- *Corresponding Author:
- V. Alagarsamy
Medicinal Chemistry Laboratory, J. S. S. College of Pharmacy, Mysore-570 015, India
E-mail: samy_veera@yahoo.com
| Date of Submission | 24 November 2003 |
| Date of Revision | 20 April 2005 |
| Date of Acceptance | 23 February 2006 |
| Indian J Pharm Sci, 2006, 68 (1):108-111 |
Abstract
A series of 2-Substituted (1,3,4) thiadiazolo quinazolines were synthesized by the cyclocondensation of 3-amino-2-mercapto quinazolin-4(3H)-ones with various one-carbon donors and screened for their CNS activities (analgesic, anti-inflammatory, sedative-hypnotic and anticonvulsant). Compound III showed good CNS depressant activity, and it is comparable with the reference standard diazepam. While all the test compounds offered significant protection against strychnine-induced and hypoxic induced convulsion, compound III exhibited equivalent activity with the standard diazepam at the dose tested, and it was found to be significant when compared to control.
Quinazolines and condensed quinazolines are found to possess potent CNS activities like analgesic [1], anti-inflammatory [2], sedative-hypnotic [3] and anticonvulsant [4]. The thiadiazoloquinazoline nucleus is associated with diverse pharmacological activities such as antibacterial [5,6], antifungal [7], phosphodiesterase inhibitory [8], anti-inflammatory [9], platelet aggregation inhibitory [10] and antihypertensive [11,12]. In spite of the fact that a large number of condensed quinazolines have been prepared and studied extensively, the 1,3,4-thiadiazolo quinazoline nucleus is relatively unexplored. In fact, the first report on the synthesis of 1,3,4-thiadiazolo (2,3-b) quinazoline appeared in 1970, and very few reports have appeared since then. Prompted by these reports and to develop our earlier reported 2,3-disubstituted quinazolines [1,2], herein we report the analgesic, anti-inflammatory, sedative-hypnotic and anticonvulsant activities of some 2- substituted (1,3,4) thiadiazolo (2,3-b) quinazolines.
Melting points were determined in open capillary tubes on a Thomas Hoover apparatus and are uncorrected. IR spectra were recorded in KBr on a Perkin Elmer-841 grating spectrometer (cm-1); mass spectra on a Varian Atlas CH-7 mass spectrometer at 70 eV; and NMR spectra on a Varian A-60 or EM-360 spectrometer, using tetramethylsilane as internal standard. Elemental analyses were performed on Carlo Erba 1108.
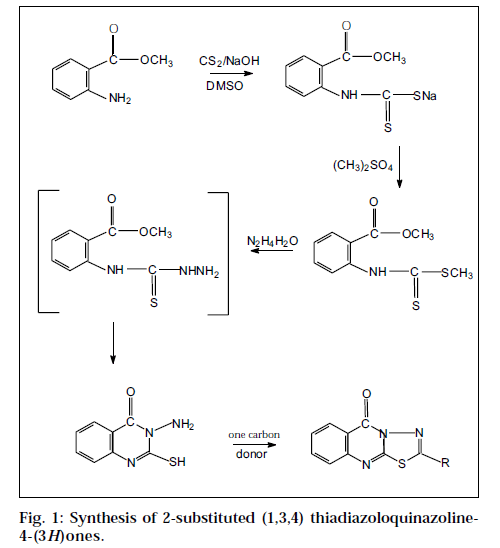
The title compounds were synthesized by the cyclization of 3-amino-2-mercapto quinazolin-4(3H)-one with a variety of one-carbon donors [13]. The starting material 3-amino-2- mercapto quinazolin-4(3H)-one, in turn, was synthesized from anthranilic acid (Fig. 1).
