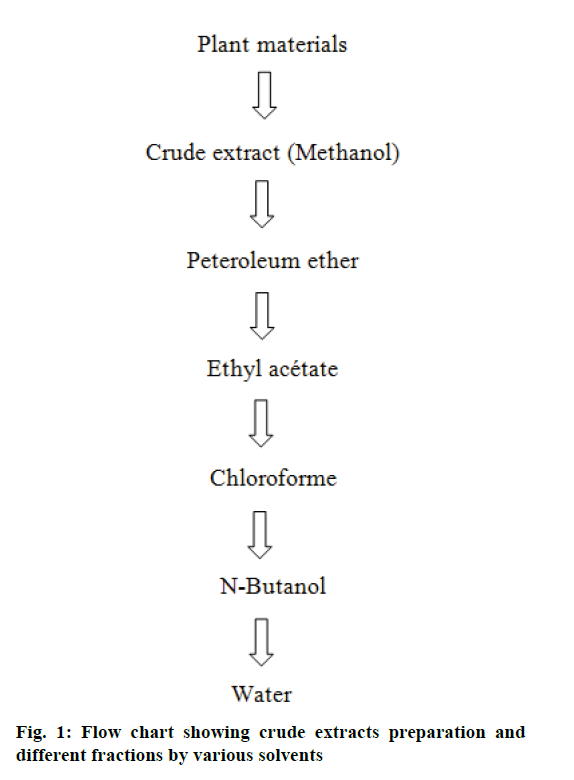
Hundred grams of the crude extract was dissolved in 500 ml sterile distilled water, mixed with 300 ml petroleum ether, shaken gently and allowed to stand for 15 min to separate the two phases. The upper petroleum ether phase was obtained the lower aqueous phase was re-extracted three times with fresh petroleum ether. All fractions of petroleum ether were pooled together, dried at 45° under vacuum using a rotary evaporator. The same procedures of fractionation were carried out with chloroform, ethyl acetate and n-butanol [31]. The lower aqueous phase at the end of the procedure was dried as described previously (Figure 1).
Preparation of media:
Nutrient broth was used for shaking incubation, standardization and nutrient agar medium for culturing to grow all microorganisms [31]. Known quantities of nutrient agar and nutrient broth were poured into conical flasks. Twenty milliliters per test tube of the nutrient broth was also poured. All the media flasks and test tubes were sterilized, poured aseptically into sterilized petri plates and allowed to solidify for about an hour. After 24 h, uncontaminated plates were used for culturing of bacteria and fungi.
Disc diffusion susceptibility assay:
Antifungal activity of different extracts were carried out using the disc diffusion assay as described by Ramdas et al.[32] and Bauer et al.[33]. Nutrient agar media plates were inoculated with 18-24 h cultures of microbial inoculums (Standard inocula 1-2×107 CFU/ ml 0.5 McFarland Standard). Three discs of Whatman No. 1 filter paper (6 mm in diameter) were placed on the media in petri plates with the help of a sterile forceps. Plant extracts in concentration of 1 and 2 mg/ disc in 6 and 12 μl volumes were applied on the discs. Antibiotics as positive control and dimethyl sulfoxide (DMSO) (12 μl/disc) as negative control were also applied on the discs in separate petri plates. Inoculated plates were kept at 37° for 18-24 h. The next day, zone of inhibition (ZOI) was recorded in mm around the discs in each plate. The experiments were conducted in triplicate and the ZOI was determined by the following Eqn., percent inhibition (%) = (zone of sample)/(zone of control)×100.
Positive controls and test organisms:
For Gram-positive bacteria; ciprofloxacin 50 μg per 12 μl, for Gram-negative bacteria; ciprofloxacin 50 μg per 12 μl, for fungal strain; clotrimazole 50 μg per 12 μl were used as positive controls. Antimicrobial activity was tested against seven bacteria (Escherichia coli, Salmonella typhi, Pseudomonas aeruginosa, Providencia sp., Proteus mirabilis, Shigella sonnei and Citrobacter sp.) and three fungal strains (Aspergillus fumigatus, Penicillium chrysogenum and Rhizopus sp.).
Determination of analgesic activity, the hot plate method:
Albino mice of either sex weighing 18-22 g were selected for this experiment. The analgesic activity of the plant’s extract was measured using the hot-plate method [34]. Thirty albino mice were randomly assigned to 5 treatment groups of 6 mice each. One group received 0.9% saline on weight basis (control) and another group was given standard and the remaining three groups were given the extract at three dose levels, 50, 100 and 150 mg/kg, respectively. Each mouse was weighed properly and the dose of the test samples and control materials were adjusted accordingly. All drugs were given orally to the respective group of mice as a suspension and normal saline, standard drug was administered intraperitoneal route (IP) to the mice. A 30 min interval was given to ensure proper absorption of the administered substances. After 30 min, the animals that showed fore paw licking or jumping response within 6-8 s were selected for the study. The mice were placed on a hot plate maintained at 55 ± 0.5°. And the time lapse for the mouse to respond to the thermal pain (reaction time) was noted. Rubbing of palms or jumping out was used as endpoint. A cut off time of +10 s was followed to avoid any thermal injury to the paws. The reaction time was recorded before and after +30, +60, +90, +120 and +180 min following administration of test or standard drugs.
Evaluation criteria:
The mean reaction time for each treated group was determined and compared with that obtained from each group before treatment. Percentage increase in reaction time (I %), was derived, using the following Eqn., I% = {(It – I0)/I0}×100, where, It is the reaction time at time (t) and I0 is the reaction time at time zero (0 h). The animals were subjected to the same test procedure at +30, +60, +120 and +180 min after the administration of test/standard/control drug [35]. All animals were sacrificed at the end of experiment.
