- Corresponding Author:
- Beatriz Cedillo-Carvallo
Clinical Laboratories of Puebla of Bioequivalence
E-mail: bcedillo@clinicaruiz.com
| Date of Submission | 17 October 2014 |
| Date of Revision | 23 April 2014 |
| Date of Acceptance | 30 April 2014 |
| Indian J Pharm Sci 2014; 76(4): 281-286 |
Abstract
Clinical response to clopidogrel varies widely due to under-dosing, drug interactions and intrinsic interindividual differences resulting from genetic polymorphisms. Cytochrome P450-2C19 is the principal enzyme involved in the activation of the prodrug and loss-of-function alleles have been described. Upon expiration of the pharmaceutical patent of clopidogrel, generic manufacturers have started to subject interchangeable formulations to bioequivalence studies. The purpose of the current investigation was to study the effect of selection of volunteers homozygous for the CYP2C19*1 haplotype on the bioavailability of clopidogrel. A regular 2×2 bioequivalence study between two formulations of clopidogrel was performed in volunteers selected and unselected for relevant CYP2C19 haplotypes for the Mexican population. It was found that selection of volunteers homozygous for the CYP2C19*1 haplotype, increased the stringency of bioequivalence statistics and resulted in bioinequivalence of a generic clopidogrel compound that otherwise proved equivalent when tested in an open unselected population. Augmentation of bioequivalence strictness is expected to result from pharmacogenetic selection of volunteers.
Keywords
Clopidogrel, pharmacogenomics, pharmacogenetics, bioequivalence, polymorphism, CYP2C19
Clopidogrel is a prodrug that requires oxidation to its intermediate metabolite, 2-oxo-clopidogrel, and then to the thiol derivative, 2-oxo-clopidogrel [1]. This active thiol metabolite inhibits adenosine diphosphate (ADP)-induced platelet aggregation by blocking the platelet P2Y12 receptor resulting in important reduction in ADP-mediated platelet aggregation [2]. Clopidogrel is standard of care in most patients undergoing percutaneous coronary intervention and those experiencing acute coronary syndromes. However, it has been suggested that response to clopidogrel varies widely with nonresponse rates ranging from 4% to 30% at 24 h [3,4]. Suggested mechanisms for this variability have included under-dosing, drug interactions and intrinsic interindividual differences resulting from genetic polymorphisms in the pathways of clopidogrel pharmacokinetics and pharmacodynamics [5,6].
Cytochrome P450-2C19 (CYP2C19) is one of the principal enzymes involved in the bioactivation of the prodrug [7,8]. A common loss-of-function allele, CYP2C19*2 (c.681G>A; rs4244285), is associated with increased risk for serious adverse cardiovascular events in both heterozygous and homozygous patients with acute coronary syndromes who are receiving clopidogrel, particularly among those undergoing percutaneous coronary intervention [9,10]. Guidelines for CYPC19 genotype? directed antiplatelet therapy have been published [11-14].
Upon expiration of the pharmaceutical patent of clopidogrel in May 2012 (Daily Finance Posted 02/27/11), generic companies have started to manufacture interchangeable formulations of this drug and hence, to subject them to bioequivalence studies. Herein, we proved that selection of volunteers homozygous for the CYP2C19*1 haplotype, increased the stringency of bioequivalence statistics and resulted in bioinequivalence of a generic compound that otherwise proved equivalent when tested in an open population.
Materials and Methods
Volunteers
A total of 36 Mexican-Mestizo volunteers of both genders were included in the study. All were accrued through open invitation and proved to fulfill the criteria established by Mexican Federal bylaws and regulations. Briefly, volunteers (20 male) had a median age of 26 years (range 18-54), their weight, height and body mass index were 65.2±8.45 kg, 166.1±6.39 cm and 23.6±2.18, respectively (mean±standard deviation); careful clinical examination ruled out present or past relevant diseases, vital signs were within normal as were laboratory results (complete blood cell count, basic clinical chemistry, hepatic enzymes, urine analysis, drugs of abuse and pregnancy test), electrocardiogram and chest X-rays. All volunteers were aware of the risks and signed a Clinical Investigation Agreement to participate in the study.
