- *Corresponding Author:
- Huiling Cao
Department of Pharmacy, Shaanxi University of Chinese Medicine, Xianyang, Shaanxi 712046, China
E-mail: caohuiling_jzs@xiyi.edu.cn
| Date of Received | 17 January 2021 |
| Date of Revision | 08 November 2022 |
| Date of Acceptance | 06 May 2023 |
| Indian J Pharm Sci 2023;85(3):565-580 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Coumarin is a kind of lactone compound with skeleton of benzo-alpha-pyranones, which have favorable druggability due to its advantages of outstanding pharmacological activities, little drug-resistance, lowtoxicity, simple skeleton, easy synthesis and structural modification, and extensive sources. The review summarizes the classification, synthesis methods, pharmacological effects of coumarin and its derivatives. It focuses on their latest progresses in anti-bacteria, anti-virus, anti-inflammation and anti-rheumatism, anti-autoimmune diseases, anti-oxidation, anti-coagulation, anti-cancer and antiangiogenic effects in detail. Especially, coumarins exhibited outstanding effects on clinical difficult miscellaneous diseases with rare drugs, difficult cure and bad prognosis, such as coronavirus disease-19, rheumatoid arthritis, autoimmune neuroinflammation, systemic lupus erythematosus, idiopathic pulmonary fibrosis, etc. The review would provide new skeletons and promising lead compounds with little drug-resistance, high-efficiency and lowtoxicity for new drug development for related diseases based on coumarins.
Keywords
Coumarin, coronavirus disease-19, rheumatoid arthritis, autoimmune neuroinflammation, systemic lupus erythematosus, idiopathic pulmonary fibrosis
Coumarin, a natural product, is widely found in the secondary metabolites of various plant sources, such as Leguminosae, Umbelliferae, Solanaceae, Rutaceae, Compositae, Daphnaceae, as well as in animals and microorganisms[1,2]. The first coumarin was extracted from legume Melilotus luteus by Vogel in the 18th century, and named “coumarin” because of its hay smell[3]. Coumarin is a kind of lactone compound with skeleton of benzo alpha (α)-pyranones and with the chemical formula C9H6O2 and molecular weight of 146.143 (fig. 1A). They often exist in free forms or a few in glycosides in nature[4]. Coumarins appear as colorless or light-yellow flake or powder with aromatic properties, which are insoluble in cold water and soluble in hot water, alcohol, chloroform, ether, and oil.
At present, more than 2000 natural coumarin compounds have been isolated from nature, which exhibit various pharmacological effects such as anti-bacteria[5], anti-virus[6], anti-inflammation[7] and anti-rheumatism[8], anti-autoimmune diseases[9], anti-oxidation[10], anti-coagulation[11], anti-cancer[12] and antiangiogenic effects[13] in detail. Especially, coumarins exhibited favorable effects on clinical difficult miscellaneous diseases with rare drugs, difficult cure and bad prognosis, such as Coronavirus Disease (COVID-19)[14], rheumatoid arthritis, autoimmune neuroinflammation[15], systemic lupus erythematosus[16], Idiopathic Pulmonary Fibrosis[17], etc. Coumarin and its derivatives present favorable druggability due to its advantages of favorable pharmacological activities[18], little drug-resistance, low-toxicity[19], simple skeleton[20], easy synthesis and structural modification and extensive sources.
At present, many coumarins as drugs have been used in clinic. There are three coumarin-related anticoagulants used in the clinical practice, such as warfarin potassium, warfarin sodium and phenylprusside[21]. Neomycin cannot only inhibit Deoxyribonucleic Acid (DNA) helicase, but also eliminate plasmids and has bactericidal effects on sensitive bacteria at high concentration. Novobiocin as a representative of coumarin Gyrase B (GyrB) inhibitors discovered in 1955, have not been widely used for an anti-infective therapy because of their low bioavailability and high toxicity[22,23]. Psoralen can be used to treat vitiligo, psoriasis, alopecia areata, seborrheic dermatitis and so on.
The review summarizes the classification, synthesis methods, pharmacological effects of coumarin and its derivatives, which would provide new skeletons and promising lead compounds with little drug-resistance, high-efficiency and low-toxicity for new drug development for related diseases based on coumarins.
Classification of Coumarins
Coumarins can be divided into simple coumarins, furanocoumarins, pyrocoumamarins and other coumarins according to their chemical structures, substituent position and characteristics[24].
Simple coumarins:
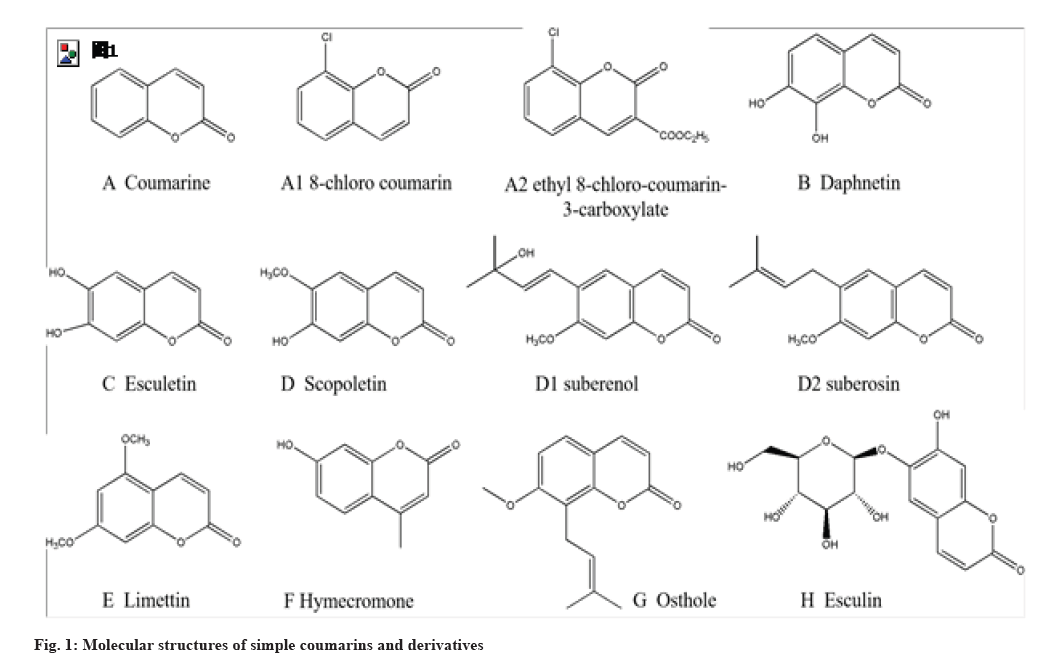
Simple coumarins are such compounds with substituents only on the benzene ring. Generally, the oxygen containing groups at C-7 position is the majority, such as -OH, -OCH3, -OCHCH=C(CH3)2, etc. The isopentenyl group is often connected at the C-6 and C-8 positions, which can be connected not only to the carbon chain but also to the oxygen. Typical simple coumarins include daphnetin (fig. 1B), esculetin (fig. 1C), scopoletin (fig. 1D), limettin (fig. 1E), osthole (fig. 1G), esculin (fig. 1H) etc.
Furanocoumarins:
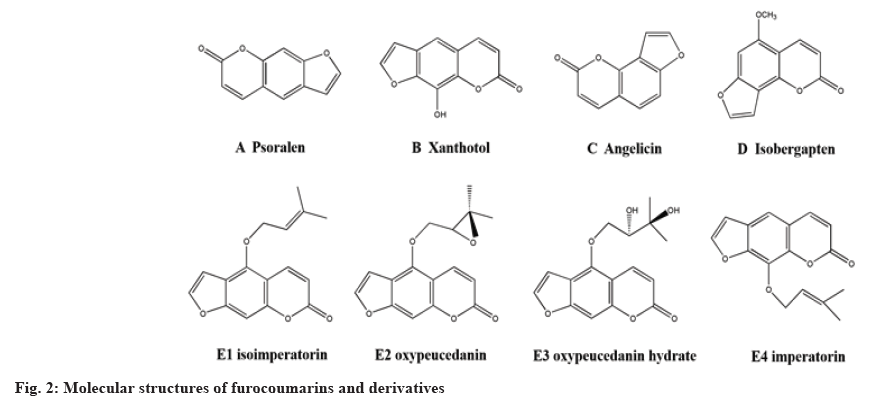
Furanocoumarins are the fusion of furan or dihydrofuran ring and coumarin skeleton. The two rings are fused in different ways to form different linear or angular furanocoumarins. The linear furanocoumarin is formed by the closed loop reaction of C-7 phenol hydroxy and C-6 isopentenyl, while the angular furanocoumarin is a lactone compound formed by the cyclization reaction of C-8 isopentenyl and ortho-phenol hydroxy. Representative linear furanocoumarins include psoralen (fig. 2A) and xanthotol (fig. 2B) and angular furanocoumarins include angelicin (fig. 2C) and isobergapten (fig. 2D), etc.
Pyranocoumarins:
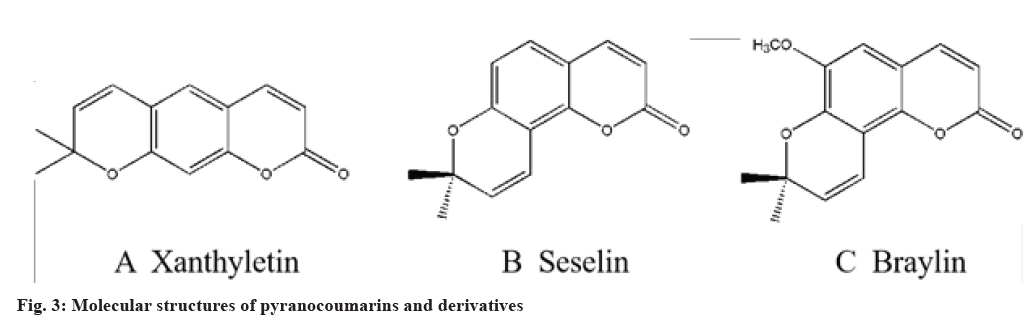
Pyranocoumarins are similar to furanocoumarins in the structure and biosynthetic pathway. The only difference is different rings, a pyran ring for pyranocoumarins formed by isoprene, not a furan ring. The pyran ring (or dihydropyran ring) is fused by the phenolic hydroxyl group at C-7 with the isopentenyl group at C-6 or C-8, respectively. The different linear or angular pyranocoumarins are formed by different closed loop modes of the pyran ring. Representative linear furanocoumarins include xanthyletin (fig. 3A) and angular furanocoumarins include seselin (fig. 3B) and braylin (fig. 3C), etc.
