- *Corresponding Author:
- S. B. Chavan
Research and Development and Bhasma Section, Atharva Nature Healthcare Pvt. Ltd., Pune, Maharashtra 412207, India
E-mail: ictrcdrugresearch2016@gmail.com
| Date of Received | 13 July 2021 |
| Date of Revision | 27 July 2023 |
| Date of Acceptance | 02 January 2024 |
| Indian J Pharm Sci 2024;86(1):170-186 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Lauha Bhasma being incinerated metals, minerals and animal products attributed with specific therapeutic value needs standardization. Present study validates the pharmaceutical process of Lauha Bhasma/incinerated iron and characterizes it using traditional as well as modern techniques. Lauha Bhasma was prepared by traditional method. Pure iron turnings were sequentially quenched, 7 times each, into liquid media (1:4 w/v, for each) viz., sesame oil, cow’s butter milk, cow’s urine, sour gruel, Dolichus biflorus seed decoction and decoction of equally mixed pericarp of three myrobalans (Triphala). It was further mixed with sulphur, triturated with Aloe barbadensis leaf pulp and incinerated at 650°-850°, till the achievement of desired characters. The obtained reddish-violet powder contained 60 %-65 % of iron while characterized as crystalline ferric oxide, alpha haematite, on X-ray diffraction simulation. Vibrating sample magnetometer revealed hysteresis with less magnetisation saturation but high coercivity. Thermo-gravimetric analysis showed loss of ~3.5 % w/w between 300°-900°. Both, scanning and transmission electron microscopy visualised variably sized agglomerates of spherical to irregularly shaped particles with smooth edges. Dynamic light scattering analysis illustrated distribution of agglomerates as <250 nm (10 %), <515 nm (50 %) and <1050 nm (90 %) while negative zeta potential upto 14 mV. Elemental assay depicted heavy metals within permissible range and presence of trace elements up-to 2.75 %. Such Lauha Bhasma, characterized as alpha haematite, is traditionally prescribed in blood, liver, intestine and eye disorders. This study will help to explore this micro-nanomedicine as a therapeutic agent using in vitro and in vivo mechanistic studies.
Keywords
Biomaterials, biomedicine, characterization, hematite, incinerated iron, Lauha Bhasma, standardization, quenching
Lauha Bhasma (LB) incinerated iron is one of the important ingredients in various Ayurvedic formulations like Arogyavardhini Gutika, Navayas Lauha, Tapyadi Lauha and Trailokyachintamani rasa[1]. According to Rasashastra, the pharmaceutical branch of Ayurveda, Lauha is included in the metal group (dhatu varga) and more precisely as pure metal (shuddha lauha). Further, in Rasashastra, three broad types of iron (Lauha) are mentioned viz., Magnetite (kanta lauha), Wrought iron (teekshna lauha) and Pig iron (munda lauha). Though kanta lauha is mentioned as the best type, the latter two are also indicated for medicinal use[2].
Classically, LB is attributed with bitter, sweet and astringent tastes; cold potency and eye tonic (chakshushya), scraping (lekhana), anti-aging (vayasya) and Kapha-Pitta pacifying properties. It is mainly recommended in inflammation (shotha), haemorrhoids (arsha), splenomegaly (pleeharoga), anaemia (pandu), obesity (medoroga), diabetes (meha), worms (krumi) and skin disorders (kushtha) with a therapeutic daily dose of 125-250 mg[3]. Recent trials in animals and humans with iron deficiency anaemia and obesity have shown significant effect of LB as compared to standard drug ferrous sulphate[4]. However, if iron is not properly purified and incinerated as mentioned in classical texts, such LB can cause life threatening conditions like anaemia (pandu), skin disorders (kushtha), cardiac disorders (hrudroga), stomach ache (shoola), renal calculus (ashmari) and may also lead to death[2].
In textual methods, all metals before their medicinal use are physically and chemically treated by a general purification process in a defined set of media (samanya shodhana)[5]. Further, iron is specifically purified by quenching it into the juice of Musa paradisiaca rhizome/quenching in decoction of three myrobalans (triphala) or cow’s urine or sprinkling decoction of three myrobalans on red hot iron (vishesh shodhana)[2]. Further, LB preparation method i.e., incineration (Marana) is mentioned with various triturating media viz., three myrobalans/Boerrhavia diffusa leaf juice/ Aloe barbadensis juice/Calotropis procera latex/ Phyllanthus emblica fruit juice/Adhatoda vasica leaf juice, cow’s urine, rice gruel and lemon juice with or without cinnabar/metacinnabar and sulphur[2]. However, pharmaceutical standardization of a single preparatory method with comprehensive characterization is unavailable, even in Ayurvedic Formulary and Ayurvedic Pharmacopoeia of India (API).
Recent reports on pharmaceutical standardization of LB have focussed on manufacturing processes viz., quenching/purification followed by heating procedures viz., indirect heating under sun (bhanupaka), heating in a pan (sthalipaka) and heating with dried cow-dung cakes or muffle furnace (putapaka). So far, LB has been prepared either using decoction of three myrobalans or four different methods using combinations viz., iron, sulphur and Aloe vera; iron, cow’s ghee and decoction of three myrobalans; iron triturated with pomegranate leaf juice; and iron, black sulphide of mercury and Aloe vera[6-8].
In the present study, LB was prepared at industrial scale by upgrading the traditional method[2]. Pure iron turnings were sequentially quenched, 7 times each, into liquid media viz., sesame oil, cow’s buttermilk, cow’s urine, rice gruel, Dolichus biflorus seed decoction and decoction of three myrobalans. It was further mixed with processed sulphur, triturated with fresh Aloe barbadensis leaf pulp and incinerated, repeating the trituration and incineration cycles till the desired characters were achieved. The final product, LB, obtained through this standardized pharmaceutical process was characterized to develop a monograph. Advanced analytical techniques like scanning and transmission electron microscopy, X-Ray Diffraction (XRD), vibratory sample magnetometry and dynamic light scattering were used to study the physicochemical properties. Since, LB is widely used in Ayurvedic medicines, a comprehensive study conducted on industrial scale batches will help in quality control and quality assurance.