The research protocol and informed consent (LCPB-11- 009 Dated 2011/07/15 DI-F012 Rev.0/2011-05-11 and LCPB-11-009 Dated 2011/07/15 UC-F003 Rev.0/2009- 01-09) were approved on July 25th 2011 by the Ethics Committee of Centro de Hematología y Medicina Interna, Laboratorios Clínicos de Puebla y Laboratorios Clínicos de Puebla de Bioequivalencia, and further endorsed by the Federal Commission for the Protection Against Sanitary Risks (COFEPRIS Approval Number CAS/OR/01/CMN/113300410B0192-3259/2011) on August 9th 2011.
Clinical study design
A single-dose administration, cross-over study was used, with a wash-out period of seven days (?21 times T½). After randomization of sequences, either a 75 mg tablet of generic clopidogrel or a similar tablet of Plavix® were administered to the fasting volunteers in the first period, and the opposite sequence during the second session. A total of 13 blood samples along a 36 h period were drawn into heparin-containing tubes from each subject in each experimental session to measure clopidogrel plasma levels. An additional EDTA pretreatment sample was obtained from each volunteer into an EDTAK2- containing tube for genotyping.
Measurement of clopidogrel plasma levels
Ultra-performance liquid chromatography (UPLC) coupled to tandem mass spectrometry (MS/MS) was used to develop a method to measure clopidogrel plasma concentrations in the volunteers? samples [15]. Solid phase extraction (SPE) was performed at acidic pH employing Oasis MCX™ (Waters) 96-well plates. An Acquity® UPLC BEH C18, 1.7 µm, 2.1×50 mm column (Waters®, Part No 186002350) was used with isocratic acetonitrile:formic acid 0.1% (75:25 v/v). The total run time was 3.5 min and the retention times were 1.897±0.02 and 1.450±0.03 min for clopidogrel and ticlopidine (internal standard, IS), respectively. Chromatography and tandem spectrometry were performed in a Waters Quattro Premier XETM tandem quadrupole mass spectrometer with Acquity UPLCTM system and analyzed in the multiple reaction monitoring mode using the respective [M+H]+ ions, m/z 321.9>154.75 and 263.95>153.77 for clopidogrel and IS, respectively. The method was validated in accordance with the recommendations of both the United States Food and Drug Administration and the Mexican Comisión Federal para la Protección contra Riesgos Sanitarios [16,17]. The overall mean recovery, using SPE extraction, was found to be 82.70, 82.06 and 80.0 %, for low, medium and high concentrations, respectively. Calibration curves were linear in the concentration range of 50-6000 pg/ml, the mean correlation coefficient during the validation was 0.998959.
Table 1 summarizes the precision and accuracy results of calculated concentrations of calibration samples. The lower limit of quantification (LLOQ) was 50 pg/ml. The LLOQ, 50 pg/ml, was sensitive enough for detecting terminal phase concentrations of the drug. Inter-batch precision of the method ranged from 5.23 to 5.82%, while Inter-batch accuracy ranged from 96.68 to 104.32%. Intrabatch precision ranged from 5.00 to 6.36%, while Intra-batch accuracy ranged from 94.40 to 102.05% at concentrations of 150 pg/ml (LQC), 3000 pg/ml (MQC) and 5000 pg/ml (HQC). Samples were subjected to freeze storage (?196°) during the entire period covered by the bioequivalence study, i.e., from the first day of volunteer sample collection up to the last day of sample analysis. The long-term stability for clopidogrel in plasma was proved in samples that were stored frozen for a period of 4 months [18].
| Dependent | Hypothesis | SS | F stat | P value |
|---|---|---|---|---|
| Ln (Cmax) | Sequence | 1.2263 | 0.90 | 0.3498 |
| Ln (Cmax) | Period | 0.1921 | 1.95 | 0.1732 |
| Ln (AUC0-t) | Sequence | 0.4551 | 0.45 | 0.5052 |
| Ln (AUC0-t) | Period | 0.0367 | 0.48 | 0.4922 |
| Ln (AUC0-8) | Sequence | 0.3786 | 0.42 | 0.5208 |
| Ln (AUC0-8) | Period | 0.0297 | 0.49 | 0.4910 |
SS: Sum of squares, F stat: statistic F value, P: probability, AUC: area under curve
Table 1: Summary of Anova to Test Effects of Sequence and Period
Genotyping by TaqMan assays
Genomic DNA was extracted from peripheral blood. The DNA was quantified by spectrophotometry and diluted to 5 ng/µl. Relevant (allele frequencies >0.01) CYP2C19 haplotypes for the Mexican Mestizo population, as detailed in Table 2, were analyzed using validated genotyping assays (Applied Biosystems, Foster City, California) according to the manufacturer?s instructions. If none of the analyzed SNPs of a given gene was detected a homozygous *1/*1 genotype was assumed [19-21].