Acetic acid (AA)-induced writhing test in mice:
Albino mice of either sex, weighing 18-22 g were used. Animals were withdrawn from food and water 2 h before the start of experiment. Mice were divided into 5 groups, each group consisting of six animals. One group served as negative control (normal saline ml/ kg), second group served as positive control (aspirin 5 mg/kg), while third, fourth and fifth groups received the extract of both plants orally in doses of 50,100,150 mg/kg. Writhing behaviour was tested in those mice in which 1% AA was administered IP and number of abdominal constrictions occurring over the period of 20 min was counted just after 5 min of administering 1% AA (10 ml/kg). The test samples (50, 100 and 150 mg/kg) or aspirin 100 mg/kg or 0.9% sodium chloride was administered IP 30 min before 1% AA administration. Percent analgesia was calculated with the help of following Eqn., % protection = 100– (number of writhes in experimental group×100/number of writhes in control).
Antipyretic activity:
Antipyretic activity of crude extract of F. tenacissima was measured by slightly modifying the method described by Adams et al. using mice (30-35 g) of either sex [36]. The selected animals were healthy and adjusted to laboratory conditions before the start of experiment. Pyrexia was induced by injecting 20% w/v brewer's yeast suspension (10 ml/kg) subcutaneously into the animals' dorsum region. Normal body temperature of each mouse was recorded using a digital thermometer [37]. All groups were fasted overnight but allowed free accesses to drinking water and after 24 h rectal temperature of each mouse was recorded. The induction of pyrexia was confirmed by rise in temperature more than 0.7° F, while animals showed rise in temperature less than 0.7° F were excluded from experiment.
Animal grouping:
The animals were divided into five groups each group consist of six mice. The group I received saline (10 ml/ kg) as a negative control, group II received paracetamol (150 mg/kg) as a standard drug while the remaining groups III, IV and V received 100, 200 and 300 mg/ kg F. tenacissima crude extract orally, respectively [37].
After drug administration, rectal temperature was again recorded periodically at 0.5, 1, 2, 3 and 4 h of drug administration.
Statistical analysis:
Data are presented as mean values of three replicates. MSTATC computer software (version 6.1) was used to carry out statistical analysis [38]. Significant difference among means was compared using Least Significant Difference (LSD) test [39].
Results and Discussion
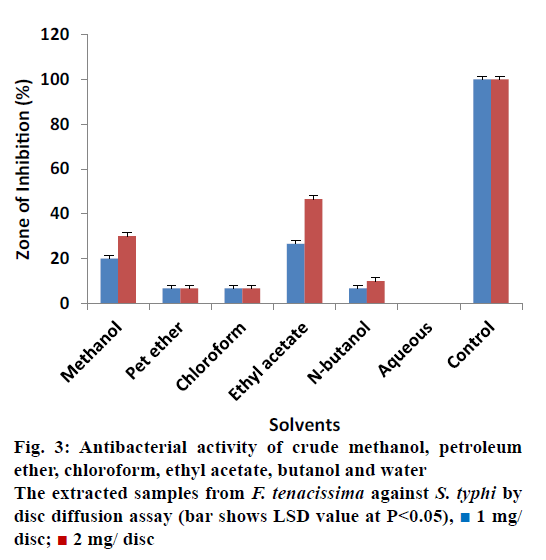
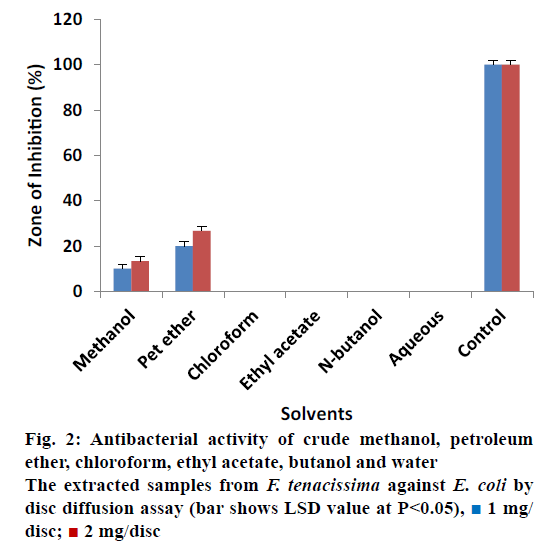
The antibacterial activity of all extracted samples from F. tenacissima against E. coli is shown in Figure 2. The data revealed that most of the extracts showed no activity against E. coli at concentration of 1 and 2 mg/ disc except the crude methanol and petroleum ether extracts. Crude methanol extract measured inhibitory zone of 10% ± 0.20 and 13.33% ± 0.17 at concentrations 1 and 2 mg/disc correspondingly. Similarly, petroleum ether extracted samples showed 20% ± 0.30 ZOI at a concentration of 1 mg/disc and 26.67% ± 0.40 ZOI at concentration of 2 mg/disc. These results revealed that all the extracts showed activity against S. typhi except the aqueous extract (Figure 3). Most effective antibacterial activity against S. typhi was exhibited by the ethyl acetate extract (26.67% ± 0.50 and 46.67% ± 0.50 ZOI at 1 and 2 mg/disc concentrations, respectively). Crude methanol extracts on the other hand showed activity of 20% ± 0.30 at 1 mg/disc and 30% ± 0.30 at 2 mg/ disc concentration. Petroleum ether, chloroform and n-butanol extracts showed minor activities in the range of 6.67 to 10%.