Materials and Methods
Collection of raw material:
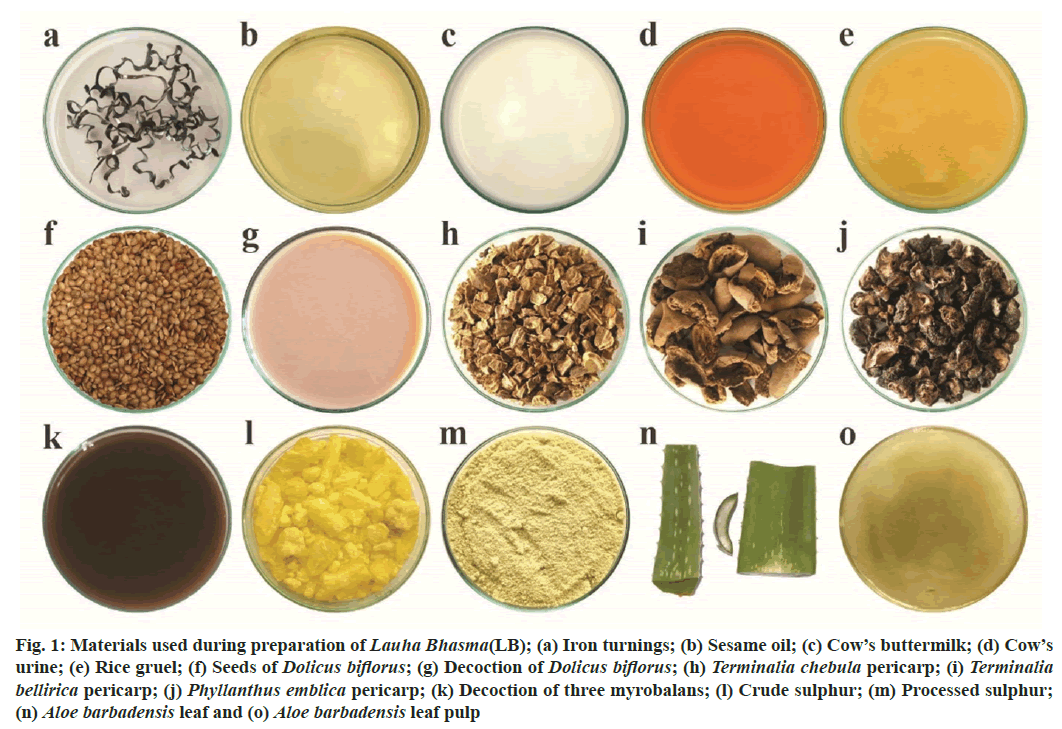
Iron turnings (Grade SAE1029) were purchased from Yukta Engineers, Pune, India while sulphur was obtained from S. V. Ayurvedic Bhandar, Navi Mumbai, India. Cow’s ghee and sesame oil were purchased from B. V. Chitale Group, Pune and Pitambari Products, Thane, India respectively. Rice, turmeric powder and Dolicus biflorus Syn. Vigna unquiculate (Linn.) Walp. seeds were procured from local market while three myrobalans/Triphala i.e., pericarp of Terminalia chebula Retz./ Haritaki (TC), Terminalia bellirica/ Bibhitaki Willd. (TB) and Phyllanthus emblica Linn. Syn. Emblica officinalis Gaertn. (PE) from Manakarnika Aushadhalay, Pune. Cow’s milk, cow’s buttermilk, cow’s urine and Aloe barbadensis mill. Syn. Aloe vera was obtained from Bharatiya Sanskriti Darshan Trust, Pune. Crude herbs were identified and authenticated at Indian Drug Research and Laboratory, Pune.
Preparation and storage of media:
All the processes were carried out in Atharva Nature Healthcare Pvt. Ltd., Pune; in triplicate. Buttermilk was prepared by adding 28 l of water in 14 kg of cow’s curd, churned it to de-fat and strained to get 28.5 l. Sour gruel (28 l) was prepared by boiling 3 kg rice in 42 l of water and reduced to 30 l of starchy water. This water was self-cooled; turmeric (150 g), rock salt (75 g) and sesame oil (300 ml) were added and allowed to ferment in a tightly closed porcelain jar for 15 d to get rice gruel. Decoctions of crushed Dolicus biflorus seeds and coarse powder of Triphala, were prepared separately from 7 kg of material, each, by adding 112 l of water and boiled to get 28.5 l of decoction, each[2]. Crude sulphur was purified (shodhana)[3] wherein sulphur (total 10 kg, 250 g aliquot) admixed with cow’s ghee (total 2.5 kg, 62.5 g aliquot) were melt in iron pan and poured through a muslin cloth into cow’s milk (total 40 l, 1 l aliquot) kept in a stainless-steel vessel. The solidified lump was cooled, crushed, washed with hot water, dried and powdered to get purified sulphur. Fresh leaves of Aloe barbadensis were cut, the pulp scrapped and ectoderm was discarded. The pulp was grinded and strained through #22 (710 µm).
Purification (Shodhana):
In common purification process[2], iron turnings (1 kg) were heated for 5-10 min till they turned red hot (900°-1000°) and then dipped into sesame oil kept in a stainless-steel container (304 Grade, 6 l capacity; 19 cm×21 cm). The process of heating and quenching was repeated for 6 more times in sesame oil. Then, these turnings were further heated and quenched for 7 times in each of the media viz., cow’s buttermilk, cow’s urine, rice gruel and Dolicus biflorus decoction, sequentially. Further, iron turnings obtained after quenching from the last cycle of Dolicus biflorus decoction, were heated and quenched 7 times in the Triphala decoction. For each quenching, 4 l of fresh media was used. Organoleptic and weight change of iron turnings were recorded while temperature and physicochemical change in the medium before and after each quenching.
Incineration of iron (Marana):
The process of incineration was carried out after initial grinding and trituration, sequentially[2] with some modifications like mechanized grinding and trituration techniques. For the first incineration cycle, equal quantity of purified iron turnings (~800 g) and purified sulphur (~800 g) were mixed thoroughly in a stone mortar (8 l capacity, ovoid, maximum inner dimensions as 13 cm×24 cm×51 cm). For 2nd and 3rd incineration cycles, 200 g of purified sulphur was added to the mixture obtained from previous incineration. The mixture was grinded each time for 3 h using a stone pestle (weight-2 kg, length-25.5 cm, basal circumference-22 cm and diameter-6 cm). The trituration (bhavana) of grinded iron and sulphur mixture with 200-400 ml of Aloe barbadensis juice was carried out in the stone mortar manually, for the first four incineration cycles. Further, an electrically operated wet grinder (MK-GW200, Panasonic India Pvt. Ltd., Chennai, India) was used for both, grinding and trituration and 3 h duration was set in each case to maintain uniformity.
Coin like round, flat pellets (thickness-5 mm, diameter-3 cm) were manually casted out of the triturated material in earthen vessels (diameter-27 cm, inner height-4 cm, thickness-4-5 mm, capacity-1.2 l) and dried in shade at room temperature (18 h). The dried pellets were covered with another earthen vessel and their joints were sealed with wet clay smeared cotton cloth (width-8 cm, length-1 m) and kept in shade for drying. During every cycle, the triturated material was pelletized equally in 2 or 4 vessels. The assemblage of concealed earthen vessels was subjected to incineration as reported previously with slight modification[9]. Here, four patterns as 15:7.5, 18:9, 20:10 and 24:12 kg of cow dungs (below: above) were used. The product after each incineration was grinded to powder and sieved through #40, #150 and #200 mesh, sequentially. The incineration procedure was repeated till the desired form was achieved.
Organoleptic and physicochemical evaluation of raw, in-process and finished products:
Raw material, processing media, intermediates and finished products were subjected to organoleptic evaluation. Liquid media were evaluated for pH and specific gravity. Sesame oil was additionally assessed for refractive index while Aloe barbadensis juice for viscosity. Processed sulphur was analysed for loss on drying, bulk/tapped density, sieve analysis, oil and sulphur content. LB was evaluated for Fe content, Loss on Drying (LOD), loss on ignition, acid insoluble ash, pH, bulk/tapped densities and sieve analysis[10,11].
Physical characterisation of LB:
Scanning Electron Microscopy (SEM) on Leica Cambridge 440 (Leica Cambridge Ltd., Cambridge, UK) and Transmission Electron Microscopy (TEM) on TECNAI 20ST with 200 kV, LaB6 source (FEI Company, Oregon, USA) were carried out to obtain the size and morphology of the LB particles[9]. For TEM, sample was dispersed in acetone by ultra-sonication, dropped onto a carbon coated copper grid (#200) and dried in air. The images were captured at magnification scales between 500-20 nm and micrometric measurements were recorded.
The particle size by Dynamic Light Scattering (DLS) technique and Zeta Potential (ZP) were measured on 90 Plus instrument (Brookhaven Instruments Corp., Holtsville NY, USA) equipped with 35 mW solid state laser and a highly sensitive avalanche photo diode detector[9]. ZP was measured by taking sample in a square disposable cuvette and an electrode designated for aqueous suspensions was used for the electric field mobility measurements. Ten technical replicates were performed for each. Magnetic measurements were made on EG&G PAR 4500 Vibrating Sample Magnetometer (VSM-Princeton Applied Research, TN, USA) Magnetization as a function of applied magnetic field in the range ±15 kOe was measured.