| Gene | Haplotype | Characteristic | Assay code | Observed |
|---|---|---|---|---|
| SNP | MAF1 | |||
| CYP2C19 | *2 | rs4244285 | C__25986767_70 | 0.07 |
| (NG_008384.1:g. | ||||
| 24154G>A) | ||||
| *17 | rs12248560 | C____469857_10 | 0.10 | |
| (NG_008384.1:g. | ||||
| 4195C>T) |
1Minor Allele Frequency observed in the Mexican Mestizo population (unpublished results), MAF for *2 are comparable to those reported previously [20, 21], SNP: Single nucleotide polymorphisms, MAF: minor allele frequency
Table 2: Analyzed Alleles
Statistical analysis
Analysis of variance (ANOVA), pharmacokinetics and bioequivalence statistics were performed with the aid of the WinNonLin® software package. Criteria for bioequivalence were those required by the Mexican Comisión Federal para la Protección contra Riesgos Sanitarios [22]. The results of all initially accrued subjects (n=36) were analyzed without prior knowledge of their genotype; however, in a second step, only subjects that were homozygous for the CYP2C19 *1/*1 genotype (n=20) were included in the statistical assay.
Results
Overall study features
The clinical study was successfully completed without contingences and drug-related adverse effects. All the blood samples were drawn timely and the volunteers? good health was ascertained until the end of the follow-up period (seven days after completion of the second experimental session). The analytical method was robust during the monitoring of all of the 936 plasma samples
Pharmacogenetic selection
Twenty of the 36 volunteers showed to have a CYP2C19 *1/*1 homozygous genotype, only two presented the *1/*17 combination of alleles, and the remaining had a *1/*2 genotype. As shown in Tables 4-7, pharmacokinetic and bioequivalence statistics were estimated with the results of all the 36 subjects that participated in the study, but also with the homogeneous subgroup of them who had a *1/*1 genotype.
Analysis of variance
ANOVA was used to evaluate the effects of sequence and period on the log-transformed pharmacokinetic parameters peak concentration [Ln(Cmax)], area under the curve from time zero to the last measured point [Ln(AUC0-t)]and area under the curve from time zero and projected to infinite [Ln(AUC0-8)]. As shown in Table 3, ANOVA showed that there were no significant sequence or period effects, hence, the correct design and conduction of the clinical study was confirmed.
| Dependent | Hypothesis | SS | F stat | P value |
|---|---|---|---|---|
| Ln (Cmax) | Sequence | 1.2263 | 0.90 | 0.3498 |
| Ln (Cmax) | Period | 0.1921 | 1.95 | 0.1732 |
| Ln (AUC0-t) | Sequence | 0.4551 | 0.45 | 0.5052 |
| Ln (AUC0-t) | Period | 0.0367 | 0.48 | 0.4922 |
| Ln (AUC0-8) | Sequence | 0.3786 | 0.42 | 0.5208 |
| Ln (AUC0-8) | Period | 0.0297 | 0.49 | 0.4910 |
SS: Sum of squares, F stat: statistic F value, P: probability, AUC: area under curve
Table 3: Summary of Anova to Test Effects of Sequence and Period
Pharmacokinetic analysis
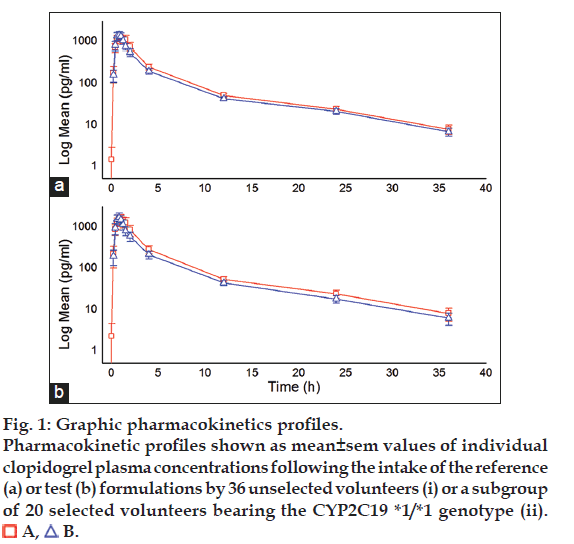
Tables 4 and 5 summarize descriptive statistics of pharmacokinetic parameters Cmax, AUC0-t and AUC0-8 in either unselected volunteers or selected according to their CYP2C19 haplotype, while fig. 1 depict the mean±standard error of the mean of the log transformed individual measurements of plasma clopidogrel levels in unselected and selected volunteers.