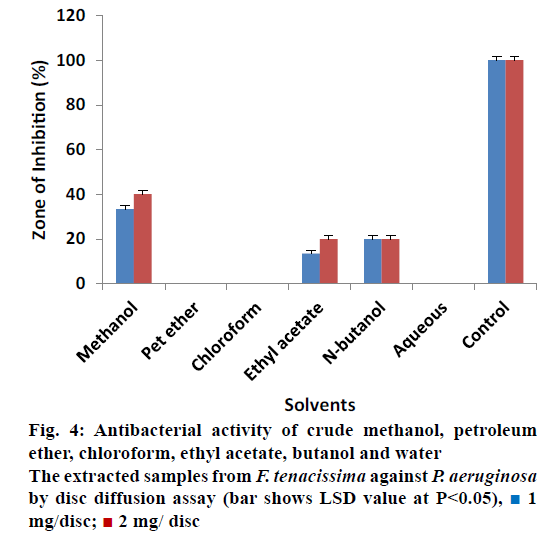
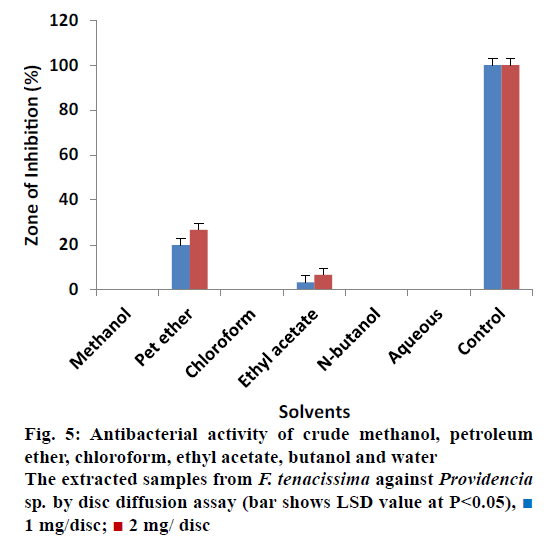
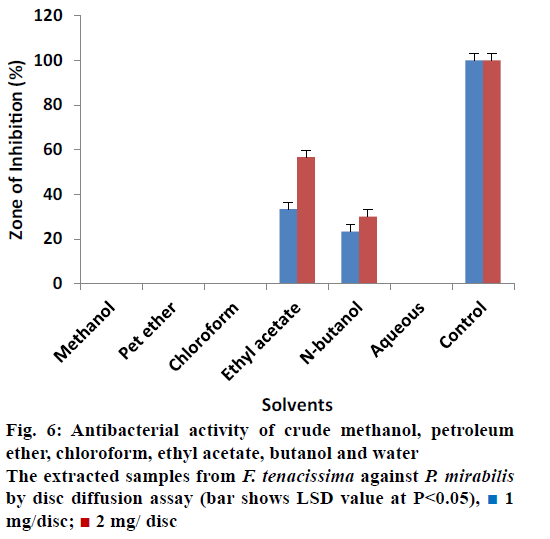
All extracts of F. tenacissima were also evaluated against P. aeruginosa (Figure 4). The data indicated that crude methanol extract showed ZOI of 33.33% ± 0.30 at 1 mg/disc and 40% ± 0.34 at 2 mg/disc concentration. Ethyl acetate extract yielded 13.33% ± 0.17 and 20% ± 0.50 activity at 1 and 2 mg/disc, respectively. N-butanol extract on the other hand showed ZOI of 20% ± 0.50 at concentrations of 1 and 2 mg/disc each. Petroleum ether, chloroform and aqueous extracts did not show any activity against P. aeruginosa. These results indicated that almost all extracts were inactive against Providencia sp. except petroleum ether and ethyl acetate extracts, which showed minimum activity (Figure 5). Petroleum ether extract produced ZOI of 20% ± 0.20% and 26.67% ± 0.17 at concentrations of 1 and 2 mg/disc, while, ethyl acetate extract yielded ZOI of 3.33% ± 0.00 at 1 mg/disc and 6.66% ± 0.50 at 2 mg/disc concentrations. The data revealed that almost all the extracts were ineffective to control the growth of Proteus except ethyl acetate and n-butanol, which showed activity compared to controls (Figure 6). Ethyl acetate extracted samples showed significant results revealing 33.33 ± 0.50% ZOI at concentration of 1 mg/ disc and 56.66 ± 0.20% ZOI at concentration of 2 mg/ disc. Similarly, n-butanol extracted samples measured 23.33 ± 0.51% and 30.00 ± 0.17% ZOI at 1 and 2 mg/disc concentrations, respectively.