Other coumarins:
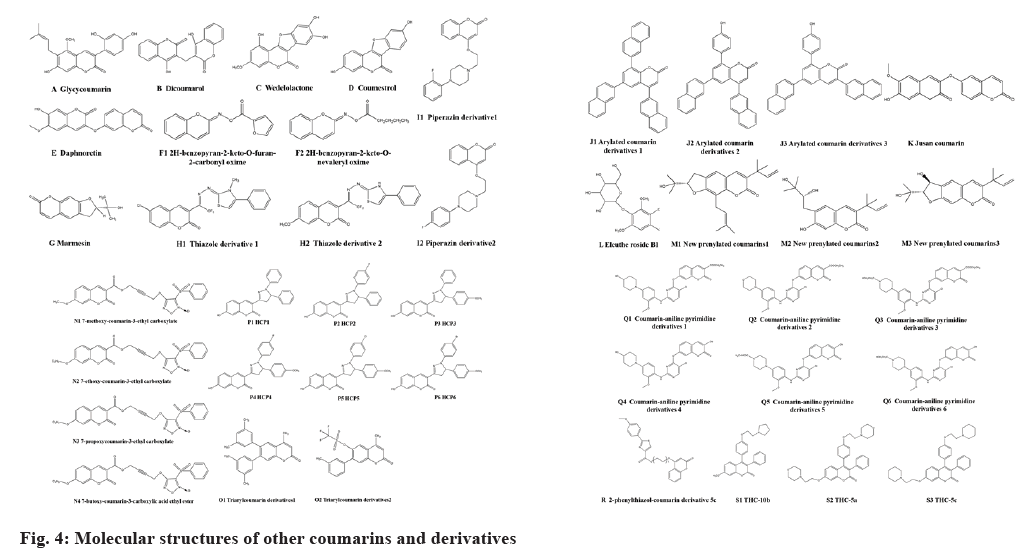
Other coumarins refer to substituent coumarins at C-3 or C-4 positions of benzo connected with α-pyranone ring and coumarin polymers. In addition to the presence of the phenyl at C-3 and C-4 positions, there are also 3,4-benzo structures, such as glycycoumarin (fig. 4A), dicoumarol (fig. 4B), etc. 4-oxycoumarins including wedelolactone (fig. 4C), coumestrol (fig. 4D), etc., and dimer coumarins include daphnoretin (fig. 4E), etc.
Synthesis of Coumarins
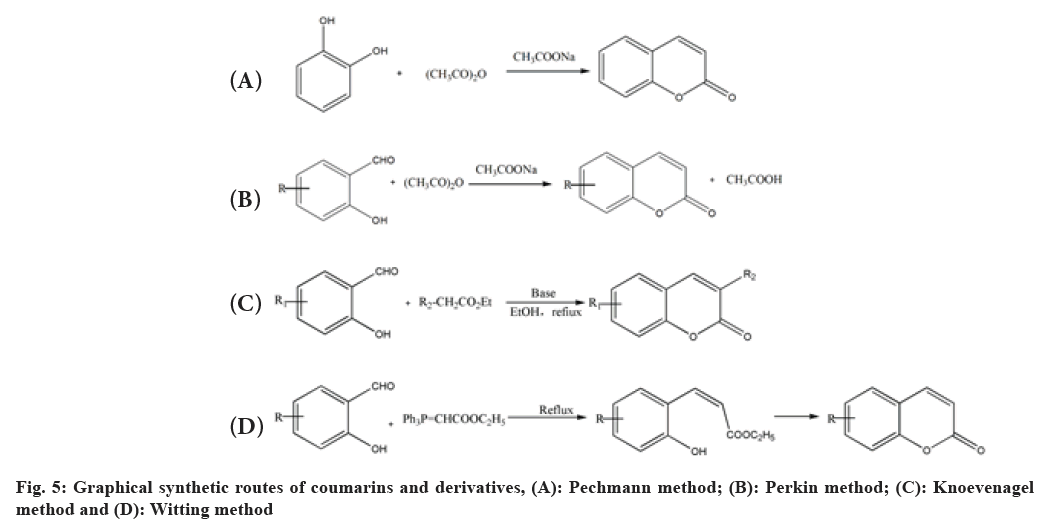
Due to its advantages such as simple skeletons and easy synthesis and structural modification, chemists developed many synthetic methods to obtain coumarin and its derivatives, such as Pechmann method[25,26], Perkin method[27], Knoevenagel method[28] and Wittig method, etc.,[29].
Pechmann method:
The Pechmann method (fig. 5A) was first developed by the German chemist Hansvon Pechmann. Coumarin derivatives are obtained by cyclization reaction using phenol and beta (β)-ketone acid or ketone acid ester as raw materials and acids such as Trifluoroacetic acid (CF3COOH), Aluminum trichloride (AlCl3) or concentrated Sulfuric acid (H2SO4) as catalysts (fig. 5A). It is a basic synthesis method of coumarin derivatives with simple operations and high yields. However, the method requires harsh reaction conditions, such as high temperature, a large number of acid catalysts, and a long reaction time, especially along with more by-products. Later, many scholars improved the method, such as using new catalysts, using microwave-assisted to improve its yields and so on.
Perkin method:
Perkin method (fig. 5B) is one of the classic methods of coumarin synthesis in a large-scale industrial production. The method prepares coumarin derivatives by the condensed cyclization reaction using weak base sodium acetate as a catalyst, salicylaldehyde and acetic anhydride as raw materials (fig. 5B). The method also has the disadvantages of a high reaction temperature, a long reaction time, low yields, complex and diverse by-products.
Knoevenagel method:
Knoevenagel method (fig. 5C) is an improvement one based on Perkin method. 3-substituted coumarin derivatives are prepared by dehydration and condensation to form unsaturated carbonyl compounds using compounds with α-hydrogen atoms (such as ethyl acetoacetate, etc.) and aldehydes or ketones as raw materials, the weak base (triethylamine, etc.) as catalysts (fig. 5C). As the reactants containing active methylene are used in the reaction, the basic catalyst only needs an ordinary organic base, which also reduces the reaction temperature and reaction time. Therefore, the Knoevenagel method has the advantages of a short reaction time, mild conditions and high yields.
Witting method:
Compared with the three methods mentioned above, the Witting method (fig. 5D) is less used in the synthesis of coumarin derivatives, which introduces substituents at positions 3 or 4 of the coumarin skeleton. In the method, coumarin derivatives are prepared by refluxing in solvents using salicylaldehyde and ethoxycarbonyl-methylidene phosphorus ylide as raw materials (fig. 5D). Witting method showed a fine functional group tolerance, simple operations, mild reaction conditions, which makes it possible as a promising large-scale synthesis method for coumarin derivatives.
Pharmacological Advances
Coumarins exhibited excellent pharmacological effects, little drug-resistance and low-toxicity on anti-bacterium[30], anti-virus[31], anti-inflammation[32], anti-rheumatism, anti-autoimmune diseases, anti-oxidation[33], anti-coagulation[34,35], anti-cancer[36,37] and so on. Especially, coumarins exhibited outstanding effects on clinical difficult miscellaneous diseases such as COVID-19, rheumatoid arthritis[38], autoimmune neuroinflammation[39], systemic lupus erythematosus, IPF, etc. The diseases got into troubles of rare drugs, difficult cure and bad prognosis. Therefore, coumarins would provide new skeletons and promising lead compounds with little-drug-resistance, high-efficiency and low-toxicity for new drug development for related diseases.
Anti-pathogenic microorganism:
With the large-scale clinical application of antibiotics, antibiotic resistance is increasingly becoming a serious problem. There are rare effective anti-fungus or anti-virus drugs in a clinical practice. Coumarins exhibit favorable anti-bacteria, anti-fungus and anti-virus effects with little drug-resistance, which has been a research hotspot for anti-infection treatment and overcoming drug-resistance.
Anti-bacteria: Some natural coumarins as new antibiotics, such as novobiocin, chlorobiocin and coumermycin A1, present outstanding effects on infection diseases caused by Gram-positive bacteria with little drug-resistance.
Coumarins and its derivatives exhibit favorable anti-bacteria activities. Zhou et al.[18] found that esculin from a Chinese herb namedfraxetin presented bacteriostatic activity on Escherichia coli (Minimal Inhibitory Concentration (MIC), 40 μg/ml) with little drug-resistance in a concentration- and time-dependent way. Its bactericidal rate reached 68.2 % treated 72 h with 40 μg/ml esculin. Wang et al.[40] declared that esculin could inhibit proliferation of Staphylococcus aureus. Li et al.[20] synthesized two piperazin-coumarin derivatives 1 (fig. 4 I1) and 2 (fig. 4 I2) and found that the two derivatives exhibited excellent bacteriostatic activity against Staphylococcus aureus, Bacillus subtilis, Escherichia coli and Pseudomonas aeruginosa by double dilution assay. The bacteriostatic activity of derivative B was better than derivative A. The derivative B exhibited the best bacteriostatic activity against Bacillus subtilis (MIC, 0.337 μg/ml) and the derivative A presented the best performance against Pseudomonas aeruginosa (MIC, 0.675 μg/ml).
The favorable bacteriostatic activity of esculin was attributed to inactivating topoisomerase I and topoisomerase II to inhibit the synthesis of bacterium DNA and Ribonucleic Acid (RNA), or inhibiting bacterium protein synthesis, or increasing the permeability of cell membrane, but not resulting in disintegration of cell walls or cell membrane. The elimination of bacteria plasmid was responsible for the little drug-resistance of esculin.