| A | B | ||||||
|---|---|---|---|---|---|---|---|
| Cmax (pg/ml) | AUC0-t (pg*h/ml) | AUC0-8 (pg*h/ml) | Cmax (pg/ml) | AUC0-t (pg*h/ml) | AUC0-8 (pg*h/ml) | ||
| Mean | 1702 | 4177 | 4446 | 1532 | 3593 | 3815 | |
| SD | 1986 | 3955 | 4056 | 1731 | 3301 | 3278 | |
| CV | 116 | 94 | 91 | 112 | 91 | 85 | |
SD: Standard deviation, SEM: standard error of the mean, CV: coefficient of variation, AUC: area under curve
Table 4: Descriptive Statistics of Pharmacokinetic Parameters in the Full Group of 36 Unselected Volunteers
| A | B | ||||||
| Cmax (pg/ml) | AUC0-t (pg*h/ml) | AUC0-8 (pg*h/ml) | Cmax (pg/ml) | AUC0-t (pg*h/ml) | AUC0-8 (pg*h/ml) | ||
| Mean | 2039 | 4656 | 4867 | 1736 | 3860 | 4045 | |
| SD | 1830 | 3561 | 3687 | 1569 | 3015 | 3154 | |
| SEM | 409 | 796 | 824 | 351 | 674 | 705 | |
| CV | 90 | 76 | 76 | 90 | 78 | 78 | |
SD: Standard deviation, SEM: standard error of the mean, CV: coefficient of variation, AUC: area under curve
Table 5: Descriptive Statistics of Pharmacokinetic Parameters in the Subgroup of 20 Selected Volunteers Showing the Cyp2c19 *1/*1 Genotype
Figure 1:Graphic pharmacokinetics profiles.
Pharmacokinetic profiles shown as mean±sem values of individual
clopidogrel plasma concentrations following the intake of the reference
(a) or test (b) formulations by 36 unselected volunteers (i) or a subgroup
of 20 selected volunteers bearing the CYP2C19 *1/*1 genotype (ii).
 A,
A,
 B.
B.
It seems evident that removal of the volunteers with genotypes other than the homozygous *1/*1 allele combination, resulted in a slight reduction of variance and an increased mean value for Cmax, AUC0-t and AUC0-8 of both reference (A) and test (B) formulations, but also in a much clearertendency of the reference formulation to be supraequivalent. Visual analysis of the graphics (fig. 1) confirms that there is clear although slight tendency of the plasma clopidogrel levels obtained with formulation A, to be above of those obtained with formulation B, all along the kinetic profile, and this tendency becomes more evident ?and significant, see below- when only homogeneous CYP2C19 *1/*1 volunteers are included in the analysis.