Chemical characterisation of LB:
Raw iron turnings and LB were analysed on PANalytical X’PERT Pro X-ray diffractometer (Lelyweg, Almelo, Germany) using Cu-Kα radiation (λ=1.5418 Å) for XRD and Thermogravimetric Analysis (TGA)-Q5000 from TA Instruments Inc. (New Castle, DE, USA) as reported earlier[9]. Elemental assay by Inductively Coupled Plasma- Optical Emission Spectrometer (ICP-OES) was performed using an established methodology[9]. Samples weighing 100 mg were digested on Ethos Easy Advanced Microwave Digestion System (Milestone SRL, Milan, Italy) and diluted samples were aspirated on ICP-OES (Thermo Fisher Scientific, Waltham, MA, USA). The intensities for K, Li, Na, Ba, Ga, Sr, Ca, Ag, In, Cu, Al, Cr, Mg, Tl, Fe, Mn, B, Co, Cd, Bi, Ni, Pb, Zn, As, Hg and S were measured at wavelengths 766.490, 670.784, 588.995, 455.403, 417.206, 407.771, 393.366, 328.068, 325.609, 324.754, 309.271, 283.563, 279.553, 276.787, 259.940, 257.610, 249.773, 228.616, 226.502, 223.061, 221.647, 216.999, 213.856, 189.042, 184.950, 180.731 nm respectively. Fe and S were measured in radial view while others in the axial view.
Results and Discussion
All the raw materials used for LB preparation are displayed in fig. 1. Raw iron was identified as bluish black metallic shavings of varying length (10-15 mm), having magnetic property and soluble in HCl. It contained 99.6 % w/w of Fe as measured by titration. The results of other raw materials are detailed in Table 1.
| Raw Material | Results |
|---|---|
| Sesame oil (n=8) | Refractive index 1.54±0.002, specific gravity 0.92±0.002, iodine value 80.97±10.79, acid value 3.14±0.75, saponification value 166.76±12.35; unsaponifiable matter 4.58±1.36 % and moisture content 0.17±0.06 % w/w |
| Butter milk (n=8) | Specific gravity 1.03±0.02, pH of 3.44±0.02 |
| Cow’s urine (n=3), | Specific gravity 1.03±0.002, pH 8.78±0.79 |
| Rice gruel (n=4) | Specific gravity 0.98±0.001, pH 3.26±0.07 |
| Decoction of Dolicus biflorus (n=3) | Specific gravity 1.05±0.002, pH 4.79±0.32 |
| Terminalia chebula | LOD 2.6, total ash 4.7, acid insoluble ash (AIA) 0.17, alcohol extractive value (AEV) 46.5, water extractive value (WEV) 57.6 (all % w/w); Thin Layer Chromatography (TLC) of ethanolic extract on mobile phase toluene: ethyl acetate:formic acid (5:4:1) showed Rf 0.08, 0.31, 0.43, 0.49, 0.91 (all grey) at UV 254 nm; 0.03 (yellow), 0.84, 0.91 (both greenish blue) at UV 365 nm and 0.09, 0.36 (both grey) on spraying 1 % Ferric chloride (FeCl3) |
| Terminalia bellirica | LOD 5.3, total ash 4.6, AIA 0.4, AEV 27.4, WSE 43.0 (all %w/w); TLC of ethanol extract using mobile phase chloroform:ethyl acetate:formic acid (5:4:1) showed Rf- 0.06, 0.20, 0.39,0.73 (all black) at UV 254 nm; 0.06 (grey), 0.20, 0.39, 0.73 (all black) at UV 365 nm and 0.03, 0.39 (both black) after spraying with 1 % FeCl3 |
| Phyllanthus emblica | LOD 6.9, total ash 3.1, AIA 0.7, AEV 30.2 and WEV 39.3 (all % w/w); TLC of ethanol extract on solvent system acetone:chloroform:formic acid:toluene (2:2.5:0.5:3) showed Rf 0.05, 0.36, 0.60, 0.69, 0.90 (all grey) at UV 254 nm; 0.05 (grey), 0.60, 0.90 (both blue) at UV 365 nm and 0.13, 0.20, 0.30 (all black) after spraying with 1 % FeCl3 |
| Decoction of three myrobalans (n=3) | Specific gravity 1.08±0.002, pH 2.85±0.01 |
| Purified Sulphur (n=3) | Light yellow coloured, oily powder, with characteristic odour, soluble in carbon disulphide; LOD 0.29±0.03 %, bulk density 0.70±0.01 g/cc, tapped density 0.88±0.05 g/cc, oil content 8.97±0.8 % w/w, sulphur content 90.52±0.7 % w/w, retention of 93.35±1.41 % on passing through #100 sieve |
| Fresh Aloe barbadensis (AB) leaves (n=4) | LOD 96.62±0.72, total ash 0.53±0.1, AIA 0.16±0.05, AEV 1.85±0.14, WEV 1.51±0.07 (all % w/w); TLC of ethanol extract using solvent system toluene:ethyl acetate:formic acid (5:4:1), displayed Rf value of 0.05, 0.33, 0.81 (all blue) at 254nm; 0.33 (red) at 365nm and 0.33 (blue) after spraying with anisaldehyde H2SO4 reagent |
| Fresh AB pulp (n=3) | Specific gravity 1.002±0.001, pH 4.92±0.32, viscosity 3.31±0.41 mP |
Table 1: Basic Analysis of Raw Materials Used in Preparation of Incinerated Iron/ Lauha Bhasma (LB)
Fig. 1: Materials used during preparation of Lauha Bhasma(LB); (a) Iron turnings; (b) Sesame oil; (c) Cow’s buttermilk; (d) Cow’s urine; (e) Rice gruel; (f) Seeds of Dolicus biflorus; (g) Decoction of Dolicus biflorus; (h) Terminalia chebula pericarp; (i) Terminalia bellirica pericarp; (j) Phyllanthus emblica pericarp; (k) Decoction of three myrobalans; (l) Crude sulphur; (m) Processed sulphur; (n) Aloe barbadensis leaf and (o) Aloe barbadensis leaf pulp
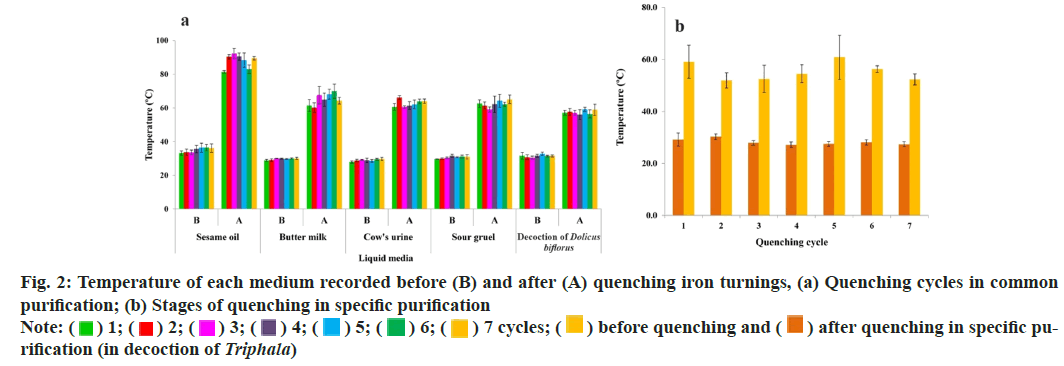
The temperature records of media during quenching are displayed in Table 2 while change is illustrated in fig. 2. The changes in iron turnings after each purification and incineration cycle are displayed in fig. 3. The weight change during both the purification processes and incineration cycles is depicted in fig. 4a. The average weight loss during common and specific purification was 54±1.73 (~5.4 %) and 51.33±15.5 (~5.13 %) g, respectively while total yield after complete incineration was 94.07±0.91 g (93 %-95 %) with a loss of 5.93±0.91 g (5 %-7 %). A weight gain between 4 %-11 % was noted after I1 while a weight loss between 0.8 %-3.5 % was recorded during successive incinerations. During Incineration (I), sulphur was used in equal amount of (814±19.64 g) for I1 and 200 g for next 3 successive cycles. Similarly, 366.67±70.71 of AB juice was required for trituration. For cycles 1 to 5 (I1-I5) and 6 onwards (I6), 2 and 4 vessels, respectively, were required to accommodate the dried pellets of triturated material.