The Institutional Animal Ethics Committee approved the protocol used for experiments. Test for analgesic activity [14] was performed by tail-flick technique, using Wistar albino mice (25-35 g) of either sex selected by random sampling technique. Diclofenac sodium at a dose level of 10 mg/kg was administered as standard drug for comparison. The test compounds at a dose level of 10 mg/kg were administered orally as suspension in 1% sodium carboxymethyl cellulose (Na CMC). The reaction time was recorded at 1, 2 and 3 h after the treatment. The cutoff time was 10 s. The percent analgesic activity (PAA) was calculated by the following formula: PAA = (T2/T1) × 100, where T1 is the reaction time (s) before treatment, T2 is the reaction time (s) after treatment. All the test compounds exhibited mild to moderate analgesic activity and are significant when compared to control; however, none of the compounds exhibited comparable activity with that of standard diclofenac sodium as shown in Table 1.
| Code | Percent analgesic activity | Percent anti-inflammatory activity | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 h | 2 h | 3 h | 30 min | 1 h | 2 h | 3 h | 4 h | 5 h | |
| I | 180.61 ± 0.28++ | 233.72 ± 0.38+ | 202.98 ± 0.47++ | 17.6 ± 2.19+ | 18.8 ± 2.41++ | 20.0 ± 0.23++ | 19.4 ± 4.12++ | 16.8 ± 2.16+ | 15.7 ± 0.13+ |
| II | 162.6 ± 0.24+ | 179.5 ± 0.24++ | 170.82 ± 0.42++ | 16.5 ± 0.63++ | 17.5 ± 0.23++ | 18.3 ± 0.96++ | 17.3 ± 2.76+ | 15.3 ± 1.36+ | 15.0 ± 0.31+ |
| III | 156.8 ± 0.13+++ | 169.71 ± 0.38++ | 155.61 ± 0.33++ | 14.13 ± 0.16+ | 14.6 ± 2.17++ | 16.6 ± 0.29++ | 17.2 ± 0.16+ | 14.1 ± 0.61+ | 12.1 ± 2.36+ |
| IV | 146.81 ± 0.13++ | 158.51 ± 0.39+++ | 145.62 ± 0.26++ | 15.1 ± 0.23++ | 17.1 ± 4.60++ | 17.8 ± 5.10++ | 15.6 ± 0.19++ | 15.0 ± 0.14+ | 13.3 ± 5.30+ |
| V | 162.59 ± 0.39+ | 179.58 ± 2.12++ | 165.13 ± 2.63++ | 16.1 ± 4.11++ | 16.3 ± 0.41++ | 18.2 ± 0.31++ | 19.1 ± 1.16++ | 17.3 ± 2.48++ | 14.2 ± 1.36+ |
| VI | 196.06 ± 0.46++ | 236.45 ± 0.29+ | 200.01 ± 0.69++ | 19.1 ± 5.11+ | 21.1 ± 0.79++ | 22.1 ± 1.10+ | 20.1 ± 4.16++ | 18.4 ± 0.76+ | 18.5 ± 2.15+ |
| Control | 4.0 ± 1.17 | 6.0 ± 2.37 | 4.0 ± 1.64 | 3.19 ± 0.96 | 4.11 ± 0.06 | 5.32 ± 0.27 | 3.16 ± 0.23 | 2.50 ± 0.51 | 3.60 ± 1.12 |
| Standard | 311.11 ± 1.56++ | 275.6 ± 2.23+ | 230.6 ± 2.40+ | 31.2 ± 0.96+ | 35.7 ± 1.13++ | 39.1 ± 1.52++ | 30.6 ± 1.69 | 28.3 ± 1.16++ | 25.6 ± 0.96+ |
Each value represents the mean ± SD (n=6). + denotes significant differences from control at P<0.001. Diclofenac sodium used as reference standard at the dose of 10 mg/kg; dose of test compounds 10 mg/kg
Table 1: Analgesic and Anti-Inflammatory Activities Of Test Compounds
Anti-inflammatory activity was performed by carrageenan-induced paw oedema test in rats [15]. Diclofenac sodium 10 mg/kg was administered as standard drug for comparison. The test compounds were administered orally as suspension in 1% Na CMC at a dose level of 10 mg/kg. The paw volumes were measured using the mercury displacement technique with the help of a plethysmograph immediately before and 30 min, 1, 2, 3, 4 and 5 h after carrageenan injection. The percent inhibition of paw oedema was calculated by using the following formula: percent inhibition I=100[1-(a-x)/(b-y)], where ‘x’ is the mean paw volume of rats before the administration of carrageenan and test compounds or standard compound, ‘a’ stands for mean paw volume of rats after the administration of carrageenan in the control group, ‘b’ is the mean paw volume of rats before the administration of carrageenan in the control group, ‘y’ is mean paw volume of rats after the administration of carrageenan in the control group. While the test compounds protected the rats from carrageenan-induced inflammation moderately, none of the compounds showed equipotent anti-inflammatory activity with the reference standard diclofenac sodium at the dose tested.