Anti-fungus :There are rare effective antifungal drugs in clinic or in agricultural practice. Coumarins exhibited excellent antifungal activity with little drug-resistance[41,42], which would provide promising lead compounds with little drug-resistance for new antifungal drug development. Jia et al.[43] found that the coumarins presented favorable antifungal activity against Candida albicans. Liu et al.[44] declared that esculin exhibited excellent antifungal activity against Monilia krusei (MIC, 32 μg/ml), Candida glabrata (MIC, 8 μg/ml) and Cryptococcus neoformans (MIC, 8 μg/ml). Additionally, esculin improved the drug-resistance of antifungal drug fluconazole against Candida albicans.
Coumarin derivatives exhibited excellent antifungal activity. Wei et al.[45] synthesized a series of 8-substituted coumarin derivatives and the screening results indicated that they had favorable antifungal activity against four plant pathogenic fungi, including Acrida cinerea, Collum anthracis, Fusarium oxysporum and Fusarium wilt. The optimal ones were 8-chlorocoumarins (fig. 1 A1) (Median Effective Concentration (EC50), 85 μM) and 8-chlorocoumarin-3-ethyl carboxylate ((fig. 1 A2) (EC50, 78 μM). The antifungal activity was promoted by introducing appropriate small, hydrophilic and electron withdrawing groups at C-3 or C-8 of coumarins. Yuan et al.[19] designed and synthesized 18 new coumarin oxime ester derivatives by introducing the oxime ester group to coumarins. The screening results indicated that the coumarin and its derivatives (50 μg/ml) presented inhibitory activity against three plant pathogenic fungi such as apple tree paresis bacteria, tomato gray mould bacteria and riceBacillus. The derivative 2H-benzopyran-2-keto-O-furan-2-carbonyl oxime (fig. 4 F1) showed better inhibitory activity (EC50, 4.44 μg/ml) against tomato gray mould bacteriathan the positive control drug trifloxystrobin (EC50, 9.54 μg/ml). The derivative 2H-benzopyran-2-keto-O-furan-2-carbonyl oxime (EC50, 3.65 μg/ml) and 2H-benzopyran-2-keto-O-nonanal oxime (fig. 4 F2) (EC50, 3.45 μg/ml) exhibited better inhibitory activity against riceBacillus than the coumarin (EC50, 13.75 μg/ml) or positive control drug trifloxystrobin (EC50, 4.58 μg/ml). The results suggested that coumarin oxime ester derivatives had outstanding antifungal activity. Yang et al.[46] designed and synthesized a series of coumarin thiazoles containing trifluoromethyl and studied their antifungal activity. The results indicated that some coumarin derivatives have fine antifungal activity against three plant pathogenic fungi including Fusarium moniliformis, Fusarium graminearum and Curvularia Zea. Among them, thiazole derivative 1 (fig. 4 H1) had the strongest antifungal activity against Fusarium moniliformis with an inhibition rate of 74 %, while thiazole derivative 2 (fig. 4 H2) had the best antifungal activity against Fusarium gramineum and Curvularia Zea, with inhibition rates as high as 89 % and 93.4 %, respectively. The analysis of structure-activity relationship showed that the introduction of trifluoromethyl groups greatly improved the antifungal activity of the coumarin thiazole derivatives, which would provide promising lead compounds for new plant antifungal agents’ development.
Anti-virus: The clinic is lack of effective antivirus drugs. Coumarins exhibited favorable antivirus activity with little drug-resistance, which would provide new skeletons and promising lead compounds with little drug-resistance for new antivirus drug development, such as COVID-19, influenza virus, Human Immunodeficiency Virus (HIV) and so on.
COVID-19, a disease caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), was declared a pandemic from 2019 to 2022 worldwide[47]. The results from molecular dynamics simulations indicated that three coumarin derivatives have potential inhibitory effects on COVID-19, such as arylated coumarin derivatives 1, 4,6,8-tri(naphthalen-2-yl)-2H-chromen-2-one (NF1) (fig. 4 J1), arylated coumarin derivatives 2, 8-(4-hydroxyphenyl)-4,6-di (naphthalen-2-yl)-2H- chromen-2-one (NF-12) (fig. 4-2 J2) and arylated coumarin derivatives 3, 8-(4-hydroxyphenyl)- 3,6-di (naphthalen-2-yl)-2H-chromen-2-one (NF- 13) (fig. 4-2 J3). The three coumarin derivatives showed favorable binding ability with spike-protein/ Angiotensin-Converting Enzyme 2 (ACE2) protein complex with minimal energy by molecular dynamics simulation and Molecular Mechanics Poisson- Boltzmann Surface Area (MMPBSA) studies, which suggested they had potential anti-COVID-19 effects. The chymotrypsin like protease of SARS-CoV-2 plays an important role in the viral replication. The molecular docking results indicated that more than half of the coumarins had favorable interaction with the protease[48]. The jusan coumarin (fig. 4K), which was isolated from the aerial parts of Artemisia glauca, demonstrated very similar binding activity to X77, the ligand of COVID-19 protease[49].
HyaluronicAcid(HA) isanacidicmucopolysaccharide, which is divided into large molecules and small molecules. The small molecular HA are important inflammatory mediators to cause lung lesions[50]. HA was massively accumulated in lungs of critical COVID-19 patients with Acute Respiratory Distress Syndrome (ARDS) along with a loss of lymphocytes. The results in vivo also confirmed that HA was the key factor in the formation of lung Ground-Glass Lesion (GGO) and lung consolidation in COVID-19 patients. It was suggested that inhibiting HA synthesis would be a promising strategy for alleviating lung lesions in COVID-19 patients.
Hymecromone (fig. 1F), a coumarin compound isolated from Artemisia scoparia Waldst.et of the Compositae family, is a HA synthesis inhibitor. Li et al. demonstrated that hymecromone inhibited HA synthesis to significantly improve lung lesions and promoted lymphocyte recovery in COVID-19 patients, which would be a promising treatment agent to prevent severe outcome of COVID-19 patients. Yang et al. found that HA could directly cause lung lesions in mice. Hymecromone significantly reduced HA synthesis by down regulating the expression of Hyaluronan Synthase 2 (HAS2)/HAS3. Interestingly, 89 % of COVID-19 patients treated with hymecromone had lung lesion absorption, while only 42 % of patients in the control group. Additionally, lymphocyte recovery of patients treated with hymecromone was faster than the control group.
It was reported that there were about 1 billion influenza new cases worldwide per year, of which 3 million to 5 million were severe cases, resulting in 290 000 to 650 000 deaths[51].
Coumarin and its derivatives presented favorable anti-influenza virus effects. Lee et al.[52] isolated four furanocoumarins, such as isoimperatorin, (fig. 2 E1), oxypeucedanin (fig. 2 E2), oxypeucedanin hydrate (fig. 2 E3) and imperatorin (fig. 2 E4) from 70 % ethanol extracts of Angelica dahurica root. The results indicated they exhibited inhibitory activity against influenza A virus (H1N1 and H9N2) by inhibiting Cytopathic Effect (CPE). The derivative 2 oxidized imperatorin was the best one (EC50, 5.98 μM). Bizzarri et al.[53] synthesized different catechol and pyrogallol coumarin derivatives by the oxidation reaction with 2-iodoxybenzoic acid and coumarin in Dimethyl sulfoxide (DMSO) at 25°. The novel derivatives could effectively inhibit the replication of influenza A/PR8/H1N1 virus, which suggested that highly oxidized coumarins improved antiviral activity through intracellular redox reaction. Wang et al.[54] extracted 6 coumarin derivatives from Chinese herb coral and the results indicated that eleutheroside B1 (fig. 4L) presented favorable antiviral activity against influenza A (H1N1) virus (106 μg/ml). Eleutheroside B1 exhibited excellent antiviral activity treated in early stage of virus replication cycle (0-6 h) and decreased 60 % of H1N1 viral titers. It had no effects on H1N1 virus treated after 8 h.
The favorable antiviral activity of coumarins was attributed to inhibiting Neuraminidase (NA), which an antiviral drug target to facilitate influenza virus was leaving infected host cells to infect new ones as a glycoprotein located on the viral envelope. Coumarins inhibited NA to regulate the expression of apoptosis-related proteins to promote the apoptosis of influenza virus. Meanwhile, coumarins could inhibit viral transcription to inhibit the early stages of the viral replication cycle. The results suggested that coumarins would provide promising lead compounds for antiviral new drug development.
Acquired Immunodeficiency Syndrome (AIDS) is an infectious disease caused by the HIV featured with a rapid spread and high mortality rate[55]. Coumarin and its derivatives exhibited favorable anti-HIV effects[56-58], which would provide valuable lead compounds for anti-HIV new drug development.
Liu et al.[58] isolated three new prenylated coumarins1-3 (fig. 4) and nine known pentenylated coumarins. The results indicated that the three newly ones presented excellent inhibitory activity against HIV (EC50, 0.29 μM, 0.68 μM, 0.17 μM).
Hamdy et al. prepared triarylcoumarin by coupling reactions using 4-methyl-6,7-dihydroxy-coumarin as raw materials. Two triarylcoumarin derivatives (fig. 4) showed favorable anti-HIV activity (EC50, 4.57 μM, 13.20 μM). Jesumoroti et al. designed and synthesized a series of new coumarin-3-carbonic hydrazide derivatives by introducing hydrazine groups at C3-position of the coumarin skeleton. The coumarin derivatives presented excellent inhibitory activity against HIV-1 IN (Half-maximal Inhibitory Concentration (IC50), 13 µg/ml) while showed non- cytotoxic to normal human cells.