Statistical analyses for bioequivalence
Table 6 summarizes the result of the statistics that resulted from the analysis of all 36 volunteers. The Mexican regulations, as well as the United States Food and Drug Administration and other international bodies, consider two products bioequivalent if the 90% CI of the relative mean Cmax, AUC0-t and AUC0-8 of the test to reference should be within 80.00% to 125.00% in the fasting state; therefore, the conclusion of the study heretofore is that the test (B) and reference (A) formulations of 75 mg clopidogrel are indeed bioequivalent.
| 90% CI | Power | ||
|---|---|---|---|
| Ln (Cmax) | 84.21 | 109.93 | 0.8692 |
| Ln (AUC0-t) | 80.91 | 102.22 | 0.9335 |
| Ln (AUC0-8) | 81.56 | 100.59 | 0.9675 |
CI: Confidence interval
Table 6: Summary of Statistics to Assess Bioequivalence in 36 Unselected Volunteers
However, as seen on Table 7, bioequivalent statistics of the selected homogeneous group, despite showing sufficient statistic power, values exceed the 90% confidence limits to accept equivalence and hence, in this exercise, formulations A and B should be declared bioinequivalent.
| 90% CI | Power | ||
|---|---|---|---|
| Ln (Cmax) | 73.68 | 103.31 | 0.8012 |
| Ln (AUC0-t) | 72.62 | 98.81 | 0.8080 |
| Ln (AUC0-8) | 74.37 | 99.51 | 0.8161 |
CI: Confidence interval
Table 7: Summary of Statistics to Assess Bioequivalence in 20 Selected Volunteers Bearing the Genotype Cyp2c19 *1/*1
Discussion
Over the past decade, concerns have been expressed increasingly regarding the difficulty for highly variable drugs and drug products (%CV greater than 30) to meet the standard bioequivalence criteria using a reasonable number of study subjects. The topic has been discussed on numerous occasions at national and international meetings [23-29]. Despite the lack of a universally accepted solution for the issue, regulatory agencies generally agree that an adjustment of the traditional bioequivalence limits for these drugs or products may be warranted to alleviate the resource burden of studying relatively large numbers of subjects in bioequivalence trials. An alternate solution to the problem of highly variable drugs/products is to employ subpopulations of pharmacogenetically homogeneous volunteers in bioequivalence studies, in order to reduce variability due to genetically defined metabolic inter-individual differences. This approach is quite tempting because both bioavailability and bioequivalence studies could be carried out in small numbers of homogeneous subjects, thus rendering these clinical studies easier and cheaper.
This report proves that pharmacogenetic selection of volunteers with homozygous highly functional CYP2C19 haplotypes, reduces the ?variability? introduced by the inclusion of less functional CYP2C19 variant carriers but, in turn, increases the stringency of bioequivalence criteria. The inclusion of both CYP2C19 *1/*2 and *1/*17 carriers, resulted in a decrease of the mean values for Cmax, AUC0-t and AUC0-8, for both reference and test formulations, contributed a certain degree of ?tolerance? to the statistical analysis, and led to the conclusion of bioequivalence of both products, although a tendency of the test product to be sub-equivalent to the reference one was evident. Exclusion of volunteers with less functional CYP2C19 genotypes reduced such ?tolerance? and resulted in a bioinequivalence declaration for the same two formulations of clopidogrel.
From the standpoint of the consumer, increased stringency for bioequivalence is an advantage in as much as the bioavailability of generic formulations should be almost identical to that of the reference formulation to fulfill bioequivalence criteria, thence; efficacy and safety are better guaranteed. However, from the manufacturers? standpoint, selection of volunteers by means of pharmacogenetic criteria might significantly decrease the chance of generic formulations to ?pass? currently valid bioequivalence criteria.
Regulatory agencies in several countries debate on the desirability and convenience to reduce the number of volunteers in bioavailability and bioequivalence studies through pharmacogenetic selection [30-34]. There are drugs that are only or mainly transported or metabolized by a single enzyme, while others involve several complex pathways, enzymatic or otherwise. Obviously, the degree of homogeneity that can be accomplished when selecting subgroups of volunteers will depend on the number of enzymes or metabolic steps, their relative activities, their interactions and the allele frequency of the involved genes. The more homogeneous the selected population sample, the higher bioequivalence stringency is expected to result; that is, two formulations have to be practically identical to display the same bioavailability in practically identical individuals. Perhaps in the near future we will be seeing changes in both, the genomic selection criteria for individuals to participate in pharmaceutical clinical trials, as well as the criteria for similarity of two formulations to be considered interchangeable by regulatory agencies worldwide. In conclusion, pharmacogenetic selection of volunteers according to their CYP2C19 haplotype, increased the strictness of bioequivalence for a generic clopidogrel formulation.