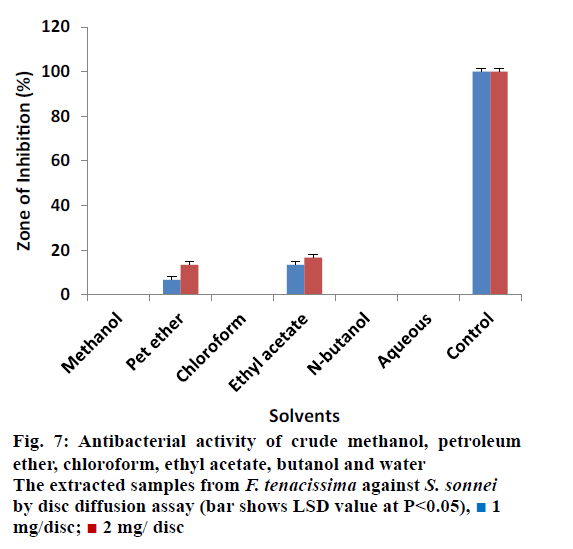
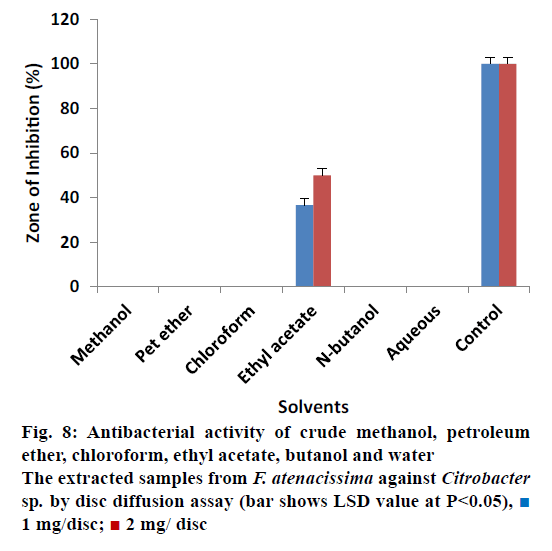
It was also observed that almost all the extracts were non effective against Shigella except petroleum and ethyl acetate extracted samples (Figure 7). Petroleum ether extracted samples showed 06.66 ± 0.80% ZOI at 1 mg/ disc and 13.33 ± 0.78% ZOI at 2 mg/disc concentrations. Petroleum ether extracted also showed minimum activity. Ethyl acetate extracted samples measured inhibitory zone of 13.33 ± 0.30% and 16.66 ± 0.50% at 1 mg/disc and 2 mg/disc concentrations, respectively. Different extracted samples from F. tenacissima, were screened against Citrobacter as shown in Figure 8. Our results indicated that all the tested extracts were not effective to control the activity of against Citrobacter except ethyl acetate extracted samples, which inhibit the activity of the tested microbes by 36.66 ± 0.26% at 1 mg/disc and 50.00 ± 0.35% at concentration of 2 mg/disc.
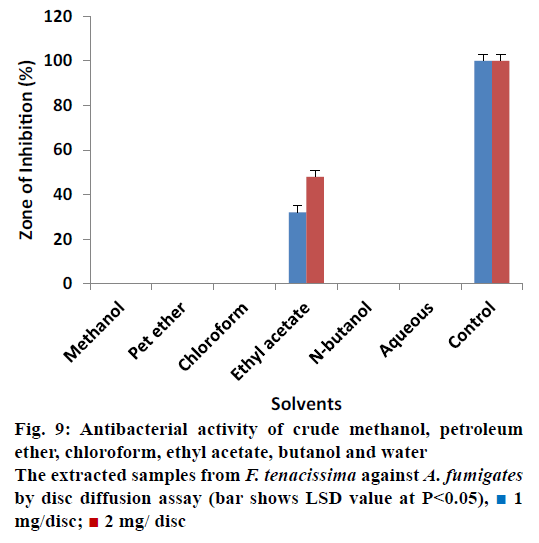
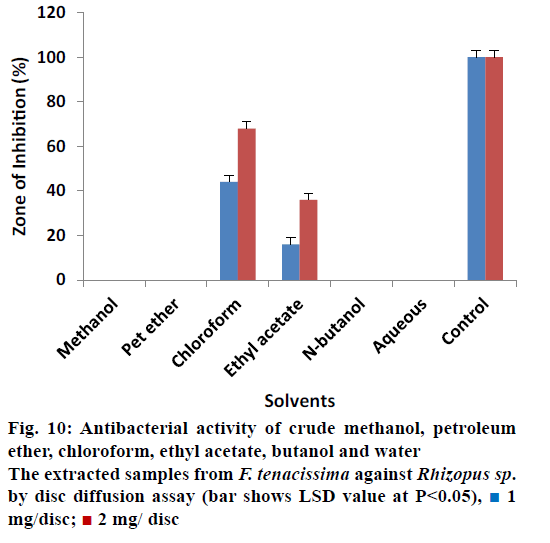
The antifungal activity of different solvent extracted samples of F. tenacissima that almost all extracts showed 0% ZOI against A. fumigatus at concentration of 1 and 2 mg/disc except ethyl acetate extracted samples (Figure 9). The data indicated that ethyl acetate extracted samples reduced the activity of A. fumigatus by 32.00 ± 0.40% at 1 mg/disc and 48.00 ± 0.40% at 2 mg/disc concentration when compared with controls. Data regarding the antifungal activity of extracted samples of F. tenacissima is shown in Figure 10. The data indicated that, most of the extracts showed no activity (ZOI) against Rizophus except chloroform and ethyl acetate extract. Chloroform extracted samples reduced the activity of the tested microbe by 44.00 ± 0.50% at concentration of 1 mg/disc and 68.00 ± 0.50% at 2 mg/disc. Similarly, ethyl acetate extracted samples showed activity of 16.00 ± 0.44% at a 1 mg/disc and 36.00 ± 0.70% at a concentration of 2 mg/disc.