Sedative-hypnotic activity was done by measuring the reduction in motor activity, using actophotometer [16]. Mice were chosen as test animals in a group of six. Basal activity score was taken and then test compounds and reference standard diazepam were administered orally at the dose of 10 mg/kg as suspension in 1% Na CMC. Scores were recorded at 30 min, 1h and 2h after the drug administration. The percent reduction in motor activity was calculated by the following formula: % reduction in motor activity = [(A-B)/A] × 100, where A = basal score, B = score after drug treatment.
The results of sedative-hypnotic activity reveal that all the test compounds depressed the CNS at varying degree. The compound III showed good activity, while the other compounds showed moderate CNS depression.
Anticonvulsant activity by strychnine-induced convulsion method [17] was performed in albino rats. Animals were divided into various groups each consisting of six animals. The test compounds (I-VI) were administered orally at a dose of 50 mg/kg as an aqueous suspension in 1% Na CMC and the standard diazepam was administered intraperitonially at a dose of 4 mg/kg, while the control group was fed with the same volume of 1% Na CMC suspension. After 30 min, strychnine was administered at a dose of 4 mg/kg intraperitonially. The latency of convulsions and mortality was assessed for each animal and is shown in Table 2.
| Code | Strychnine Test | Hypoxic Stress Test | Percent CNS depression | ||||
|---|---|---|---|---|---|---|---|
| Onset of convulsion (min) | Death time (min) | Onset of convulsion (min) | Death time (min) | 30 min | 1 h | 2h | |
| I | 9.2 ± 0.44+++ | 10.1 ± 1.7+ | 24.0 ± 2.55+++ | 6.05 ± 0.19+ | 26.3 ± 1.7++ | 39.6 ± 2.1++ | 35.6 ± 1.7++ |
| II | 11.7 ± 2.6++ | 12.4 ± 2.4+ | 25.5 ± 0.63+++ | 7.21 ± 4.4++ | 31.4 ± 2.3++ | 42.1 ± 3.7++ | 36.5 ± 2.6++ |
| III | 13.2 ± 0.58+++ | 13.5 ± 2.2+ | 30.06 ± 1.29+ | 8.13 ± 0.25+++ | 33.6 ± 1.9++ | 56.5 ± 2.1++ | 49.9 ± 2.9++ |
| IV | 8.9 ± 1.32+ | 9.3 ± 1.58+ | 26.3 ± 1.69++ | 5.44 ± 2.15+ | 27.5 ± 3.1+ | 40.1 ± 1.6+ | 35.7 ± 1.7+ |
| V | 10.1 ± 0.32++ | 11.12 ± 0.00++ | 26.2 ± 1.19+ | 7.16 ± 6.27+ | 24.2 ± 2.3++ | 33.2 ± 1.2+ | 26.4 ± 2.3+ |
| VI | 10.9 ± 0.56+ | 12.17 ± 0.00++ | 28.6 ± 5.66+ | 7.74 ± 3.63++ | 23.4 ± 1.6+ | 35.6 ± 1.6+ | 24.6 ± 1.7+ |
| Control | 4.4 ± 2.56 | 6.3 ± 1.99 | 20.9 ± 1.54 | 1.98 ± 0.33 | 03.5 ± 1.4 | 02.3 ± 0.96 | 04.2 ± 0.9 |
| Diazepam | 15.4 ± 2.06++ | 16.5 ± 1.93+ | 31.1 ± 1.60++ | 8.98 ± 1.37+++ | 69.1 ± 2.6++ | 85.9 ± 1.3+++ | 71.5 ± 1.5++ |
Each value represents the mean ± SD (n=6). + denotes significant differences from control at P<0.001. For anticonvulsant activity dose of test compounds 50 mg/kg; reference standard diazepam 4 mg/kg. For sedative-hypnotic activity dose of test compounds and diazepam 10 mg /kg.
Table 2: Anticonvulsant And Sedative-Hypnotic Activities Of Test Compounds I-Vi.