Anti-parasitism: Coumarins showed anti-parasitism effects, whichwouldprovidenewideasforanti-parasite new drug development. Daphnetin is a benzopyrone compound named 7,8-dihydroxyeoumarin, which is extracted from the endemic plant Daphne sylvestris in the Changbai Mountains. Liu et al.[59] found that daphnetin (300 μg/ml and 500 μg/ml) shortened the life span of Caenorhabditis elegans (C. elegans) and the 500 μg/ml group showed better performance. The daphnetin exhibited higher the life-shortening rate of F2 generation C. elegans than that of F1 generation, indicating that daphnetin had a cumulative toxicity against C. elegans. In addition, with the increase of daphnetin concentration, abnormal development of C. elegans was observed and showed an increasing trend, indicating that daphnetin had concentration- dependent and cumulative toxicity against C. elegans. Daphnetin is an iron chelator, which can act on the iron-containing enzymes of C. elegans, affecting their metabolism to inhibit or kill C. elegans.
Anti-inflammation, anti-immunity, anti-oxidation and anti-coagulation:
Inflammation, oxidative stress and immunologic dysfunction are closely related to occurrence and development of many diseases, for instance, rheumatoid arthritis, autoimmune neuroinflammation, systemic lupus erythematosus, IPF, etc. The clinical difficult miscellaneous diseases got into troubles of rare drugs, difficult cure and bad prognosis. Coumarins exhibited favorable effects with multi- target collaboration on the difficult miscellaneous diseases, which would provide promising lead compounds for new drug development for related- diseases.
Anti-rheumatoid arthritis: Coumarins presented excellent anti-rheumatoid arthritis effects, which would provide new skeletons and promising lead compounds for anti-rheumatoid arthritis new drug development. Zhang et al. built a mouse model with Collagen Induced Arthritis (CIA) induced by bovine type II collagen and explored anti-CIA effects of psoralen with a 21 d treatment. The results indicated that psoralen (fig. 2A) could significantly improve the degree of ankle swelling, movement limitation, spleen index (34.86±3.32) of mice with CIA compared with the model group (54.23±5.12) and downregulated the expression of inflammatory factors such as Interleukin-6 (IL-6), IL-lβ and Tumor Necrosis Factor-α (TNF-α), therefore, improving CIA. It is a significant treatment strategy for rheumatoid arthritis to promote cell apoptosis of Fibroblast-Like Synoviocytes (FLS). Zong et al. found that 7-hydroxycoumarin improved posterior foot swelling and arthritis index, relieved joint pathological injury of mice with CIA.
Psoralen improving CIA was attributed to immunomodulatory effects by regulating a balance of leukomonocyte and inhibiting release of inflammatory factors. 7-hydroxycoumarin inhibited Wnt/β-catenin pathway by down regulating the expression of related proteins (Wnt1, β-catenin, phospho Glycogen Synthase Kinase-3β (p-GSK- 3β), Low-Density Lipoprotein Receptor Protein 6 (LRP6), cyclin D1 and cellular Myc (c-Myc)) to inhibit cell proliferation and promote cell apoptosis of FLS, therefore improving CIA.
Anti-autoimmune encephalomyelitis: Coumarins such as daphnetin (fig. 1B) and osthole (fig. 1G), significantly inhibited the production and release of early inflammatory factors, therefore, improved autoimmune encephalomyelitis, which would provide promising lead compounds with high efficiency and low toxicity for anti-autoimmune neuroinflammation new drug development based on coumarins. Wang et al. built a mouse model of Experimental Autoimmune Encephalomyelitis (EAE) and explored the effects of daphnetin on anti-EAE by treating 28 d with daphnetin (8 mg/kg). The results indicated that daphnetin significantly alleviated spinal inflammation and the degree of demyelination to alleviate the pathological symptoms of EAE mice, which was attributed to inhibitory effects of daphnetin on the activation, maturation and antigen presentation ability of Dendritic Cells (DCs). Additionally, daphnetin presented low toxicity, which suggested that daphnetin had good safety and druggability. Chen et al. built a C57BL/6 mouse model with EAE by immunizing mouse with myelin oligodendroglia glycoprotein (MOG35-55) and explored the effects of cnidiadin on anti-EAE by treating on 7 d (subclinical stage) or 13 d (clinical stage) after modeling. The results demonstrated that cnidiadin significantly alleviated spinal inflammation and the degree of demyelination, therefore, alleviated the pathological symptoms of EAE mice. The therapeutic effects of cnidiadin with early intervention were better than that of late intervention.
Coumarins could inhibit Nuclear Factor-kappa B (NF-κB) pathway and activate Heme Oxygenase 1 (HO1) to inhibit the release of related inflammatory factors (IL-1β, IL-6 and TNF-α) to inhibit activation, maturation and antigen presentation ability of DCs, therefore, improving the pathological symptoms of EAE mice.
Anti-systemic lupus erythematosus: Systemic Lupus Erythematosus (SLE) is an inflammative desmosis related to unknown autoimmunity and involvement of several visceral organs characterized by erythema or excessive deposition, or loss of skin pigmentation. As one of difficult miscellaneous diseases, it got into troubles of difficult cure, poor prognosis and rare drugs. Daphnetin exhibited favorable effects on anti-systemic lupus erythematosus, which would provide promising lead compounds for anti-systemic lupus erythematosus new drug development based on coumarins. Li et al. built a NZB/WF1 mouse model with SLE and explored the effects of daphnetin on anti-SLE effects treated with intraperitoneal injections of daphnetin once a day for 12 w. The results demonstrated that daphnetin treatment could significantly improve the survival rate of SLE-prone mice, reduce renal damage and blood urea nitrogen levels and inhibit the production of serum autoantibodies, thus improving the pathological symptom of SLE mice.
Protein A20 is an effective anti-inflammatory protein to maintain immune balance of body and down regulated protein A20 in cells leads to a marked phenotype of auto inflammation. Daphnetin could inhibit NF-κB pathway to up regulate the expression of protein A20 and down regulate the expression of related inflammatory factors (IL-1β, IL-6 and TNF-α), therefore inhibiting activated T cell to alleviate the inflammation and injury in SLE mice.
Anti-IPF and anti-asthma: IPF is a progressive disease characterized by excessive deposition of Extracellular Matrix (ECM) and chronic inflammation. Du et al. built a mouse model with IPF by intratracheal injection of bleomycin (BLM) and explored the effects of psoralen on IPF administered for 14 d after modeling. The results demonstrated that psoralen alleviated BLM-induced lung parenchymal inflammation and pulmonary fibrosis in IPF mice, therefore, increase survival rate of IPF mice while presenting little effects on mice weight. Psoralen could downregulate the expression of Transforming Growth Factor-β1 (TGF-β1), IL-1β, inhibit fibroblast proliferation and collagen synthesis to alleviate inflammatory cascades or respiratory dysfunction, therefore, improving pathological symptom in IPF mice.
Li et al.[60] built a BALB/c mouse model with asthma with ovalbumin and explored the effects of imperatorin on chronic airway inflammation and airway remodeling in an asthma model mouse. The results indicated that imperatorin could significantly inhibit inflammatory cell infiltration and goblet cell proliferation, reduce mucus secretion and collagen deposition, decrease the numbers of inflammatory cells and levels of IL-4, IL-5 and IL-13 and increase the level of Interferon-gamma (IFN-γ) in the bronchoalveolar lavage fluid in lung tissue of the asthma model mice, therefore, alleviating airway inflammation and airway remodeling in the asthma model mice[61].
Anti-oxidation: Coumarins exhibited excellent anti- oxidation effects; which would be potential drugs to treat many diseases related to lipid peroxidation injury. Mogadem et al.[62] built a rat model with hepatotoxicity induced by Carbon tetrachloride (CCl4) and explored the effects of daphnetin on anti- oxidation. The results demonstrated that daphnetin alleviated CCl4-induced lipid peroxidation and increased antioxidant capacity; therefore, improving liver and kidney function of rats. Zhang et al.[63] found that esculetine and daphnetin presented favorable free radical scavenging capacity with a Diphenyl Picrohydrazine (DPPH) assay, which suggested that esculetine and daphnetin exhibited excellent anti- oxidant capacity. Zhang et al.[64] extracted the active ingredient of natural coumarins from Chinese herb Angelica by an ultrasonic method with ethanol as solvent. The results indicated that Angelica dahurica extracts (0.6 mg/ml) exhibited better free radical scavenging capacity than that of the same amount of VC with a DPPH assay, which suggested that coumarins from Angelica dahurica would be natural antioxygen with high-efficiency and low-toxicity.
Witaicenis et al.[65] built a rat model with intestinal inflammation by enema treatment with trinitrobenzene sulfonic acid and explore the antioxidant effects of six coumarin derivatives (daphnetin, esculin, fraxetin, scopoletin, scoparone and 4-methyl-umbeliferone) by an intragastric administration with the target coumarin derivatives. The rat colons were obtained for evaluation after 48 h. The results demonstrated that the six coumarin derivatives exhibited favorable antioxidant capacity by a lipid peroxidation assay and a DPPH assay. Daphnetin and esculetin presented excellent antioxidant capacity by inhibiting lipid peroxidation. Except for 7-hydroxy-4-methyl coumarin, the rest five derivatives exhibited favorable antioxidant capacity by increasing glutathione levels and inhibiting myeloperoxidase activity.
Anti-coagulation: There are three coumarin-related anticoagulants used in clinic, such as warfarin potassium, warfarin sodium and phenylprusside. Warfarin is a commonly used anticoagulant targeting to Vitamin K Epoxide Reductase (VKOR)[66]. Warfarin blocks the vitamin K functional cycle by inhibiting the activity of VKOR and preventing VKO from being converted into vitamin K and Vitamin K2 (VK2). In addition, it inhibits the γ-carboxylation of vitamin K-dependent clotting factors. Therefore, it acts as a favorable anticoagulant[67].