| Quenching cycle | SesameOil | Cow’s buttermilk | Cow’s urine | Sour gruel | Decoction of Dolicus biflorus | Decoction of three myrobalans | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | A | B | A | B | A | B | A | B | A | B | A | |
| 1 | 36 | 83 | 28 | 55 | 29 | 64 | 30 | 59 | 27.7 | 56 | 33 | 48.8 |
| 32 | 80 | 29 | 68 | 28 | 61 | 29 | 62 | 33.7 | 55 | 30 | 70.8 | |
| 32 | 81 | 30 | 61 | 27 | 57 | 29 | 67 | 33.4 | 60 | 25 | 57.7 | |
| Mean | 33 | 81 | 29 | 62 | 28 | 61 | 30 | 63 | 31.6 | 57 | 29 | 59.1 |
| SD | 2.2 | 1.3 | 1.2 | 6.2 | 1.2 | 3.5 | 0.4 | 4 | 3.38 | 2.4 | 4.3 | 11.1 |
| 2 | 37 | 89 | 27 | 57 | 30 | 65 | 30 | 65 | 28.2 | 61 | 32 | 47.9 |
| 33 | 89 | 30 | 66 | 29 | 64 | 29 | 58 | 33.1 | 54 | 29 | 57.7 | |
| 31 | 93 | 30 | 57 | 27 | 68 | 31 | 62 | 30.9 | 57 | 30 | 50.3 | |
| Mean | 34 | 90 | 29 | 60 | 29 | 66 | 30 | 61 | 30.7 | 58 | 30 | 51.9 |
| SD | 3.1 | 2.3 | 1.5 | 4.9 | 1.5 | 2.1 | 0.8 | 3.6 | 2.45 | 3.5 | 1.9 | 5.11 |
| 3 | 36 | 92 | 30 | 57 | 30 | 59 | 31 | 57 | 29.7 | 57 | 27 | 44.2 |
| 33 | 87 | 30 | 74 | 30 | 62 | 30 | 58 | 32.3 | 54 | 30 | 62.2 | |
| 31 | 98 | 30 | 72 | 29 | 60 | 31 | 62 | 29.5 | 59 | 27 | 51.1 | |
| Mean | 34 | 92 | 30 | 68 | 29 | 61 | 31 | 59 | 30.5 | 57 | 28 | 52.5 |
| SD | 2.4 | 5.2 | 0.2 | 9.1 | 0.6 | 1.4 | 0.9 | 2.7 | 1.56 | 2.6 | 1.6 | 9.08 |
| 4 | 40 | 89 | 29 | 59 | 32 | 57 | 33 | 53 | 33.2 | 56 | 26 | 55.2 |
| 34 | 88 | 30 | 72 | 28 | 63 | 30 | 65 | 31.2 | 51 | 29 | 60.1 | |
| 33 | 95 | 30 | 63 | 27 | 65 | 31 | 68 | 30.4 | 61 | 26 | 48.2 | |
| Mean | 36 | 91 | 30 | 65 | 29 | 61 | 32 | 62 | 31.6 | 56 | 27 | 54.5 |
| SD | 3.8 | 3.7 | 0.5 | 6.8 | 2.4 | 4 | 1.7 | 8.3 | 1.44 | 5 | 1.9 | 5.98 |
| 5 | 42 | 89 | 29 | 65 | 30 | 67 | 31 | 57 | 34.1 | 60 | 28 | 48.2 |
| 34 | 81 | 30 | 74 | 28 | 59 | 30 | 69 | 33.4 | 57 | 29 | 76.9 | |
| 34 | 95 | 30 | 65 | 28 | 60 | 31 | 67 | 30.7 | 61 | 26 | 57.4 | |
| Mean | 36 | 88 | 30 | 68 | 29 | 62 | 31 | 64 | 32.7 | 59 | 28 | 60.8 |
| SD | 4.6 | 7.5 | 0.6 | 5.1 | 1.5 | 4.6 | 0.5 | 6.7 | 1.8 | 2.3 | 1.6 | 14.7 |
| 6 | 40 | 88 | 30 | 62 | 31 | 65 | 32 | 60 | 31.2 | 52 | 28 | 56.3 |
| 34 | 80 | 31 | 71 | 29 | 61 | 29 | 63 | 32.4 | 59 | 30 | 58.5 | |
| 36 | 81 | 30 | 77 | 29 | 65 | 32 | 64 | 30.8 | 58 | 27 | 54.1 | |
| Mean | 37 | 83 | 30 | 70 | 30 | 64 | 31 | 62 | 31.5 | 56 | 28 | 56.3 |
| SD | 3.3 | 4.3 | 0.6 | 7.3 | 1.1 | 2.4 | 1.5 | 2.2 | 0.83 | 4.1 | 1.8 | 2.2 |
| 7 | 41 | 88 | 29 | 61 | 31 | 66 | 33 | 61 | 31.5 | 52 | 27 | 48.7 |
| 34 | 90 | 31 | 68 | 28 | 62 | 29 | 64 | 32.5 | 61 | 29 | 52.3 | |
| 34 | 91 | 30 | 64 | 30 | 64 | 32 | 70 | 30.5 | 63 | 26 | 56 | |
| Mean | 36 | 90 | 30 | 64 | 30 | 64 | 31 | 65 | 31.5 | 59 | 27 | 52.3 |
| SD | 4.3 | 1.7 | 1.2 | 3.4 | 1.7 | 2.3 | 2.2 | 4.5 | 1 | 5.7 | 1.7 | 3.65 |
Table 2: Temperature Recorded During Purification (Quenching) of Iron
Fig. 3: Changes in iron turnings during LB (Batch 1) preparation, (a-f) Iron turnings after 7th quenching in sesame oil, cow’s buttermilk, cow’s urine, rice gruel, decoction of Dolicus biflorus and decoction of Triphala, respectively; (g-o) Conversion of purified iron turnings into powder form after each incineration i.e. from I1-I9 respectively
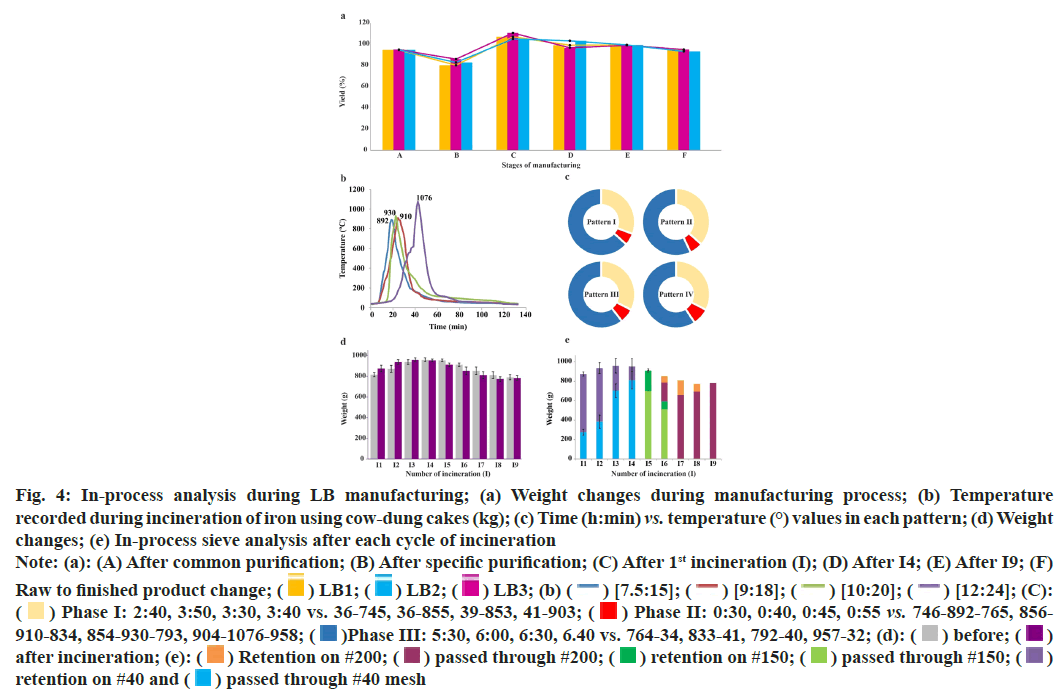
Fig. 4: In-process analysis during LB manufacturing; (a) Weight changes during manufacturing process; (b) Temperature
recorded during incineration of iron using cow-dung cakes (kg); (c) Time (h:min) vs. temperature (°) values in each pattern; (d) Weight
changes; (e) In-process sieve analysis after each cycle of incineration
Note: (a): (A) After common purification; (B) After specific purification; (C) After 1st incineration (I); (D) After I4; (E) After I9; (F) Raw to finished product change;  [12:24]; (C):
[12:24]; (C):  Phase I: 2:40, 3:50, 3:30, 3:40 vs. 36-745, 36-855, 39-853, 41-903;
Phase I: 2:40, 3:50, 3:30, 3:40 vs. 36-745, 36-855, 39-853, 41-903;  Phase II: 0:30, 0:40, 0:45, 0:55 vs. 746-892-765, 856-910-834, 854-930-793, 904-1076-958;
Phase II: 0:30, 0:40, 0:45, 0:55 vs. 746-892-765, 856-910-834, 854-930-793, 904-1076-958;  Phase III: 5:30, 6:00, 6:30, 6.40 vs. 764-34, 833-41, 792-40, 957-32; (d):
Phase III: 5:30, 6:00, 6:30, 6.40 vs. 764-34, 833-41, 792-40, 957-32; (d):  after incineration; (e):