Table 1 showed the data concerning the crude methanol extract of F. tenacissima with 50, 100 and 150 mg/ kg concentration for its analgesic activity using hot plate method. Different latency times were shown by different doses at different intervals of time. Extract at 50 mg/kg showed a minor increase in reaction time after 30 and 60 min, which was 14.16 ± 1.94 and 12.33 ± 2.065 s, respectively in comparison to the initial time and the corresponding measurement in the saline control group. Extract at 100 mg/kg was not effective, however, showed a little rise in latency time after 90 and 120 min, which was 13.83 ± 2.13 and 13.5 ± 1.37 s, respectively. Similarly, in comparison to its initial latency time and that in the control group. The extract at 150 mg/kg showed increment in reaction time after 60, 90, 120 and 180 min. i.e. 16.66 ± 1.63, 17.83 ± 0.98, 17 ± 1.89 and 14.83 ± 2.71 s, respectively. The gradual decrease of latency observed indicated that the effect of these extracts decreased with time. The reaction time of saline control was normal throughout the experiment and no increase in reaction time was observed. Morphine significantly (P<0.05) increased latency of thermally-induced pain on the hot plate. It is clear that crude extract of F. tenacissima at different concentrations exhibited no significant results, except at high concentration (150 mg/kg) showed effects similar to the standard morphine after 60, 90 and 120 min. Extract at 100 mg/kg dose also showed effect after 60 and 120 min.
| Group | Dose mg/kg |
Mean pain latency (s) | |||||
|---|---|---|---|---|---|---|---|
| At 0 min |
After 30 min | After 60 min | After 90 min | After 120 min | After 180 min | ||
| Normal saline |
--- | 11.5 ± 1.048 | 11.83 ± 0.75 | 12.5 ± 1.048 | 12.16 ± 1.47 | 10.83 ± 1.16 | 11.83 ± 1.16 |
| Standard morphine | 5 | 11.66 ± 1.63 | 17.16 ± 1.47 | 19.16 ± 1.47 | 20 ± 1.26 | 10.33 ± 0.81 | 11.16 ± 0.75 |
| F. tenacissimaextract | 50 | 11.66 ± 1.21 | 14.16 ± 1.94 | 12.33 ± 2.065 | 11.5 ± 2.42 | 9.66 ± 1.36 | 11.33 ± 1.50 |
| 100 | 12 ± 1.41 | 10.66 ± 1.96 | 9.83 ± 1.32 | 13.83 ± 2.13 | 13.5 ± 1.37 | 12.5 ± 1.51 | |
| 150 | 12.5 ± 2.073 | 12.16 ± 1.169 | 16.66 ± 1.63 | 17.83 ± 0.98 | 17 ± 1.89 | 14.83 ± 2.71 | |
Table 1: Effect of crude methanol extract of Forsskaolea Tenacissima on thermally induced pain in mice
Different doses of the crude methanol extract of F. tenacissima (50, 100 and 150 mg) were also tested for analgesic property (Table 2). Extract at 50 mg concentration displayed 12.07% inhibition of writhes and the mean numbers of writhes recorded against this extract were 66.83 ± 5.11, which showed non-significant results as compared to standard aspirn (20.67 ± 3.98). Extract at 100 mg/kg exhibited a 21.49% inhibition of writhes produced by AA, which were 59.67 ± 1.37, hence the dose of 100 mg/kg was not effective as compared to the standard aspirin (20.67 ± 3.98), although extract did produce some effect as compared to the normal saline group (76 ± 4.15). Extract at 150 mg/kg dose exerted 26.32% inhibition of abdominal constrictions produced by 1% AA and the number of writhes recorded were 56 ± 3.74, which were comparatively less than that of the normal saline group (76 ± 4.15).
| Group | Treatment | dose (mg/kg) |
Number of writhes Mean ± SEM |
Inhibition (%) |
|---|---|---|---|---|
| Control | Normal saline | ----- | 76 ± 4.15 | |
| Standard | Aspirin | 50 | 20.67 ± 3.98 | 72.80 |
| Crude methanol extract | ----- | 50 | 66.83 ± 5.11 | 12.07 |
| ----- | 100 | 59.67 ± 1.37 | 21.49 | |
| ----- | 150 | 56 ± 3.74 | 26.32 |
Table 2: Effect of crude methanol extract of Forsskaolea Tenacissima on acetic acid-induced writhing in mice
Table 3 showed the antipyretic activity of F. tenacissima crude extract at different doses in Swiss albino mice. The data indicated that the average temperature of control group before brewer‘s yeast injection was 99 ± 0.28° F and after brewer’s yeast injection the average temperature rose to 101.58 ± 0.49° F. These results revealed that brewer yeast increased the rectal temperature of control group. The control group was then given normal saline and temperature was recorded at 0.5, 1, 2, 3 and 4 h, which was 102.016 ± 0.65° F, 102.21 ± 0.50° F, 102.21 ± 0.50° F, 101.58 ± 0.49° F and 100.95 ± 0.64° F, respectively. A slight fall in the rectal temperature of control group was observed after 3 and 4 h, however, this temperature did not fall to normal body temperature, which was 99 ± 0.28° F before brewer’s yeast induction. The slight fall in temperature was due to a reduction in the effect of brewer’s yeast with the passage of time. Normal saline had no direct effect on decreasing temperature of mice. In the group treated with paracetamol, rectal temperature before injecting brewer’s yeast was 98.9 ± 0.20 and after brewer’s yeast injection the temperature rose to 101.96 ± 0.65. After treating with paracetamol, the temperature reduced continuously and quickly after 0.5, 1, 2, 3 and 4 h, which was 101.35 ± 0.61° F, 100.03 ± 0.40° F, 99.36 ± 0.38° F and 99.26 ± 0.27° F, respectively. This rapid and continues fall of body temperature was due to the antipyretic effect of the standard paracetamol, which brought the temperature to the normal body temperature (99.26 ± 0.27° F) in the same way the crude extract of F. tenacissima was administered orally at doses of 100, 200 and 300 mg and the rectal temperature was recorded at different time intervals.