Anticonvulsant activity by hypoxic stress induced convulsion method [18] was performed using mice as testing animals. Mice were divided into various groups, each consisting of six animals. Test compounds and standard diazepam were administered as mentioned above, while the control group was fed with the same volume of 1% Na CMC. After 30 min, mice were subjected to hypoxic stress by putting them individually in a glass container of 370 ml capacity. The containers were tightly closed with greased glass lids to ensure air tightness. Under these circumstances, the animals showed convulsions and mortality due to hypoxia. The latency for convulsions and death was assessed and shown in table, and the results were analysed statistically by the Student’s t test.
While all the compounds delayed the onset of convulsions and death time in hypoxic stress and strychnine induced convulsions (Table 2), the compound III exhibited almost equivalent activity with that of standard diazepam at the test dose in both the methods tested and is significant when compared to control. The compounds I and VI showed comparable activity with that of diazepam in hypoxic stress induced convulsion method. None of the test compounds protected the animals from mortality.
The title compounds showed a wide spectrum anticonvulsant profile against chemical and hypoxic convulsions. The activity of test compounds in the strychnine test showed that the thiadiazolo quinazolines act through inhibitory glycine receptors. Mice subjected to acute hypoxic stress showed increased respiratory rate, tremors and convulsions followed by death. Exposure to stress led to physiological and biochemical disequilibrium. The observed physiological changes such as convulsions and death following hypoxic stress, appears to be due to cerebral hypoxia [19]. Since drugs that inhibit the formation of free radicals are reported to possess protective effects against hypoxia [20], the test compounds might possibly inhibit the formation of free radicals.
Acknowledgements
The authors are grateful to the Principal and management of J. S. S. College of Pharmacy, Mysore, and to the management S. B. College of Pharmacy, Sivakasi, for providing necessary facilities to carry out this research work.
References
- Alagarsamy, V., Raja Salomon, V., Vanikavitha, G., Paluchamy, V., Ravichandran, M., Arnold Sujin, A., Thangathirupathy, A., Amuthalakshmi, S. and Revathi R., Biol. Pharm. Bull., 2002, 25, 1432.
- Alagarsamy, V., Muthukumar, V., Pavalarani, N., Vasanthanathan, P. and Revathi R., Biol. Pharm. Bull., 2003, 26(4), 557.
- Chaurasia, M.R. and Sharma, S.K., Arch. Pharm., 1982, 315, 377.
- Manabu, H., Ryvichi, I. and Hideaki, H., Chem. Pharm. Bull., 1990, 38, 618.
- Pandey, V.K. and Raj, N., Curr. Sci., 1996, 55, 785.
- Tita, T.T. and Kornet. M.J., J. Heterocycl. Chem.,1998, 25, 262.
- Modi, S.K., Kumar, V. and Narang, K.S., Indian J. Chem.,1970, 8(B), 716.
- Russo, F. and Santagati, S., Farmaco. Ed. Sci., 1981, 36, 292.
- Shaffiee, A. and Kalezari, I., J. Heterocycl. Chem., 1975, 12, 675.
- Kanchien, L. and Chung, C., J. Ching. Pharm., 1992, 32, 65.
- Kazayuki, M., Japan Kokai, 1976, 317, 118494; Chem. Abstr., 1981, 84, 39786.
- Vasant, M., Japan Kokai, 1977, 115; Chem.Abstr., 1987, 107, 63408.
- Pathak, U.S., Devani, M.B., Shishoo, C.J., Kulkarni, R.R., Rakhola, V.M., Bhadti, V.S., Ananthan, S., Dave, M.G. and Shah, V. A., IndianJ. Chem., 1986, 25(B), 489.
- Kulkarni, S. K., Life Sciences, 1980, 27, 185.
- Winter, C.A., Risely E.A. and Nu, G.N., Proc. Soc. Exp. Biol., 1962, 11, 54719.
- Amour R.E., Smith D.L., J. Pharm. Expt. Therap., 1941, 72, 74.
- Impizumi, S.L., Kayama, T. and Suzuki, J., Stroke, 1964, 15, 1061.
- Winter C.A., Risely E.A., Nu, G.N., Proc. Soc. Exp. Biol., 1982, 111, 544.
- Spagnoli, A. and Tognoni, G., Drugs, 1983, 26, 44.
- Kulkarni. S.K., Dandiya. P.C., Int. J. Med., 1975, 63, 462.