after incineration; (e):  passed through #150;
passed through #150;  retention on #40 and
retention on #40 and  passed through #40 mesh
passed through #40 mesh
Incineration of iron was carried out in four heating ranges (low to high), based on the quantity of cow-dung cakes and the amount of sulphur. The graphical presentation of the temperature patterns recorded is given in fig. 4b (representative batch, LB1) while the duration and the range of temperature observed in all the patterns (of LB1) in fig. 4c. Maximum temperature reached during Pattern-1 (used in I2) was 892° while 910° reached in Pattern-2 (used for I1 and I3). Maximum temperature recorded during Pattern-3 (for I4 and I5) was 930° while in Pattern-4 it raised up to 1076° (used from I6 to I9). Hence, for all the 4 patterns, the maximum temperature during incineration ranged between 892°-1076°. Each pattern used in this traditional method of incineration of iron can be divided into three phases with respect to the highest temperature (Tmax). Phase I as the duration required to attain temperature equivalent to (Tmax- 150°) from the point of ignition, Phase II as the duration for which the highest temperature range (from ascending and descending Tmax-150°) was sustained while Phase III as self-cooling duration. Tmax is the temperature attained outside the vessel and Tmax-150° is the assumed temperature reached inside the vessels for the chemical changes. Therefore, the maximum temperature for each pattern at which the conversion might occur in vessels can be deduced as 742, 780, 760 and 926° inside the vessels i.e., Tmax (892, 910, 930 and 1076)–150°, respectively.
The weight variation before and after incineration during each incineration cycle is illustrated in fig. 4d. During each cycle of incineration (I1- I9), in-process material was sifted through #40, #150 and #200 sieves. It was observed that 811.7±90.9 g and 142.3±78.3 g of material passed and retained, respectively, through #40 sieves, till I4; while 509.3±255.8 g and 83.3±52.4 g passed and retained, respectively, through #150 sieves, after I5. Similarly, 196.3 g and 63.7 g passed and retained, respectively, through #200 sieve after I6. Finally, 782.5±18.37 g of LB passed through sieve #200 after I9, with negligible residue (fig. 4e).
After common purification, bluish tinge faded and black to dark grey colour appeared on iron turnings. Further, after specific purification, reddish brown tinge was noticed, sharp edges became blunt as well as brittleness increased.
Classical characteristics of LB were evaluated as lustreless (nishchandra), soft in touch (shlakshnata, mruduta), entered into the crevices of finger tips (rekhapurna, sukshmata); tasteless (swadahina); reddish brown (colour like pakva jambuphala-i.e ripen fruit of Syzygium cumini) in colour and floats on stagnant water surface (varitara).
Incinerated iron was a fine powder, insoluble in water but soluble in dilute HCl. It showed 0.08±0.05 % w/w LOD, loss on ignition of 0.33±0.2 % w/w, acid insoluble ash as 5.77±2.4 and pH value as 5.87±0.7 in 1 % distilled water. The bulk and tapped densities were 1.36±0.29 g/ cc and 1.68±0.06 g/cc respectively. The Fe content was found to be 64.86±2.29 % w/w and sulphur (as SO4) was absent as assessed by gravimetric method[10]. It passed 100 % through mesh size of #100 (150 µm) while showed retention of 2.7±0.6 % and 98.1±0.4 % through mesh #200 (75 µm) and #300 (53 µm), respectively.
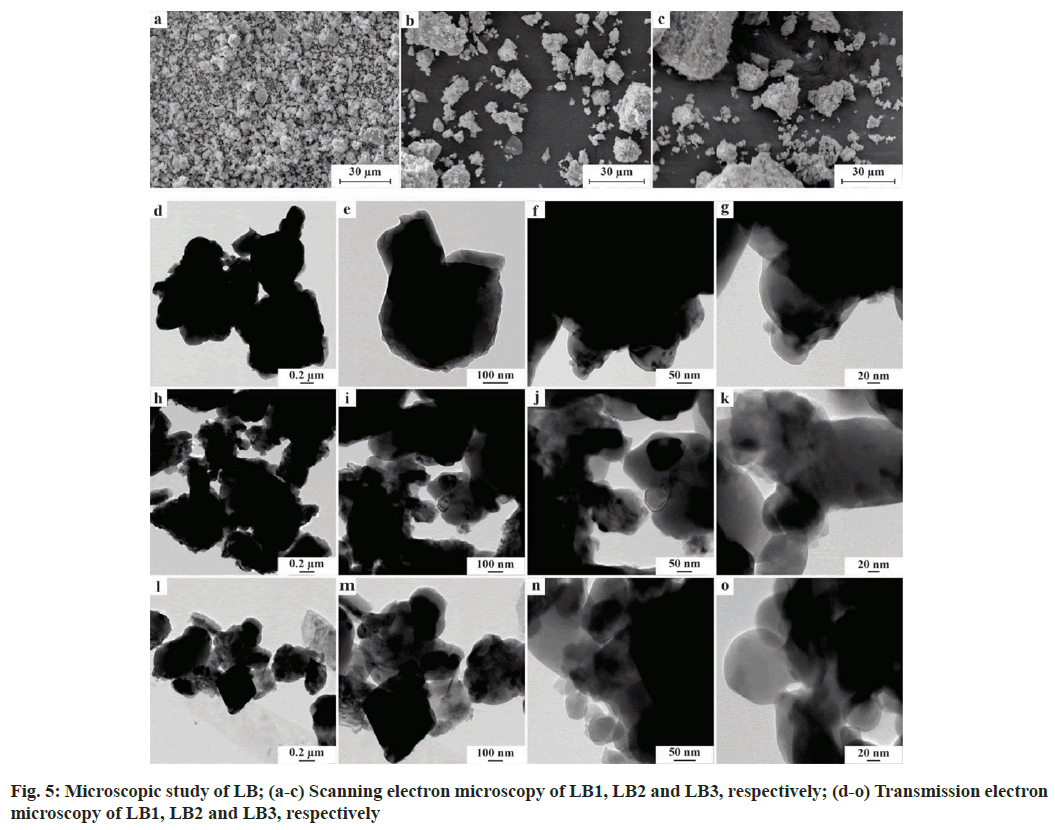
LB examined under SEM showed distinct morphology and uniform pattern at all the magnifications (representative images of 1000X magnification in fig. 5a-fig. 5c). The LB particles were irregularly shaped, in cluster form wherein smaller particles were seen adhered to the larger ones. The approximate size of the agglomerates ranged from 2-5 μm where smaller particles were less than 2 μm. TEM depicted irregularly shaped smooth-edged agglomerates of LB (comparative images illustrated in fig. 5d-fig. 5o). The smaller particles were seen to be spherically shaped and adhered to the bigger particles. The size of the agglomerates was up-to 1 μm but the smaller particles were in nano-range, smallest up-to 20 nm.