Thrombosis and detachment are responsible for adverse cardiovascular events. Coumarin and its derivatives as clinical drugs presented outstanding anticoagulant and anti-platelet aggregation effects with low-toxicity, which would provide new skeletons and promising lead compounds for anticoagulant new drug development based coumarins. For instance, warfarin and dicoumarin, derivatives of 4-hydroxycoumarin, widely used as oral anticoagulant drug, especially warfarin, which is the most commonly used coumarin in a clinical practice, can be rapidly and completely absorbed by the gastrointestinal tract and exhibits favorable anticoagulant activity, however, it has severe side effects[68]. As a result, it is indispensible to develop anticoagulant new drug with high-efficiency and low-toxicity.
Many natural coumarins presented favorable anticoagulant effects. The results indicated that suberenol (fig. 1 D1) and suberosin (fig. 1 D2) isolated from ferulic plants exhibited favorable anticoagulant activity and little damage of livers and kidneys. Compared with the control, suberenol and suberosin significant prolonged mice Prothrombin Time (PT). Mira et al. isolated three coumarins from Chinese herb named Angelica sinensis. The results indicated that hyuganin C prolonged mice PT in a concentration-dependent way and inhibited platelet aggregation induced by adenosine diphosphate.
Bang et al.[69] synthesized a series of coupled- coumarin derivatives with 7-hydroxycoumarin as raw materials. The results demonstrated that the optimal derivative (PT, 13.10 s) prolonged mice PT better than that of warfarin (PT, 7.97 s). ARY company developed a new coumarin derivative named tecarfarin to be used for inhibiting excitation of vitamin K-dependent coagulation factor II, VII, IX and X, which was expected to treat coagulation disorder of high-risk groups, attenuate pathological symptom, improve life quality, prevent acute and chronic complications, lower the mortality[70]. Amin et al.[71] synthesized twenty-three new 6-substituted coumarin derivatives and evaluated their anticoagulant effects in mice. Four derivatives showed excellent anticoagulant effects (PT, 36.5 s, 37.8 s, 38.5 s, 42.3 s), which was similar to positive control warfarin (PT, 42.3 s).
Anti-cancer:
Coumarins exhibited favorable anti-cancer effects with little drug-resistance on lung cancer[72,73], gastric carcinoma[74], hepatic carcinoma[75], breast cancer[76,77], leukemia and so on. Coumarin and its derivatives have excellent druggability due to its advantages of favorable pharmacological activities, multi-target collaboration, little drug-resistance and low-toxicity, simple skeleton, easy synthesis and structural optimization and extensive sources, which would play an important role in anti-cancer new drug development.
Anti-lung cancer: Lung cancer, the leading death cause worldwide, is characterized by low 5 y survival rate and high drug-resistance, which is divided into Small Cell Lung Cancer (SCLC) and Non-Small Cell Lung Cancer (NSCLC)[78,79]. Coumarin and its derivatives presented favorable anti-lung cancer effects with little drug-resistance, which would provide new skeletons and promising lead compounds for anti-lung cancer new drug development.
It was indicated that osthole significantly inhibited cell proliferation, promoted cell apoptosis of human NSCLC cell line H1299 cells. Wan et al.[80] found that aesculetin inhibited cell proliferation, induced cell cycle arrest in Synthesis-phase (S-phase) and promoted cell apoptosis of human NSCLC cell line H460 cells in vitro. Aesculetin significantly inhibited tumor growth of lung cancer-bearing Lewis mice model and improved its immunological function in vivo, suggesting coumarins presented excellent anti- lung cancer activity.
Wei et al.[81] synthesized a series of coumarin pyrazoline derivatives HCP1-HCP6 (fig. 4), which might be Heat Shock Protein 90 (HSP90) inhibitors. The results demonstrated that the six derivatives decreased cell vitality; promote cell apoptosis of human NSCLC cell line A549 cells, which was attributed to autophagy inhibition. The molecular docking results indicated that the six derivatives had favorable molecular interaction with the Adenosine Triphosphate (ATP)-binding pocket in N-terminal domain of Hsp90 (Hsp90N) and HCP1 (fig. 4) was the optimal one with a strong binding force with Hsp90N; suggesting Hsp90N would be the drug target for HCP1. The results would provide a useful drug target for anti-lung cancer new drug development. Han et al.[82] synthesized ten new coumarin-aniline pyrimidine derivatives. The results indicated that the ten derivatives exhibited cell proliferation of human NSCLC cell line H1975 cells in different extent (IC50, 2.70-17.59 μM) and the inhibitory activity of six coumarin-aniline pyrimidine derivatives (fig. 4) were better than that of positive control drug gefitinib (IC50, 9.18 μM).
The favorable anti-NSCLC effects of osthole was attributed to activating NF-κB pathway to upregulate the expression of pro-apoptotic protein B-cell lymphoma 2 (Bcl-2)-Associated X (BAX) and downregulate the expression of anti-apoptotic protein Bcl-2 to promote apoptosis of H1299 cells.
Anti-gastric carcinoma: Coumarins presented favorable anti-gastric carcinoma effects with little drug-resistance, which would provide promising lead compounds for new drug development based on coumarins.
Jia et al.[83] found that aesculetin inhibited cell proliferation of human gastric carcinoma cell line SGC-7901 cells in a concentration-dependent way and the inhibitory rate reached 79.88 % at a large dose (IC50, 280 μM) with a 3-(4,5-dimethylthiazol- 2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay. The morphological characteristics of SGC- 7901 cells showed an apoptotic trend. Aesculetin could upregulate the expression of pro-apoptotic protein BAX and downregulate the expression of anti-apoptotic protein Bcl-2 to promote apoptosis of SGC-7901 cells. Xu et al. demonstrated that osthole significantly inhibited cell proliferation; induce cell cycle arrest at Growth 2 (G2)/Mitotic (M) phase of human gastric carcinoma cell line SGC-7901 and Human cell line (HGC-27) cells in a concentration- dependent way. Wang et al.[84] synthesized four NO donors coumarin-3-carboxylic acid derivatives (fig. 4) by Knoevenagel method using resorcinol as raw materials. The results indicated that the four derivatives exhibited excellent inhibitory effects on HGC-27, their inhibitory effects (2.12-3.80 μM) were similar to positive control drug 5-Fluorouracil (5-FU) (IC50, 12.3 μM).
Aesculetin inhibited Phosphoinositide-3-Kinase/ Protein kinase B (PI3K/AKT) pathway to downregulate the expression of cell cycle-related proteins such as cyclin B1 and Cell division control 2 (Cdc2) to induce cell cycle arrest, therefore, inhibiting the cell growth and proliferation of SGC- 7901 and HGC-27 cells.
Anti-hepatic carcinoma: Coumarins exhibited favorable anti-hepatic carcinoma effects with little drug-resistance[85,86], which would provide new skeletons for anti-hepatic carcinoma new drug development.
Psoralen and its derivatives 5-methoxypsoralen and 8-methoxypsoralen are the representatives of anti- tumor drugs of furanocoumarins. Psoralen and its derivatives have important application value in the liver carcinoma treatment. Jiang et al.[87] found that psoralen inhibited cell proliferation; promote cell apoptosis of human hepatoma cell line SMMC- 7721 cells in a concentration- and time-dependent way by a MTT assay. Wang et al.[88] demonstrated that aesculetin could promote SMMC-7721 cell apoptosis. Osthole inhibited cell proliferation and migration, induced cell cycle arrest of Hepatocellular Carcinoma (HCC).
Osthole downregulated the expression of Matrix Metalloproteinases-2 (MMP-2) and MMP-9 to inhibit cell migration and inhibited epithelial- mesenchymal transformation and downregulated the expression of mesenchymal N-cadherin and vimentin to induce cell cycle arrest, therefore, inhibiting HCC. Psoralen upregulated the expression of protein p53 to downregulate the expression of anti-apoptotic protein Bcl-2 and upregulate the expression of pro- apoptosis protein BAX and caspase-3 to promote apoptosis of SMMC-7721 cells. Aesculetin also upregulated the expression of pro-apoptosis protein caspase-3 to promote apoptosis of SMMC-7721 cells by mitochondrial pathway.
Anti-breast cancer: Breast cancer is the first female cancer killer worldwide; its occurrence is closely related to estrogen levels[89]. Coumarins exhibited favorable anti-breast cancer effects with little drug- resistance[90], which would provide new skeletons and promising lead compounds for new drug development and overcoming drug-resistance based on coumarins.
Zhao et al.[90] demonstrated that psoralen significantly inhibited cell proliferation, induced cell cycle arrest, promoted cell apoptosis of human breast cancer cell persister Michigan Cancer Foundation-7 (MCF-7)/ Adriamycin (ADR) in a concentration-dependent manner (IC50, 18.78 μg/ml).
Mokale et al. designed and synthesized a series of heterozygous molecules containing coumarin- chalcone. The results demonstrated that derivative 10 presented favorable anti-proliferation effects on human breast cancer cell lines MCF-7 cells (EC50, 10 μg/ml) and MDA-MB-435 (EC50, 75.3μg/ml) in vitro. Oral administration of derivative 30 (5 mg/kg) resulted in an effective tumor inhibition rate of 70% against a female Sprague-Dawley (SD) rat model with breast cancer induced by N-Methyl-Nitrosourea (MNU) in vivo. Feng et al. designed and synthesized four 2-phenylthiazol-coumarin derivatives. The results indicated that the four derivatives exhibited certain inhibitory effects on MCF-7 cells and human breast cancer cisplatin-resistant cells by a Cell Counting Kit-8 (CCK-8) assay. A 2-phenylthiazol- coumarin derivative 5c (fig. 4) showed the strongest inhibitory activity against MCF-7 cells and MCF-7/ cisplatin cells (inhibitory rate, 32.44 % and 17.99 %, respectively).
Psoralen upregulated the expression of protein p53 to upregulate pro-apoptosis protein caspase-3 and downregulate the expression of anti-apoptosis protein Bcl-2 to promote cell apoptosis of MCF-7/ ADR.
Anti-leukemia: Leukemia is a group of highly heterogeneous diseases that originate from the malignant transformation of hematopoietic stem/ progenitor cells in the bone marrow. It is one of the top ten malignant tumors with the highest proportion of children and people under 35 y old[91]. It is characterized by malignant proliferation of leukemia cells in bone marrow and other tissues, accompanied by differentiation and maturation disorders and apoptosis inhibition, and extensive infiltration of systemic tissues and organs[92]. Coumarins presented favorable anti-leukemia with little drug- resistance[93,94], which would provide promising lead compounds for anti-leukemia new drug development.