References
- Savi P, Pereillo JM, Uzabiaga MF, Combalbert J, Picard C, Maffrand JP, et al. Identification and biological activity of the active metabolite of clopidogrel. Thromb Haemost 2000;84:891-6.
- Weber AA, Reimann S, Schrör K. Specific inhibition of ADPinduced platelet aggregation by clopidogrel in vitro. Br J Pharmacol 1999;126:415-20.
- Gurbel PA, Bliden KP, Hiatt BL, O?Connor CM. Clopidogrel for coronary stenting: Response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation 2003;107:2908-13.
- Gurbel PA, Cummings CC, Bell CR, Alford AB, Meister AF, Serebruany VL; Plavix Reduction of New Thrombus Occurrence (PRONTO) trial. Onset and extent of platelet inhibition by clopidogrel loading in patients undergoing elective coronary stenting: The Plavix Reduction of New Thrombus Occurrence (PRONTO) trial. Am Heart J 2003;145:239-47.
- Nguyen T, Frishman WH, Nawarskas J, Lerner RG. Variability of response to clopidogrel: Possible mechanisms and clinical implications. Cardiol Rev 2006;14:136-42.
- O?Donoghue M, Wiviott SD. Clopidogrel response variability and future therapies: clopidogrel: Does one size fit all? Circulation 2006;114:e600-6.
- Brandt JT, Close SL, Iturria SJ, Payne CD, Farid NA, Ernest CS, et al. Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel. J Thromb Haemost 2007;5:2429-36.
- Kazui M, Nishiya Y, Ishizuka T, Hagihara K, Farid NA, Okazaki O, et al. Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Metab Dispos 2010;38:92-9.
- Holmes MV, Perel P, Shah T, Hingorani AD, Casas JP. CYP2C19 genotype, clopidogrel metabolism, platelet function, and cardiovascular events: A systematic review and meta-analysis. JAMA 2011;306:2704-14.
- Kreutz RP, Nystrom P, Kreutz Y, Miao J, Desta Z, Breall JA, et al. Influence of paraoxonase-1 Q192R and cytochrome P450 2C19 polymorphisms on clopidogrel response. Clin Pharmacol 2012;4:13-20.
- Mega JL, Hochholzer W, Frelinger AL 3rd, Kluk MJ, Angiolillo DJ, Kereiakes DJ, et al. Dosing clopidogrel based on CYP2C19 genotype and the effect on platelet reactivity in patients with stable cardiovascular disease. JAMA 2011;306:2221-8.
- Scott SA, Sangkuhl K, Gardner EE, Stein CM, Hulot JS, Johnson JA, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for cytochrome P450-2C19 (CYP2C19) genotype and clopidogrel therapy. Clin Pharmacol Ther 2011;90:328-32.
- Swen JJ, Nijenhuis M, de Boer A, Grandia L, Maitland-van der Zee AH, Mulder H, et al. Pharmacogenetics: From bench to byte - an update of guidelines. Clin Pharmacol Ther 2011;89:662-73.
- 14. Product Monograph, Plavix®. In: Sanofi-Aventis Canada Inc. 2012. Available from: http://www.bmscanada.ca/static/products/en/pm_pdf/ Plavix_EN_PM.pdf. [Last accessed on 2012 Dec 11].
- Karazniewicz-Lada M, Danielak D, Burchardt P, Kruszyna L, Komosa A, Lesiak M, et al. Clinical pharmacokinetics of clopidogrel and its metabolites in patients with cardiovascular diseases. Clin Pharmacokinet 2014;53:155-64.
- US Department of Health and Human Services, FDA. Guidance for Industry: Bioanalytical Method Validation. May 2001. Available from: http://www.fda.gov/downloads/Drugs/.../Guidances/ucm070107.pdf [Last accessed on 2013 May 18].
- Federal Commission for the Protection Against Sanitary Risks. Commission on Analytical Control and Extended Coverage. Criteria for the validation of physicochemical methods. Available from: http:// www.cofepris.gob.mx/TyS/Documents/TercerosAutorizados/ cvfq032011. pdf [Last accessed on 2013 May 18].