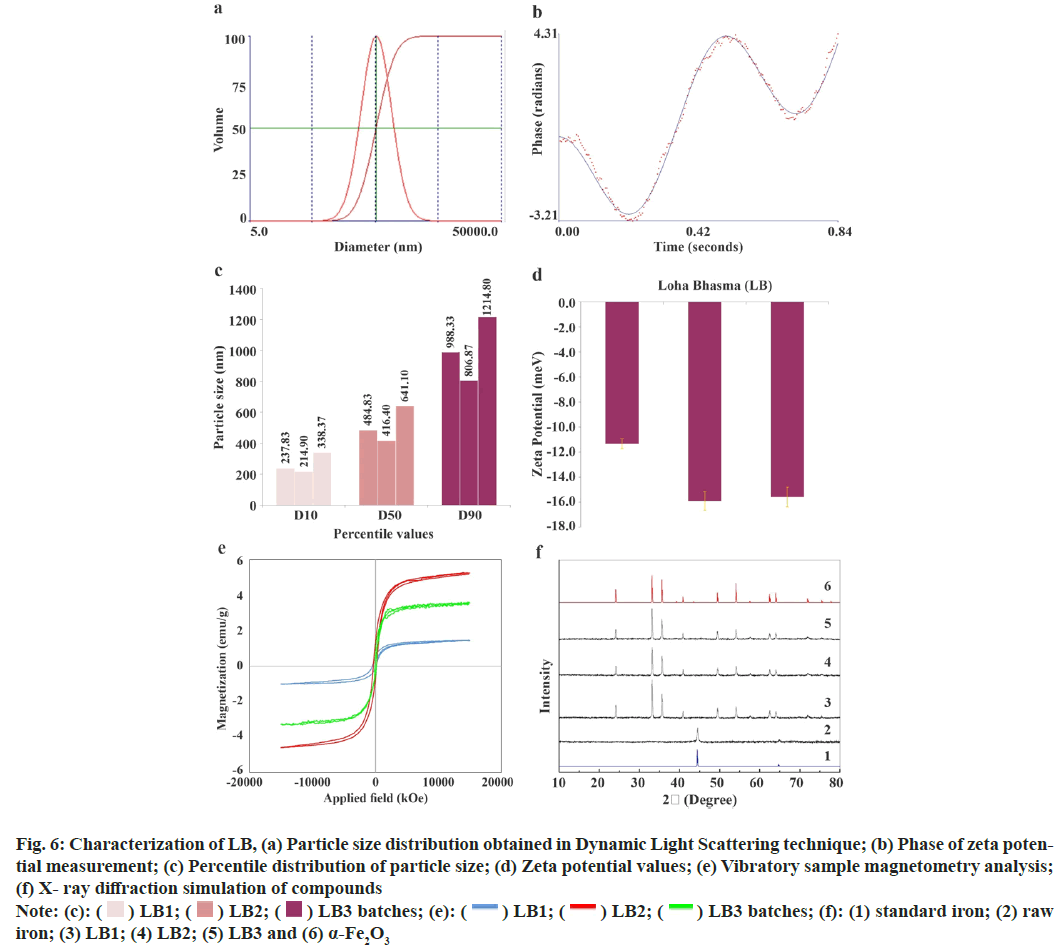
The measurements of DLS and ZP of LB are enumerated in Table 3 and Table 4, respectively, while representative graphs are illustrated in fig. 6a and fig. 6b, respectively. The finished products showed effective diameter ranging 800-1200 nm while polydispersity and span from 0.30-0.38 and 1.35-1.55, respectively. The particle size distribution of LB showed that 10 % particles were below 260 nm (<0.26 μm), 50 % of particles below 500 nm (<0.5 μm) and 90 % portion of particles were below 1000 nm (<1.0 μm). LB 1, 2 and 3 showed ZP of -11.32, -15.9 and -15.59 mV, respectively, with an average of -14.27±2.56 mV. Summary of DLS and ZP readings are depicted in fig. 6c and fig. 6d.
| Batch | Run | ED (nm) | PD | Span | D10 (nm) | D50 (nm) | D90 (nm) |
|---|---|---|---|---|---|---|---|
| LB1 | A | 1110.2 | 0.39 | 1.54 | 255.4 | 518.3 | 1051.8 |
| B | 1054.4 | 0.38 | 1.55 | 237.7 | 485.4 | 991.4 | |
| C | 981.8 | 0.39 | 1.56 | 220.4 | 450.8 | 921.8 | |
| Mean | 1048.8 | 0.38 | 1.55 | 237.83 | 484.83 | 988.33 | |
| SD | 64.38 | 0 | 0.01 | 17.5 | 33.75 | 65.05 | |
| LB2 | A | 857.6 | 0.33 | 1.43 | 225.7 | 438.2 | 850.8 |
| B | 846.23 | 0.32 | 1.41 | 230 | 443.2 | 853.9 | |
| C | 741.43 | 0.33 | 1.43 | 189 | 367.8 | 715.9 | |
| Mean | 815.09 | 0.32 | 1.42 | 214.9 | 416.4 | 806.87 | |
| SD | 64.04 | 0.01 | 0.01 | 22.53 | 42.16 | 78.79 | |
| LB3 | A | 1364.33 | 0.32 | 1.39 | 379.5 | 724.7 | 1384 |
| B | 1208.37 | 0.3 | 1.35 | 347.7 | 655.5 | 1235.8 | |
| C | 1002.73 | 0.29 | 1.36 | 287.9 | 543.1 | 1024.6 | |
| Mean | 1191.81 | 0.3 | 1.37 | 338.37 | 641.1 | 1214.8 | |
| SD | 181.37 | 0.02 | 0.02 | 46.51 | 91.65 | 180.62 | |
| LB (Mean±SD) | 1018.57±190.2 | 0.34±0.04 | 1.45±0.1 | 263.70±65.7 | 514.11±115.2 | 1003.33±204.4 | |
Note: ED: Effective Diameter; PD: Polydispersity and D10-D50-D90: Percentile values
Table 3: Dynamic Light Scattering Analysis of LB
| Run | Mean mobility | Mean zeta potential (mV) | ||||
|---|---|---|---|---|---|---|
| LB1 | LB2 | LB3 | LB1 | LB2 | LB3 | |
| 1 | -0.82 | -1.08 | -1.46 | -10.55 | -13.79 | -18.74 |
| 2 | -1 | -1.52 | -1.3 | -12.76 | -19.43 | -16.68 |
| 3 | -0.83 | -1.53 | -1.21 | -10.63 | -19.55 | -15.52 |
| 4 | -0.84 | -1.32 | -1.43 | -10.81 | -16.94 | -18.25 |
| 5 | -0.97 | -1.21 | -1.04 | -12.41 | -15.48 | -13.3 |
| 6 | -1.05 | -1.28 | -1.31 | -13.49 | -16.41 | -16.71 |
| 7 | -0.91 | -1.03 | -1.26 | -11.69 | -13.24 | -16.13 |
| 8 | -0.87 | -1.01 | -1.33 | -11.13 | -12.91 | -17.08 |
| 9 | -0.82 | -1.16 | -0.94 | -10.45 | -14.84 | -12.02 |
| 10 | -0.73 | -1.28 | -0.9 | -9.3 | -16.41 | -11.51 |
| Mean±SEM | -0.88±0.03 | -1.24±0.06 | -1.22±0.06 | -11.32±0.40 | -15.90±0.74 | -15.59±0.79 |
Note: One-way analysis of variance (ANOVA) test performed if p<0.05 showed significant difference in LB1 when compared with LB2 and LB3
Table 4: Zeta Potential Measurements of LB
Fig. 6: Characterization of LB, (a) Particle size distribution obtained in Dynamic Light Scattering technique; (b) Phase of zeta potential
measurement; (c) Percentile distribution of particle size; (d) Zeta potential values; (e) Vibratory sample magnetometry analysis;
(f) X- ray diffraction simulation of compounds
Note: (c):  LB3 batches; (f): (1) standard iron; (2) raw iron; (3) LB1; (4) LB2; (5) LB3 and (6) α-Fe2O3
LB3 batches; (f): (1) standard iron; (2) raw iron; (3) LB1; (4) LB2; (5) LB3 and (6) α-Fe2O3
Magnetization vs. applied field is graphically presented in fig. 6e. Magnetic hysteresis loop was observed for all the LB samples while the magnetization was almost saturated at the highest field of measurement (15 kOe). The values of saturation magnetization (Ms), remnant magnetization (Mr) and coercivity (Hc) were extracted from the graph. LB1, LB2 and LB3 exhibited Ms (emu/g) as 1.45, 5.27 and 3.56 (3.43±1.91); Mr (emu/g) as 0.31, 1.02, 0.57 (0.63±0.36) while Hc as (Oe) 290, 257 and 117 (221.33±91.85).