Antiangiogenic effects:
Angiogenesis can provide oxygen and nutrients to tumor cells, remove wastes from the tumor microenvironment, and promote tumor growth and invasion. The neovascularization can transfer tumor cells to lead to tumor deterioration, which plays a key role in tumorigenesis and development[95]. An imbalance between pro-angiogenic and anti- angiogenic factors can drive aberrant angiogenesis in tumor tissues[96]. Vascular Endothelial Growth Factor (VEGF) plays an important role in angiogenesis and repair of blood vessels in normal tissue or endothelial cell proliferation and migration in disease[97,98].
Coumarin exerts anti-angiogenic effects by regulating the expression of VEGF and phosphorylation level of VEGFR-2, which would provide a new antiangiogenic therapy. Park et al.[99] found that esculin could inhibit VEGF induced proliferation and DNA synthesis of Human Umbilical Vein Endothelial Cells (HUVECs) without cytotoxicity. Esculin downregulated the expression of MMP-2 in HUVECs stimulated by VEGF, thereby inhibiting the cell migration. Esculin also downregulated the phosphorylation level of VEGFR-2 and its downstream signaling pathways of Extracellular signal-regulated Protein Kinases 1 and 2 (ERK1/2) and endothelial Nitric Oxide Synthase (eNOS)/Akt, restrained microvascular growth in VEGF-treated aortic ring in vitro, and blocked VEGF induced neovascularization and hemoglobin content in Matrigel plug model in vivo. Kim et al.[100] demonstrated that marmesin (fig. 4) could inhibit VEGF-A induced endothelial cell migration, invasion and proliferation, and inhibit tumor cell angiogenesis.
Coumarin derivatives presented anti-cancer activity by inhibiting the secretion of VEGF to restrain angiogenesis of cancer cells. Cui et al. studied the anti-cancer activity and mechanism of three Tristyle-Coumarin derivatives (TCHs), TCH-10b (fig. 4), TCH-5a (fig. 4-4 S2) and TCH-5c (fig. 4-4 S3). It was indicated that compound TCH-5c had inhibitory effects on vascular endothelial cells permanent human cell line EA.hy926 and breast cancer cells, SK-BR-3 and MCF-7. Compound TCH- 5c inhibited cell proliferation and migration; induce Resting phase (G0)/G1 cell cycle arrest, changed cell cytoskeleton organization to cause cell death in EA.hy926 cells. Compound TCH-5c suppressed tumor formation in SK-BR-3 xenograft mouse model in vivo by inhibiting the secretion of VEGF to restrain endothelial angiogenesis.
Summary
There are over 2000 coumarin natural compounds from plants, animals or microorganisms in nature. Coumarin is a kind of lactone compound with skeleton of benzo α-pyrones, which can be divided into simple coumarins, furanocoumarins, pyrocoumamarins and other coumarins according to their chemical structures, substituent position and characteristics.
With the large-scale clinical application of antibiotics, antibiotic resistance is increasingly becoming a serious problem. There are rare effective anti-fungus or anti-virus drugs in a clinical practice. Inflammation, oxidative stress and immunologic dysfunction are closely related to occurrence and development of many diseases, especially clinical difficult miscellaneous diseases, for instance, COVID-19, rheumatoid arthritis, autoimmune neuroinflammation, systemic lupus erythematosus (Li et al., 2017), IPF and cancers, etc. The diseases got into troubles of difficult cure and bad prognosis, rare drugs and severe drug-resistance. Coumarins exhibited favorable effects with multi-target collaboration on anti-infection, anti-inflammatory, anti-rheumatism, anti-immunity and anti-oxidation, anti-coagulation and anti-cancer. Coumarins had favorable druggability due to its advantages of outstanding pharmacological activities, multi-target synergy, little drug-resistance, low-toxicity, simple skeleton, easy synthesis and structural modification, and extensive sources.
The structure optimization of coumarin master ring or substituent groups greatly improve their activities. Some coumarin derivatives have favorable anti- bacteria, anti-virus, anti-coagulant, anti-cancer, anti-angiogenesis. Nicousamide, a coumarinamide derivative, is a phase 2 clinical drug intended for the treatment of renal dysfunction, including diabetic nephropathy and hypertensive nephropathy. Studies have shown that nicousamide can inhibit or slow the progression of renal fibrosis by inhibiting the phosphorylation of TGF-β receptor and its downstream proteins. The coumarin derivatives would provide promising lead compounds with little drug-resistance, high-efficiency and low-toxicity for new drug development for related diseases based on coumarins.
However, most coumarins-related researches focused on pharmacological activity evaluation in vitro in a cell level, lacking of data concerning in vivo effects and clinical trials. Additionally, it is of absence to systematic and in-depth molecular mechanism. Meanwhile, some natural coumarins exhibit poor activity, low bioavailability and high toxicity, which limit its new drug development and clinical applications. It is an issue worthy of in- depth research how to modify its structure to obtain coumarin derivatives with favorable pharmacological activity and bioavailability, little drug-resistance and low toxicity. With in-depth researches concerning pharmacological effects, structural optimization, molecular mechanism of coumarins, coumarins would be a broad application prospects in the near future.
Authors’ contributions:
All authors have contributed significantly. Jie Jin, Huijin Li and Pengquan Li are responsible for the review writing and reference analysis. Lu Xing, Xiaoqiang Huang, Jie Zhang, Xin Zhou and Wei Qin, focus on the reference review and analysis. Chunxia He, Dong Zhao and Haiqing Chu devote themselves to the reference collection. Yi Ma and Huiling Cao commit themselves to the manuscript revise and polish. Jie Jin, Hui-Jin Li and Peng-Quan Li have contributed equally to this work.
Funding:
This work was supported by the National Natural Science Foundation of China (No.82200513); the Key Program of Shaanxi Provincial Science and Technology Department (Nos.2022ZDLSF05-15, 2021SF-303, 2021JQ-786 and 2021JQ-788); the Key Program of Shaanxi Provincial Education Department (Nos.17JS117, 19JS058, 20JS134 and 20JS138); the Key Program of Weiyang District Bureau of Science, Technology and Industry Information Technology (No: 202031 and 202221) and the Talent Program of Xi'an Medical University (No: 2021TD02).
Conflict of interests:
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Yang Q, Peng Y. Advances in antitumor natural coumarin drugs. J Chizhou Coll 2013;3:49-52.
- Luo Y, Luo XX, Li MK. Research progress on antibacterial activity and mechanism of coumarin compounds. Shandong Med J 2017;28:102-5.
- Vogel A. Darstellung von Benzoesäure aus der Tonka?Bohne und aus den Meliloten?oder Steinklee?Blumen. Ann Phys 1820;64(2):161-6.
- Liu W, Qiao XY, Zhang ZY. Antifungal application of esculin. China Patent 201810941384.5, 2018-11-09.
- Li W, Yang S, Xu P, Zhang D, Tong Y, Chen L, et al. SARS-CoV-2 RNA elements share human sequence identity and upregulate hyaluronan via NamiRNA-enhancer network. EBioMedicine 2022;76:103861.
[Crossref] [Google Scholar] [PubMed]
- Zhang LX, Shi CY, Liu RY, Geng HL. Synthesis and antifungal activity of multi-substituted coumarins. Chem Res Appl 2020;7:1214-26.
- Zong P. The therapeutic effect of 7-hydroxycoumarin on collagen arthritis in rats and its mechanism of regulating the proliferation/apoptosis of FLS through Wnt/β-catenin pathway. Anhui Med Univ 2022;7(11):115.
- Zhang S, Wang D, Xue N, Lai F, Ji M, Jin J, et al. Nicousamide protects kidney podocyte by inhibiting the TGFβ receptor II phosphorylation and AGE-RAGE signaling. Am J Translat Res 2017;9(1):115.
[Google Scholar] [PubMed]
- Wang D, Lu Z, Zhang H, Jin SF, Yang H, Li YM, et al. Daphnetin alleviates experimental autoimmune encephalomyelitis via regulating dendritic cell activity. CNS Neurosci Ther 2016;22(7):558-67.
[Crossref] [Google Scholar] [PubMed]
- Figueroa-Guinez R, Joao Matos M, Vazquez-Rodriguez S, Santana L, Uriarte E. Interest of antioxidant agents in parasitic diseases. Curr Top Med Chem 2015;15(9):850-6.
[Crossref] [Google Scholar] [PubMed]
- Gao L, Wang F, Chen Y, Li F, Han B, Liu D. The antithrombotic activity of natural and synthetic coumarins. Fitoterapia 2021;154:104947.
[Crossref] [Google Scholar] [PubMed]
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71(3):209-49.
[Crossref] [Google Scholar] [PubMed]
- Cui N, Lin DD, Shen Y, Shi JG, Wang B, Zhao MZ, et al. Triphenylethylene-coumarin hybrid TCH-5c suppresses tumorigenic progression in breast cancer mainly through the inhibition of angiogenesis. Anticancer Agents Med Chem 2019;19(10):1253-61.
[Crossref] [Google Scholar] [PubMed]
- Singh R, Kumar A, Rane JS, Khan R, Tripathi G, Ajay AK, et al. Arylcoumarin perturbs SARS-CoV-2 pathogenesis by targeting the S-protein/ACE2 interaction. Sci Rep 2022;12(1):17038.
[Crossref] [Google Scholar] [PubMed]
- Bao Y, Meng X, Liu F, Wang F, Yang J, Wang H, et al. Protective effects of osthole against inflammation induced by lipopolysaccharide in BV2 cells. Mol Med Rep 2018;17(3):4561-6.
[Crossref] [Google Scholar] [PubMed]
- Li M, Shi X, Chen F, Hao F. Daphnetin inhibits inflammation in the NZB/W F1 systemic lupus erythematosus murine model via inhibition of NF-κB activity. Exp Ther Med 2017;13(2):455-60.