| Treatment | Dose (mg/kg) |
Average temperature (°F) before brewer yeast injection | Average temperature (°F) after brewer yeast injection | Average temperature (°F) after brewer yeast injection at different time interval | ||||
|---|---|---|---|---|---|---|---|---|
| After 0.5 h | After 1 h | After 2 h | After 3 h | After 4 h | ||||
| Control | 99 ± 0.28 | 101.58 ± 0.49 | 102.016 ± 0.65 | 102.21 ± 0.50 | 102.21 ± 0.50 | 101.58 ± 0.49 | 100.95 ± 0.64 | |
| Paracetamol | 150 | 98.9 ± 0.20 | 101.96 ± 0.65 | 101.35 ± 0.61 | 100.03 ± 0.40 | 99.36 ± 0.38 | 99.25 ± 0.20 | 99.26 ± 0.27 |
| Crude methanol Extract | 100 | 99.06 ± 0.35 | 102.5 ± 0.36 | 102.35 ± 0.30 | 102.03 ± 0.45 | 101.85 ± 0.372 | 101.6 ± 0.4 | 100.8 ± 0.24 |
| 200 | 99 ± 0.322 | 101.91 ± 0.38 | 101.65 ± 0.31 | 101.25 ± 0.225 | 101.08 ± 0.194 | 100.9 ± 0.25 | 100.9 ± 0.167 | |
| 300 | 99.03 ± 0.15 | 102.15 ± 0.40 | 101.93 ± 0.38 | 101.43 ± 0.33 | 101.16 ± 0.34 | 101.05 ± 0.26 | 101.05 ± 0.26 | |
Table 3: Antipyretic activity of crude methanol extract of Forsskaolea Tenacissima
By giving 100 mg dose orally, it was observed that the average temperature of the mice before brewer’s yeast injection was 99.06 ± 0.35° F and after brewer’s yeast induction the temperature rose to 102.5 ± 0.36° F. After giving 100 mg dose of extract, the temperature was recorded as 102.35 ± 0.30° F, 102.03 ± 0.45° F, 101.85 ± 0.372° F, 101.6 ± 0.4° F and 100.8 ± 0.24° F at 0.5, 1, 2, 3 and 4 h in that order. From this data it could be concluded that F. tenacissima extract at 100 mg/ kg dose had only slight antipyretic activity since this dose failed reduce the hyperthermia to normal body temperature (99.06 ± 0.35° F). Similarly, at the 200-mg dose, the average temperature before (99 ± 0.322° F) and after (101.91 ± 0.38° F) injection of brewer’s yeast was recorded and there has been an increase (pyrexia) in temperature after brewer’s yeast injection. After giving the dose, the average temperature of 200 mg dose group was 101.65 ± 0.31° F, 101.25 ± 0.225° F, 101.08 ± 0.194° F, 100.9 ± 0.25° F and 100.9 ± 0.167° F at 0.5, 1, 2, 3 and 4 h, respectively. Upon scrutiny, it was observed that after 3 and 4 h the decrease in average temperature due to 200 mg dose occurred indicating that the 200 mg dose exerted some antipyretic activity. In the 300 mg treated group the average temperature recorded before (99.03 ± 0.15° F) and after (102.15 ± 0.40° F) brewer’s yeast injection, we noticed an increase in temperature, which was due to brewer yeast, which induces pyrexia in the body of mice, after giving 300 mg dose to the group we found different average temperatures i.e. 101.93 ± 0.38° F, 101.43 ± 0.33° F, 101.16 ± 0.34° F, 101.05 ± 0.26° F and 101.05 ± 0.2° F at 0.5, 1, 2, 3 and 4 h correspondingly.
The antibacterial and antifungal activity of different solvent extracted samples from F. tenacissima showed that some of the extracts were active against bacteria and fungi while some were inactive. Ethyl acetate, petroleum ether, crude methanol and n-butanol measured good activity except aqueous extract, which was non-reactive against all the bacterial strains. Ethyl acetate extract appeared to be active against Proteus, Citrobacter, S. typhi, P. aeruginosa, and Providensia at both concentrations. Similarly, petroleum ether extract reduced the growth of E. coli, Providensia, S. typhi and Shigella and zero activity against P. aeruginosa, Proteus, and Citrobacter at both concentrations. Crude methanol extract showed activity against P. aeruginosa, S. typhi, E. coli at concentrations of 1 and 2 mg/disc correspondingly while it was inactive against P. proteus, Shigella and Citrobacter. Similar results are reported by McGaw et al. who concluded that crude methanol extract of Pouzolzia mixta solms (Urticaceae) leaf and stem showed moderate results against E. coli and P. aeruginosa [40]. n-butanol extract also showed minor activity against Proteus, P. aeruginosa and S. typhi at both concentrations while it was inactive against E. coli, Providencia, Shigella, and Citrobacter.
The extracts of F. tenacissima were also tested against three fungal strains i.e. A. fumigatus, Penicillium and Rhizopus. All extract of F. tenacissima were nonreactive against Penicillium at both higher and lower concentrations hence it proved that F. tenacissima plant is not sensitive against Penicillium, and a few extracts of F. tenacissima showed some activity against A. fumigatus and Rhizopus. Only ethyl acetate extract showed activity against A. fumigatus while the rest were inactive against the same fungus. Further, the ethyl acetate and chloroform extracts showed activity against Rhizopus, at concentrations of 1 and 2 mg/disc. It was found that chloroform extracts of both plants were active against two fungal strains, A. fumigatus and Rhizopus. These results agree with Somchit et al. where the chloroform extract of Acalypha indica (Euphorbiaceae) showed good antifungal activity against Candida albicans and microsporum cani [41]. Except chloroform, all other extracts of the same plant were inactive against all the given fungi. Similar results have also been reported by Chahardehi et al. [42].
The results of the hot plate test revealed that the latency time was not significantly affected at low doses of 50 to 100 mg/kg. The effect was noticed at high dose of 150 mg/kg after 60, 90 and 120 min. It has been reported that a number of flavonoids have analgesic activities [43]. Therefore, the low analgesic property might be due to the absence of some essential flavonoids.