A direct comparison of the XRD pattern of raw iron with simulated pattern showed that iron turnings were in pure form with characteristic peak at ~45°, without any oxide content and adulterants. On the other hand, incinerated iron revealed presence of iron oxide content in the form of alpha hematite/ α-Fe2O3 having characteristic peak at ~33° and without any presence of metallic iron or sulphides (fig. 6f).
Raw iron revealed no change in the mass while the three batches of LB recorded mass loss against the increase in temperature up-to 900°. The total average mass loss for all the three batches of LB was 3.3±0.36 % w/w. The losses (% w/w) up-to various temperatures deduced from the graphs were 0.58±0.14 up-to 200°, 1.17±0.57 up-to 400°, 1.75±0.75 up-to 600° and 2.35±0.38 up-to 800°. The percentage of mass loss vs. temperature was plotted graphically (fig. 7).
ICP-OES revealed raw iron containing 98 % Fe with minor impurities of Ag, Cr, As, Fe, Mn and Cu (0.033, 0.075, 0.089, 0.217, 0.484 and 96.33 ppm, respectively) while traces of Pb, K, Co, Zn and Bi (0.002-0.009 ppm) were also detected. LB showed 59.1±0.52 % w/w of Fe and 1.7±1.34 % of S content. Heavy metals viz., Hg was absent while 0.01 ppm of Cd was detected in all the batches of finished product. Arsenic (0.01 ppm) was found in only LB2 while <0.03 ppm of Pb was present in all the batches. LB also contained essential and non-essential elements at minor levels (% mean±standard deviation); viz., Al (0.17±0.05), B (0.06±0.001), Ca (1.5±0.05), Cr (0.17±0.06), Cu (0.17±0.05), K (0.11±0.05), Mg (0.1±0.02), Mn (0.32±0.03), Na (0.02±0.006), Ni (0.04±0.00) and Zn (0.01±0.00) while negligible Bi, Ga, In and Tl (<0.03 %) were detected and Ag, Ba, Co, Li and Sr were not detected (Table 5).
| Element | Raw iron | LB1 | LB2 | LB3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration | Result | Mean | Concentration | Result | Mean | Concentration | Result | Mean | Concentration | Result | Mean | |
| Al | -0.4 | -3.54 | 0.00 | 2328.6 | 0.12 | 0.12 | 4152.7 | 0.21 | 0.21 | 3786.8 | 0.19 | 0.19 |
| -0.4 | -3.49 | 2574.3 | 0.13 | 4343.2 | 0.22 | 3773.3 | 0.19 | |||||
| As | 0.01 | 0.09 | 0.09 | -42.2 | 0 | 0.00 | 167.7 | 0.01 | 0.01 | -44.6 | 0 | 0.00 |
| 0.01 | 0.088 | -50.9 | 0 | 167.4 | 0.01 | -45 | 0 | |||||
| B | 2452.9 | 0.122 | 0.12 | 1065.1 | 0.05 | 0.06 | 1214.2 | 0.06 | 0.06 | 1246 | 0.06 | 0.06 |
| 2532.9 | 0.123 | 1278 | 0.06 | 1260.5 | 0.06 | 1274.1 | 0.06 | |||||
| Ca | -0.8 | -8.26 | 0.00 | 0.3 | 1.51 | 1.52 | 0.3 | 1.48 | 1.44 | 0.35 | 1.72 | 1.54 |
| -0.8 | -8.07 | 0.3 | 1.53 | 0.28 | 1.4 | 0.27 | 1.35 | |||||
| Cd | -28.5 | -0 | 0.00 | 103.1 | 0.01 | 0.01 | 118.7 | 0.01 | 0.01 | 123.3 | 0.01 | 0.01 |
| -29.8 | -0 | 126.2 | 0.01 | 123.7 | 0.01 | 123.4 | 0.01 | |||||
| Co | 89.8 | 0.004 | 0.01 | 62.8 | 0 | 0.00 | 65.1 | 0 | 0.00 | 56.8 | 0 | 0.00 |
| 94.9 | 0.005 | 64.5 | 0 | 68.5 | 0 | 55.9 | 0 | |||||
| Cr | 1502.1 | 0.075 | 0.08 | 1861.1 | 0.09 | 0.1 | 3813.9 | 0.19 | 0.19 | 4368.6 | 0.22 | 0.22 |
| 1543.9 | 0.075 | 2178.3 | 0.11 | 3940 | 0.2 | 4361.2 | 0.22 | |||||
| Cu | 0.02 | 0.219 | 0.22 | 0.04 | 0.19 | 0.2 | 0.04 | 0.18 | 0.19 | 0.02 | 0.11 | 0.11 |
| 0.02 | 0.214 | 0.04 | 0.2 | 0.04 | 0.2 | 0.02 | 0.11 | |||||
| Fe | 10.1 | 98.19 | 96.3 | 11.8 | 59 | 59.5 | 11.4 | 56.8 | 58.5 | 11.8 | 58.8 | 59.2 |
| 9.7 | 94.47 | 12.1 | 60.1 | 12.1 | 60.3 | 11.9 | 59.7 | |||||
| Hg | -847.3 | -0.04 | 0.00 | 1.01 | 0 | 0.00 | -4.12 | 0 | 0.00 | -9.7 | 0 | 0.00 |
| -1106 | -0.05 | 0.7 | 0 | -4.23 | 0 | -9.7 | 0 | |||||
| K | 39 | 0.002 | 0.00 | 1167.4 | 0.06 | 0.06 | 2852.8 | 0.14 | 0.15 | 2703 | 0.13 | 0.13 |
| 36.5 | 0.002 | 1249.3 | 0.06 | 3005.4 | 0.15 | 2685 | 0.13 | |||||
| Mg | -57.7 | -0 | 0.00 | 1776.4 | 0.09 | 0.09 | 2284.3 | 0.11 | 0.12 | 1896.3 | 0.09 | 0.09 |
| -3.8 | 0 | 1932.1 | 0.1 | 2399.4 | 0.12 | 1861.5 | 0.09 | |||||
| Mn | 9631.9 | 0.48 | 0.48 | 6200.1 | 0.31 | 0.32 | 6616.7 | 0.33 | 0.34 | 5815 | 0.29 | 0.29 |
| 10030.9 | 0.489 | 6688.6 | 0.33 | 6951.2 | 0.35 | 5787 | 0.29 | |||||
| Na | -32.4 | -0 | 0.00 | 219.8 | 0.01 | 0.01 | 367.3 | 0.02 | 0.02 | 353.4 | 0.02 | 0.02 |
| -38.1 | -0 | 243.8 | 0.01 | 394.7 | 0.02 | 350.1 | 0.02 | |||||
| Ni | 1258 | 0.063 | 0.06 | 787.6 | 0.04 | 0.04 | 710.1 | 0.04 | 0.04 | 709.9 | 0.04 | 0.04 |
| 1356.8 | 0.066 | 830.9 | 0.04 | 744.7 | 0.04 | 716.1 | 0.04 | |||||
| Pb | 34.1 | 0.002 | 0.00 | 410.6 | 0.02 | 0.02 | 478.5 | 0.02 | 0.02 | 506.8 | 0.03 | 0.03 |
| 35.6 | 0.002 | 516.3 | 0.03 | 512.9 | 0.03 | 499.8 | 0.02 | |||||
| S | -0.01 | -0.12 | 0.00 | 0.9 | 4.7 | 2.99 | 0.3 | 1.24 | 1.87 | 0.08 | 0.41 | 0.33 |
| -0.01 | -0.13 | 0.3 | 1.27 | 0.5 | 2.5 | 0.05 | 0.25 | |||||
| Zn | 153.9 | 0.008 | 0.01 | 191 | 0.01 | 0.01 | 175.6 | 0.01 | 0.01 | 154.4 | 0.01 | 0.01 |
| 156.8 | 0.008 | 212.4 | 0.01 | 181.7 | 0.01 | 156.2 | 0.01 | |||||
Note: Concentration of duplicate samples introduced in the argon plasma was obtained and result was calculated considering the sample weight and dilution factor followed by calculation of mean
Table 5: Inductively Coupled Plasma Optical Emission Spectrophotometry of LB
Iron, the fourth most abundant element in the earth's crust is greyish, lustrous, soft, malleable and ductile but a strong metal. In nature, it occurs in minerals, herbs and animal origin products. It reacts readily with oxygen and water to give brown-black hydrated iron oxide called as rust. Several types of irons like pig iron, wrought iron, malleable iron and grey iron are in use for several purposes[12].