[Crossref] [Google Scholar] [PubMed]
- Du MY, Duan JX, Zhang CY, Yang HH, Guan XX, Zhong WJ, et al. Psoralen attenuates bleomycin?induced pulmonary fibrosis in mice through inhibiting myofibroblast activation and collagen deposition. Cell Biol Int 2019;44(1):98-107.
[Crossref] [Google Scholar] [PubMed]
- Zhou L. Antibacterial mechanism of fraxetin against Escherichia coli. Liaoning Univ J 2013.
- Yuan H, Zhang W, Liu H, Zhang Y, Zhang C, Liu X. Synthesis and antibacterial activity of coumarin oxime ester derivatives. J Pest Sci 2022;24(5):1189-95.
- Li H, Kong D, Zhu Q, Wang S, Wang Z. Synthesis and antibacterial activity of piperazine coumarin derivatives. J Xichang Univ 2019;3:8-11
- Li YL, Li HY. Narrow-spectrum UVB combined with psoralen injection in the treatment of psoriasis vulgaris. Chin J Misdiagn 2009;4:846-7.
- Fairbrother RW, Williams BL. Two new antibiotics. Antibacterial activity of novobiocin and vancomycin. Lancet 1956;271(6954):1177-9.
[Crossref] [Google Scholar] [PubMed]
- Colville JM, Gale HH, Cox F, Quinn EL. Clinical observations on the use of novobiocin in penicillin-resistant staphylococcal septicemia. Antibiot Ann 1957;5:920-6.
[Google Scholar] [PubMed]
- Kai K, Shimizu BI, Mizutani M, Watanabe K, Sakata K. Accumulation of coumarins in Arabidopsis thaliana. Phytochemistry 2006;67(4):379-86.
[Crossref] [Google Scholar] [PubMed]
- Pechmann HV, Duisberg C. Neue bildlmgsweise der cumarine. Synmese does daplliletins. Ber Dtsch Chem Ges 1883;16(2):19-20.
- Zhang LX, Shi CY, Liu RY, Geng HL. Synthesis and antifungal activity of multi-substituted coumarins. Chem Res Appl 2020;7:1214-26.
- Perkin WH. XXIII. On the hydride of aceto-salicyl. J Chem Soc 1868;21:181-6.
- Yao PH, Kumar S, Liu YL, Fang CP, Liu CC, Sun CM. Diversity-oriented synthesis of coumarin-linked benzimidazoles via a one-pot, three-step, intramolecular knoevenagel cyclization. ACS Combinatorial Sci 2017;19(4):271-5.
- Mashraqui S, Dhaval V, Mistry H. (2005). Efficient synthesis of 3a substituted coumarins. Synthetic Commun 2005;34(17):3129-34.
- Wen SQ. New progress in the application research of coumarin antibacterial compounds. Electr J Clin Med Liter 2017;24:4707-10.
- Mishra S, Pandey A, Manvati S. Coumarin: An emerging antiviral agent. Heliyon. 2020;6(1):e03217.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Li M, Wang C, Guo M, Zhang R, Chen C. Immunomodulatory effect of psoralen on rheumatoid arthritis mouse model. Chin J Exp Zool 2017;2:207-10.
- Kostova I, Bhatia S, Grigorov P, Balkansky S, S Parmar V, K Prasad A, et al. Coumarins as antioxidants. Curr Med Chem 2011;18(25):3929-51.
[Crossref] [Google Scholar] [PubMed]
- Golfakhrabadi F, Abdollahi M, Ardakani MR, Saeidnia S, Akbarzadeh T, Ahmadabadi AN, et al. Anticoagulant activity of isolated coumarins (suberosin and suberenol) and toxicity evaluation of Ferulago carduchorum in rats. Pharm Biol 2014;52(10):1335-40.
[Crossref] [Google Scholar] [PubMed]
- Mira A, Alkhiary W, Shimizu K. Antiplatelet and anticoagulant activities of Angelica shikokiana extract and its isolated compounds. Clin Appl Thromb Hemost 2017;23(1):91-9.
[Crossref] [Google Scholar] [PubMed]
- Ahmed S, Khan H, Aschner M, Mirzae H, Küpeli Akkol E, Capasso R. Anticancer potential of furanocoumarins: Mechanistic and therapeutic aspects. Int J Mol Sci 2020;21(16):5622.
[Crossref] [Google Scholar] [PubMed]
- Kupeli Akkol E, Genç Y, Karpuz B, Sobarzo-Sánchez E, Capasso R. Coumarins and coumarin-related compounds in pharmacotherapy of cancer. Cancers 2020;12(7):1959.
[Crossref] [Google Scholar] [PubMed]
- Zheng M, Zou Y, Fu YY. Research progress on pharmacological effects of daphnetin. Chin Patent Med 2017;4:790-4.
- Chen X, Pi R, Zou Y, Liu M, Ma X, Jiang Y, et al. Attenuation of experimental autoimmune encephalomyelitis in C57 BL/6 mice by osthole, a natural coumarin. Eur J Pharmacol 2010;629(1-3):40-6.
[Crossref] [Google Scholar] [PubMed]
- Wang H, Zou D, Xie K, Xie M. Antibacterial mechanism of fraxetin against Staphylococcus aureus. Mol Med Rep 2014;10(5):2341-5.
[Crossref] [Google Scholar] [PubMed]
- Zhang Q, Zhang Y, Ding S, Zhang H, Feng G. A near-infrared fluorescent probe for rapid, colorimetric and ratiometric detection of bisulfite in food, serum, and living cells. Sensors Acta B Chem 2015;211:377-84.
- Annunziata F, Pinna C, Dallavalle S, Tamborini L, Pinto A. An overview of coumarin as a versatile and readily accessible scaffold with broad-ranging biological activities. Int J Mol Sci 2020;21(13):4618.
[Crossref] [Google Scholar] [PubMed]
- Jia C, Zhang J, Yu L, Wang C, Yang Y, Rong X, et al. Antifungal activity of coumarin against Candida albicans is related to apoptosis. Front Cell Infect Microbiol 2019;8:445.
[Crossref] [Google Scholar] [PubMed]
- Liu HM, Hua M, Zhang L, Zhu HH. Effects of esculin on immune function and tumor inhibition in Lewis lung cancer mice. Northwest Pharm J 2022;5:40-5.
- Wei Q, Ning JY, Dai X, Gao YD, Su L, Zhao BX, et al. Discovery of novel HSP90 inhibitors that induced apoptosis and impaired autophagic flux in A549 lung cancer cells. Eur J Med Chem 2018;145:551-8.
[Crossref] [Google Scholar] [PubMed]
- Yang G, Shi L, Pan Z, Wu L, Fan L, Wang C, et al. The synthesis of coumarin thiazoles containing a trifluoromethyl group and their antifungal activities. Arabian J Chem 2021;14(1):102880.
- Frenk J, Chen LC, Chandran L, Groff EO, King R, Meleis A, et al. Challenges and opportunities for educating health professionals after the COVID-19 pandemic. Lancet 2022;400(10362):1539-56.
[Crossref] [Google Scholar] [PubMed]
- Abdizadeh R, Hadizadeh F, Abdizadeh T. In silico analysis and identification of antiviral coumarin derivatives against 3-chymotrypsin-like main protease of the novel coronavirus SARS-CoV-2. Mol Divers 2022;26(2):1053-76.
[Crossref] [Google Scholar] [PubMed]
- Suleimen YM, Jose RA, Suleimen RN, Ishmuratova MY, Toppet S, Dehaen W, et al. Isolation and in silico SARS-CoV-2 main protease inhibition potential of Jusan coumarin, a new dicoumarin from Artemisia glauca. Molecules 2022;27(7):2281.
- Yang S, Ling Y, Zhao F, Li W, Song Z, Wang L, et al. Hymecromone: A clinical prescription hyaluronan inhibitor for efficiently blocking COVID-19 progression. Signal Transduct Target Ther 2022;7(1):91.
[Google Scholar] [PubMed]
- Ciminski K, Chase GP, Beer M, Schwemmle M. Influenza A viruses: Understanding human host determinants. Trends Mol Med 2021;27(2):104-12.
[Crossref] [Google Scholar] [PubMed]
- Lee BW, Ha TK, Cho HM, An JP, Kim SK, Kim CS, et al. Antiviral activity of furanocoumarins isolated from Angelica dahurica against influenza a viruses H1N1 and H9N2. J Ethnopharmacol 2020;259:112945.
[Crossref] [Google Scholar] [PubMed]
- Bizzarri BM, Botta L, Capecchi E, Celestino I, Checconi P, Palamara AT, et al. Regioselective IBX-mediated synthesis of coumarin derivatives with antioxidant and anti-influenza activities. J Nat Prod 2017;80(12):3247-54.
[Crossref] [Google Scholar] [PubMed]
- Wang Y, Yan W, Chen Q, Huang W, Yang Z, Li X, et al. Inhibition viral RNP and anti-inflammatory activity of coumarins against influenza virus. Biomed Pharmacother 2017;87:583-8.
[Crossref] [Google Scholar] [PubMed]
- Liu P, Wang B, Jiang X, Zhao J, Hu X, Fu X. Effects of daphnetin on the biological function of nematodes. J Jilin Agric Univ 2014;1:36-39.
- Hamdy AM, Khaddour Z, Al-Masoudi NA, Rahman Q, Hering-Junghans C, Villinger A, et al. Synthesis of arylated coumarins by Suzuki–Miyaura cross-coupling. Reactions and anti-HIV activity. Bioorg Med Chem 2016;24(21):5115-26.
[Crossref] [Google Scholar] [PubMed]
- Jesumoroti OJ, Mnkandhla D, Isaacs M, Hoppe HC, Klein R. Evaluation of novel N′-(3-hydroxybenzoyl)-2-oxo-2 H-chromene-3-carbohydrazide derivatives as potential HIV-1 integrase inhibitors. Med Chem Comm 2018;10(1):80-8.