The crude methanol extract of F. tenacissima at different concentrations (50, 100 and 150 mg) was tested for analgesic property. Extract at 50 mg/kg dose displayed 66.83 ± 5.11 and at 100 mg/kg dose produced 59.67 ± 1.37 mean number of writhes indicating that that even a high dose of 100 mg/kg also did not result in comparable activity to that of the standard aspirin (20.67 ± 3.98). Similarly extract with 150 mg/kg dose showed 26.32% inhibition of abdominal constriction produced by AA. These findings agree with those reported by Badilla et al. who reported that the final aqueous extract of Urera baccifera (Urticaceae) when tested for antinociceptive activity produced dosedependent reductions in the writhings [44]. Although both the plants F. tenacissima and U. baccifera belong to the same family Urticaceae, they showed different results indicating that F. tenacissima possessed low antinociceptive activity against chemical stimuli. F. tenacissima extract at 100 mg/kg dose exhibited mild antipyretic activity. Similarly extract at 200 mg/kg dose also affected the temperature slightly but only after 3 to 4 h as compared to saline control. F. tenacissima extract at 300 mg/kg dose had no antipyretic activity even after 4 h. F. tenacissima showed lower activities in comparison to other family members with petroleum ether and chloroform fractions of ethanol extract of the roots of Laportea crenulata Gaud (syn. Urtica crenulata, Fam. Urticaceae) showed significant antipyretic activity in rabbits with hyperpyrexia induced by boiled milk at a dose of 0.5 ml/kg [45].
From these results it can be concluded that ethyl acetate extract from F. tenacissima was active against most of the bacterial strains tested except E. coli and Citrobacter. The same extract was effective against two of the three fungi tested and chloroform fractions showed activity against one fungus strain. Crude methanol extract at high dose also showed increment in reaction time after 60, 90, 120 and 180 min. The F. tenacissima extract exhibited antinociceptive (chemical) activity and also reduced fever in mice to some extent.
Acknowledgements
The authors thank Prof. Dr. Furukh Hussain, Department of Botany, University of Peshawar, Pakistan for identifying the plant specimens collected.
Conflict of interest
The author (s) declares no conflict of interest.
Financial support and sponsorship
Nil.
References
- http://apps.who.int/iris/bitstream/10665/43108/1/9241562862_map.pdf.
- Balick MJ, Cox PA. Plants, People and Culture: The Science of Ethnobotany. New York: Scientific American Library; 1997.
- Samuelsson G, Bohlin L. Drugs of Natural Origin: a Textbook of Pharmacognosy. Stockholm: Taylor and Francis; 2004.
- Kudi AC, Uhoh JU, Eduvie LO, Gefu J. Screening of some Nigerian medicinal plants for antibacterial activity. J Ethnopharmacol 1999;67:225-8.
- Chiariandy CM, Seaforth CE, Phelps RH, Pollard GV, Khambay BP. Screening of medicinal plants from trinidad and tobago for antimicrobial and insecticidal properties. J Ethnopharmacol 1999;64:265-70.
- Okeke MI, Iroegbu CU, Eze NE, Okoli AS, Esimone CO. Evaluation of extracts of the root of Landolphiaowerriencefor antibacterial activity. J Ethnopharmacol 2001;78:119-27.
- Ahmad I, Beg AZ. Antimicrobial and phytochemical studies on 45 Indian medicinal plants against multi-drug resistant human pathogens. J Ethnopharmacol 2001;74:113-23.
- Kubmarawa D, Ajoku GA, Enweram NM, Okorie DA. Preliminary phytochemical and antimicrobial screening of 50 medicinal plants from Nigeria. Afr J Biotechnol 2007;64:1690-6.
- Abubakar MG, Yerima MB, Zahriya AG,Ukwuani AN. Acute toxicity and antifungal studies of ethanolic leaves, stem and pulp extract of Tamarindusindica. Res J Pharm BiolChemSci 2010;1:104.
- Bakht J, Tayyab M, Ali H, Islam A, Shafi M. Effect of different solvent extracted samples of Allium sativumon bacteria and fungi. Afr J Biotechnol 2011a;10:5910-15.
- Bakht J, Islam A, Tayyub M, Ali H, Shafi M. Antimicrobial potentials of Eclipta albaby disc diffusion method. Afr J Biotechnol 2011;10:7668-74.
- Bakht J,Ali H, Khan MA, Khan A, Saeed M, Shafi M,et al. Antimicrobial activities of different solvents extracted samples of Linumusitatissimumby disc diffusion. Afr JBiotechnol 2011;10:19825-35.
- Bakht J, Islam A, Shafi M. Antimicrobial potential of Ecliptaalbaby well diffusion method. Pak J Bot 2011;43:161-6.
- Bakht J,Azra, Shafi M. Antimicrobial activity of Nicotianatobaccum using different solvent extracts. Pak J Bot 2012;44:459-63.
- Bakht J, Khan S, Shafi M. Antimicrobial potentials of fresh Allium cepaagainst Grampositive and Gramnegative bacteria and fungi. Pak J Bot 2013;45:1-6.
- Bakht J, Azra, Shafi M. Antimicrobial potential of different solvent extractsof tobacco (Nicotianarustica) against Gram negative and positive bacteria. Pak J Bot 2013;45:643-8.
- Bakht J, Shehla K, Shafi M. In vitroantimicrobial activity of Allium cepa(dry bulbs) against Gram positive and Gramnegative bacteria and fungi. Pak J PharmaSci 2014;27:139-45.