- Takahashi M, Pang H, Kawabata K, Farid NA, Kurihara A. Quantitative determination of clopidogrel active metabolite in human plasma by LCMS/ MS. J Pharm Biomed Anal 2008;48:1219-24.
- Gurbel PA, Shuldiner AR, Bliden KP, Ryan K, Pakyz RE, Tantry US. The relation between CYP2C19 genotype and phenotype in stented patients on maintenance dual antiplatelet therapy. Am Heart J 2011;161:598-604.
- Luo HR, Poland RE, Lin KM, Wan YJ. Genetic polymorphism of cytochrome P450 2C19 in Mexican Americans: A cross-ethnic comparative study. Clin Pharmacol Ther 2006;80:33-40.
- Salazar-Flores J, Torres-Reyes LA, Martínez-Cortés G, Rubi-Castellanos R, Sosa-Macías M, Muñoz-Valle JF, et al. Distribution of CYP2D6 and CYP2C19 polymorphisms associated with poor metabolizer phenotype in five Amerindian groups and western Mestizos from Mexico. Genet Test Mol Biomarkers 2012;16:1098-104.
- Norma Oficial Mexicana NOM-177-SSA1-1998, Que establece las pruebas y procedimientos para demostrar que un medicamento es intercambiable. Requisitos a que deben sujetarse los terceros autorizados que realicen las pruebas. Nov 1998. Available from: http:// www.sitio.farmacopea.org.mx/legisla/NOM-177PruebInter7may99.pdf. [Last accessed on 2014 Apr 23].
- Haidar SH, Davit B, Chen ML, Conner D, Lee L, Li QH, et al. Bioequivalence approaches for highly variable drugs and drug products. Pharm Res 2008;25:237-41.
- Conner DP. Bioequivalence Methods for Highly Variable Drugs and Drug Products. August 2009. Available from: http://www.fda. gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/ Drugs/Advisory Committee for Pharmaceutical Scienceand Clinical Pharmacology/UCM178927.pdf. [Last accessed on 2013 May 18].
- Tothfalusi L, Endrenyi L, Arieta AG. Evaluation of bioequivalence for highly variable drugs with scaled average bioequivalence. Clin Pharmacokinet 2009;48:725-43.
- European Medicines Agency. Guideline on the Investigation of Bioequivalence. Jan 2010. Available from: http://www.ema.europa. eu/docs/en_GB/document_library/Scientific_guideline/2010/01/ WC500070039.pdf [Last accessed on 2013 Jun 21].
- US Department of Health and Human Services, FDA. Draft Guidance on Progesterone. Feb 2011. Available from: http://www.fda.gov/ downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ UCM209294.pdf. [Last accessed on 2013 May 18].
- Tothfalusi L, Endrenyi L. Sample sizes for designing bioequivalence studies for highly variable drugs. J Pharm Pharm Sci 2012;15:73-84.
- Patterson SD, Jones B. Viewpoint: Observations on scaled average bioequivalence. Pharm Stat 2012;11:1-7.
- Karalis V, Symillides M, Macheras P. Bioequivalence of highly variable drugs: A comparison of the newly proposed regulatory approaches by FDA and EMA. Pharm Res 2012;29:1066-77.
- DiLiberti CE. Why Bioequivalence of Highly Variable Drugs is an Issue. Presentation to the Advisory Committee for Pharmaceutical Sciences. April 14, 2004. Available from: http://www.fda.gov/ohrms/dockets/ac/04/ slides/4034S2_02_DiLiberti.ppt. [Last accessed on 2012 Dec 12].
- Rani S. Bioequivalence: Issues and perspectives. Indian J Pharmacol 2007;39:218-25.
- Peiró AM, Novalbos J, Zapater P, Moreu R, López-Rodríguez R, Rodríguez V, et al. Pharmacogenetic relevance of the CYP2C9*3 allele in a tenoxicam bioequivalence study performed on Spaniards. Pharmacol Res 2009;59:62-8.
- Chung JY, Jung Lee Y, Bok Jang S, Ahyoung Lim L, Soo Park M, Hwan Kim K. CYP3A5*3 genotype associated with intrasubject pharmacokinetic variation toward tacrolimus in bioequivalence study. Ther Drug Monit 2010;32:67-72.