- Liu YP, Yan G, Xie YT, Lin TC, Zhang W, Li J, et al. Bioactive prenylated coumarins as potential anti-inflammatory and anti-HIV agents from Clausena lenis. Bioorg Chem 2020;97:103699.
[Crossref] [Google Scholar] [PubMed]
- Liu X, Yu H, Li D. Research on the progress of coumarin compounds with anti-HIV activity. Chin Disabil Med 2014;6:326-7.
- Li Y, Zhao Y, Wang C, An Z, Yan, Park H. Inhibitory effect of imperatorin on chronic airway inflammation and airway remodeling in asthma. Yanbian Univ J Med 2018;3:161-4.
- Li X, Tan Y, Li H, Zhao LF, Sui F. Research progress on pharmacological effect and mechanism of imperatorin. Chin J Exp Tradit Med Formulae 2020;18:196-201.
- Mogadem AI, Hassan NS, Emam MA, Mohamed MR. Antioxidant and hepatoprotective activities of two coumarin derivatives against carbon tetrachloride-induced oxidative stress and liver damage in rats. Afr J Biol Sci 2013;9(1):65-79.
- Zhang N, Chen WJ, Liu QQ, Zhou Y, Zhong RG. Free radical scavenging and anti-tumor activity of coumarin compounds. Food Res Dev 2016;1:1-5.
- Zeng Y, Gu Y, Liu J. Antioxidant study of Angelica dahurica and Lonicera japonica extract. Agric Prod Proc 2019;9:6-8.
- Witaicenis A, Seito LN, da Silveira Chagas A, de Almeida Junior LD, Luchini AC, Rodrigues-Orsi P, et al. Antioxidant and intestinal anti-inflammatory effects of plant-derived coumarin derivatives. Phytomedicine 2014;21(3):240-6.
[Crossref] [Google Scholar] [PubMed]
- Shen G, Cui W, Zhang H, Zhou F, Huang W, Liu Q, et al. Warfarin traps human vitamin K epoxide reductase in an intermediate state during electron transfer. Nat Struct Mol Biol 2017;24(1):69-76.
[Crossref] [Google Scholar] [PubMed]
- Xu YQ, Wang YY, Li F, Qiu WH, Shen Y, Shen G. A cell-based method for determining the activity of vitamin K epoxide reductase. J Henan Normal Univ 2022;4:107-11.
- van Leeuwen Y, Rosendaal FR, van der Meer FJ. The relationship between maintenance dosages of three vitamin K antagonists: Acenocoumarol, warfarin and phenprocoumon. Thromb Res 2008;123(2):225-30.
[Crossref] [Google Scholar] [PubMed]
- Bang NC, Abyshev AZ, Ivkin DY. Synthesis and in vivo evaluation of new coumarin conjugates as potential indirect-action anticoagulants. Pharm Chem J 2019;53(5):419-22.
- Hobl EL, Jilma B. Tecarfarin: A novel vitamin K antagonist. Thromb Haemo 2017;117(11):2009-11.
- Amin KM, Gawad NM, Rahman DE, El Ashry MK. New series of 6-substituted coumarin derivatives as effective factor Xa inhibitors: Synthesis, in vivo antithrombotic evaluation and molecular docking. Bioorg Chem 2014;52:31-43.
[Crossref] [Google Scholar] [PubMed]
- Zhang ZR, Leung WN, Cheung HY, Chan CW. Osthole: A review on its bioactivities, pharmacological properties and potential as alternative medicine. Evid Based Complement Alternat Med 2015;2015:919616.
[Crossref] [Google Scholar] [PubMed]
- Peng JM, Zhu, KZ, Ye JL, Su LD, Dai YA. Effects of osthole on proliferation and apoptosis of lung cancer H1299 cells. Tianjin Med 2020;2:87-90.
- Xu X, Liu X, Zhang Y. Osthole inhibits gastric cancer cell proliferation through regulation of PI3K/AKT. PLoS One 2018;13(3):e0193449.
[Crossref] [Google Scholar] [PubMed]
- Zhang XN, Wang Y, Wu Y, Luo W. Research progress of antitumor active components and mechanism of Psoralea. Bachu Medicine 2019;1:102-6.
- Mokale SN, Begum A, Sakle NS, Shelke VR, Bhavale SA. Design, synthesis and anticancer screening of 3-(3-(substituted phenyl) acryloyl)-2H-chromen-2ones as selective anti-breast cancer agent. Biomed Pharmacother 2017;89:966-72.
- Feng YY, Song MQ, Wu WL, Qian JJ, Liu YW, Si XX. Synthesis and antitumor activity of 2-phenylthiazole-coumarin derivatives. Chem Res Appl 2022;6:1362-8.
- Liu ZC, Li ZX, Zhang Y, Zhou T, Zhang J, You W, et al. Interpretation of 2020 global cancer statistical report. J Multidiscipl Cancer Manag 2021;7(2):1-4.
- Zhang Y, Xu Y, Cui Q, Wang S, Ji S, Zou H. Research status and hotspots of immune checkpoint inhibitors in the treatment of lung cancer abroad based on Web of Science. Chin Oncol Med 2022;5:891-5.
- Wan XL, Zhu HJ, Ma LL. Effect of esculin on proliferation and apoptosis of human lung cancer H460 cells in vitro. Cancer Progr 2017;9:1023-5.
- Wei Y, Peng W, Wang D, Hao SH, Li WW, Ding F. Design, synthesis, antifungal activity, and 3D-QSAR of coumarin derivatives. J Pestic Sci 2018;43(2):88-95.
[Crossref] [Google Scholar] [PubMed]
- Han C, Wu LT, Su F, Hu XQ, Wang XX, Li MY. Synthesis and anti-non-small cell lung cancer activity of coumarin-aniline pyrimidine conjugate. Chin J Pharm 2020;3:177-82.
- Jia S, Liu B, Zhang D. Mechanism of aesculetin-induced apoptosis in human gastric cancer SGC-7901 cells. Heilongjiang Med 2012;3:365-8.
- Wang R, Zhang Y, Zhang Z, He LQ. Synthesis and antitumor activity of NO donor coumarin derivatives. Chem World 2018;11:768-773.
- Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66(2):115-32.
- Lin ZK, Liu J, Jiang GQ, Tan G, Gong P, Luo HF, et al. Osthole inhibits the tumorigenesis of hepatocellular carcinoma cells. Oncol Rep 2017;37(3):1611-8.
[Crossref] [Google Scholar] [PubMed]
- Jiang Z, Xiong J. Induction of apoptosis in human hepatocarcinoma SMMC-7721 cells in vitro by psoralen from Psoralea corylifolia. Cell Biochem Biophys 2014;70:1075-81.
[Crossref] [Google Scholar] [PubMed]
- Wang J, Wei ZM. Mechanism study on apoptosis of human hepatoma cells SMMC-7721 induced by acetin. Chin Patent Med 2012;11:2059-63.
- Wang J, Lv S, Li M. Research progress on the anti-breast cancer mechanism of active ingredients of traditional Chinese medicine in vitro. Chin J Exp 2020;17:197-203.
- Zhao WZ, Cheng K, Wang XH, Hua YT, Xu CF, Jiang JR. Effect of psoralen on cell cycle and apoptosis of drug-resistant breast cancer cell lines. Chin J Clink 2016;14:2111-5.
- Shi YY, Ma CY, Miao JJ, Chen Y, Chen JW, Li X. Study on the mechanism of multi-target reversal of multidrug resistance in leukemia by traditional Chinese medicine. Chin Mater Med 2016;47(7):1230-5.
- Baliakas P, Hadzidimitriou A, Sutton LA, Rossi D, Minga E, Villamor N, et al. Recurrent mutations refine prognosis in chronic lymphocytic leukemia. Leukemia 2015;29(2):329-36.
[Crossref] [Google Scholar] [PubMed]
- Wang CJ, Hsieh YJ, Chu CY, Lin YL, Tseng TH. Inhibition of cell cycle progression in human leukemia HL-60 cells by esculetin. Cancer Lett 2002;183(2):163-8.
[Crossref] [Google Scholar] [PubMed]
- Park C, Jin CY, Kwon HJ, Hwang HJ, Kim GY, Choi IW, et al. Induction of apoptosis by esculetin in human leukemia U937 cells: Roles of Bcl-2 and extracellular-regulated kinase signaling. Toxicol In Vitro 2010;24(2):486-94.
[Crossref] [Google Scholar] [PubMed]
- Lopes-Coelho F, Silva F, Gouveia-Fernandes S, Martins C, Lopes N, Domingues G, et al. Monocytes as endothelial progenitor cells (EPCs), another brick in the wall to disentangle tumor angiogenesis. Cells 2020;9(1):107.
[Crossref] [Google Scholar] [PubMed]
- Jaszai J, Schmidt MH. Trends and challenges in tumor anti-angiogenic therapies. Cells 2019;8(9):1102.
[Crossref] [Google Scholar] [PubMed]
- Abhinand CS, Raju R, Soumya SJ, Arya PS, Sudhakaran PR. VEGF-A/VEGFR2 signaling network in endothelial cells relevant to angiogenesis. J Cell Commun Signal 2016;10(4):347-54.
[Crossref] [Google Scholar] [PubMed]
- Koch S, Tugues S, Li X, Gualandi L, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Biochem J 2011;437(2):169-83.
[Crossref] [Google Scholar] [PubMed]
- Park SL, Won SY, Song JH, Lee SY, Kim WJ, Moon SK. Esculetin inhibits VEGF-induced angiogenesis both in vitro and in vivo. Am J Chinese Med 2016;44(01):61-76.
[Crossref] [Google Scholar] [PubMed]
- Kim JH, Kim JK, Ahn EK, Ko HJ, Cho YR, Lee CH, et al. Marmesin is a novel angiogenesis inhibitor: Regulatory effect and molecular mechanism on endothelial cell fate and angiogenesis. Cancer Lett 2015;369(2):323-30.
[Crossref] [Google Scholar] [PubMed]