- Bakht J, Shaheen S, Shafi M. Antimicrobial potentials of Menthalongifoliaby disc diffusion method. Pak J PharmaSci 2014;27:939-45.
- Bakht J, Gohar N, Shafi M. In vitroantibacterial and antifungal activity of different solvent extracted samples of Alhagimaurorum. Pak J PharmaSci 2014;27:1955-61.
- Ali N, Dawood A, Bakht J. Antibacterial activity of different solvent extracted samples from the flowers of medicinally important Plumeriaobstusa. Pak J PharmacetSci 2015;28:195-200.
- Ullah R, Bakht J, Shafi M. Antibacterial and antioxidant potential of Periplocahyaspidis. Bangladesh J Pharmacol 2015 10:645-51.
- Osbourne AE. Performed antimicrobial compounds and plant defense against fungal attack. Plant Cell 1966;8:1821-31.
- Caldentey KMO, Inze D. Plant cell factories in the post-genomic era; new ways to produce designer secondary metabolites. Trends in Plant Sci 2004;9:433-40.
- Qaisar M, Ahmad VU, Nisar M, Gilani SN, Pervez S. Bio-directed Isolation from Forsskaleatenacissima. J ChemSoc Pak 2008;30:854-9.
- Loutfy B. Flora of Egypt. Volume 1. Cairo (Egypt): Al Hadara Publishing; 1999. p. 18-20.
- Tackholm V. Students Flora of Egypt. 2nd ed. Cairo (Egypt): Cairo University Press; 1974. p. 55-8.
- Abouri M AE, Mousadik F, Msanda H, Boubaker BS, Cherifi K. An ethnobotanical survey of medicinal plants used in the Tata Province, Morocco. Intl J Med Plant Res 2012;1:99-123.
- Alali FQ, Tawaha K, El-Elimat T, Syouf M, El-Fayad M, Abulaila K, et al. Antioxidant activity and total phenolic content of aqueous and methanolic extracts of Jordanian plants: an ICBG project. Nat Prod Res 2007;21:1121-31.
- El-Hela AA, Abdel-Hady NM, Dawoud GT, Hamed AM, Morsy TA. Phenolic content, antioxidant potential and Aedesaegyptii, ecological friend, larvicidal activity of some selected Egyptian plants. J Egyptian SocParasitol 2013;43:215-34.
- Syed W, Samir K, Waqar A, Niaz A. Spasmogenic, spasmolytic and antihypertensive activity of ForsskaleatenacissimaL. Afr J Pharm Pharmacol 2010;4:381-5.
- Bakht J, Tayyab M, Ali H, Islam A,Shafi M. Effect of different solvent extracted samples of Allium sativumon bacteria and fungi. Afr J Biotechnol 2011;10:5910-15.
- Ramdas K, Suresh G, Janardhana N, Masilamani S. Antifungal activity of 1,3 disubstituted symmetrical and unsymmetrical thioureas. Pest Sci 1998;52:145-51.
- Bauer AW, Kirby WMM, Sherris JC, Turck M. Antibiotic susceptibility testing by standardized single disk method. Am J ClinPathol 1966;45:493-6.
- Eddy NB, Leimbach D. Synthetic analgesics. II. dithienylbutenyl and dithienylbutylamines. J PharmacolExpTher 1953;107:385-93.
- Vittalrao AM, Shanbhag T, Kumari M, Bairy KL, Shenoy S. Evaluation of antiinflammatory and analgesic activities of alcoholic extract of Kaempferiagalangain rats. Indian J PhysiolPharmacol 2011;55:13-24.
- Adams SS, Hebborn P, Nicholson JS. Some aspects of the pharmacologyofIbufenac, a non-steroidal antiinflammatory agent. J Pharm Pharmac 1968;20:305-12.
- Junaid N, Vikas G, Prithviraj C, Pawan K. Antiinflammatory and antipyretic activity of Aleuritismoluccanaleaves. Asian J Pharm Clin Res 2010;3:35-7.
- https://msu.edu/~freed/disks.htm.
- Steel RGD, Torrie JH, Dickey DA. Principles and procedures of statistics.A Biometrical Approach. New York (USA): McGraw Hill; 1997.
- McGaw LJ, Van der Merwe D, Eloff JN. In vitroanthelmintic, antibacterial and cytotoxic effects of extracts from plants used in South African ethnoveterinary medicine. Vet J 2007:173;366-72.
- Somchit, MN, Abdul Rashid R, Abdullah, A, Zuraini A, Zakaria ZA, Sulaiman MR.In vitro antimicrobial activity of leaves of AcalyphaindicaLinn. (Euphorbiaceae). Afr J Microbiol Res 2010;4:2133-36.
- Chahardehi AM, Ibrahim D, Sulaiman SF. Antioxidant, antimicrobial activity and toxicity test of Pileamicrophylla. Intl J Microbiol 2010;1-6.
- Gaur K, Rana AC, Nema RK, Kori ML, Sharma CS.Antiinflammatory and analgesic activity of hydroalcoholic leaves extract of Euphorbia neriifoliaLinn.Asian J PharmaClini Res 2009;2:1.
- BadillalB, Mora G, Lapa AJ, Ernim JAS. Antiinflarnmatory activity of Urerabaccifera(Urticaceae). Intl J Trop Biol Cons 1999;47:365-71.
- Khan A, Baki MA, Alim Al-Bari MA, Hasan S, Mosaddik MA, Rahman MM, et al. Antipyretic activity of roots of LaporteacrenulataGaud in rabbit. ResJ Med MedSci 2007;2:58-61.
 1 mg/
disc;
1 mg/
disc;  2 mg/disc
2 mg/disc