In Rasashastra, types of iron viz., munda lauha, teekshna lauha and kanta lauha described with specific physical, chemical and medicinal properties are correlated with cast/pig iron, wrought iron and magnetite, respectively[2,5]. Munda lauha is used for making daily household items like vessels, agricultural tools etc.; teekshna lauha (Faulad) is meant for armory equipment[5] and kanta lauha is magnetite[10], wherein the latter two are indicated for Bhasma preparation[5]. Kanta lauha as specific characteristics of destroying the fragrance of asafoetida and bitterness of Azadirachta indica leaves when their paste is applied to kanta lauha vessel[2]. Though kanta lauha is the best variety for medicine, it is not readily available[5]. In this study, industrial waste iron in the form of turnings was used for the preparation of LB and can be considered as wrought type. LB, an official medicine[3], is used in several ailments by Ayurvedic physicians. It is an important ingredient in numerous dosage forms like powders, tablets, confectioneries and hydro-alcohols[1] as well as special formulations viz., Lauha kalpa (iron formulations) and Ayaskruti (quenched dosage form of iron)[2,3,5]; but requires proper procedures for its synthesis. Different media are used to attain the desired form for easy absorption/assimilation in the body without causing toxicity[2].
In this study, red hot iron quenched in sesame oil increased the temperature of sesame oil (90°), thus rendering brittleness due to faster cooling as well removal of associated greasy matter that cannot be removed by aqueous media. Organic liquids are preferred over aqueous media, since they cause insufficient heat transfer due to excessive air bubble formation on the surface[13]. In addition, sesame oil may remove ferric ions by forming metal soaps and the repetitive thermal stress causing particle size reduction[14]. Similarly, removal of other oxide impurities may be explained due to the aqueous media viz., butter milk, urine, rice gruel and Dolicus biflorus decoction wherein temperature of all these also increased (55°-65°). Quenching in butter milk may help to remove the rust (Fe2O3) as reported earlier, where butter milk treated samples reduced Fe2O3 while used butter milk showed absence of free Fe3+ [15]. Ammoniated acid in cow’s urine is known to dissolve Fe2O3 to form water soluble Fe2+/Fe3+ complexes whereas uric acid also forms ureate-Fe3+/Fe2+ complexes, hence removal of Fe oxides in this step can be considered[16]. Rice-gruel may be helpful to remove Fe3+ through the formation of co-ordination compounds since it contains high level of phytic acid, a chelating agent reacting with Fe2+/ Fe3+ salts to give iron complexes[17]. Similarly, Dolicus biflorus decoction, rich in gallic acid forms complex with iron chloride and water soluble gallic acid-metal complexes may get formed/detached during repeated washing[18]. Further, quenching in the Triphala decoction containing gallic acid may maintain iron in Fe2+ form, without getting oxidized. Thus, after quenching, bluish-black raw iron turned reddish-brown due to partial oxidation while sharpness of the edges reduced. The attained brittleness can be strongly corroborated with stress corrosion cracking theory[19].
In the next step, iron was triturated with Aloe barbadensis juice with/without sulphur and incinerated. No quantitative data is so far available; hence the quantities of iron, sulphur, Aloe barbadensis juice and heating were standardized. Aloe barbadensis, water rich plant containing polysaccharides and saponins, used in the present study probably helped to wet grind the particles smoothly, bind them easily during pellet formation, desiccate in a short duration and leave behind negligible inorganic residue after incineration. It possibly also acts as a natural surfactant, stabilising agent[20], solvent, bio-template for nano- metal oxide synthesis and preventer of particle agglomeration; thus, highlighting the importance of green biosynthesis against harmful chemicals as precipitating and reducing agents[21].
Initial three cycles of grinding iron and sulphur mixture, trituration with Aloe barbadensis juice followed by incineration was an important step. The temperature was gradually increased from I2 to I9 cycles by increasing the fuel, except for I1 and I3, where it was kept higher to oxidize the sulphur. The gradual chemical transformation of iron from sulphide to oxide form is associated with variables such as temperature, atmosphere and particle size in this case. The gradual temperature increase in the pit, sulphur dioxide atmosphere inside the earthen vessels, and gradual decreasing trend of particle size i.e., iron turnings into fine powder are observed during this entire manufacturing process. A study reports that, pyrite (>0.045 mm grain size) gets directly and completely oxidized to hematite at ~500° in air whereas for 0.09-0.125 mm size, hematite is formed at temperature >515° while pyrrhotite (FeS) is formed at temperature <515°. Thus, a complex route reaction of FeS2 → FeS (intermediate) → Fe3O4/Fe2O3 during pyrite roasting is reported[22]. Similar situation is possible during the incineration of iron in the concealed vessels. The assumed highest temperature inside the vessels (~750° to ~900°) and SO2 atmosphere might be facilitating the gradual conversion of FeS (formed in the ascending temperature range) to oxide initially, followed by complete conversion due to Aloe barbadensis.
LB manufactured by this method was alpha hematite (α-Fe2O3) (fig. 6f) which purports similar findings on LB analysed by Mössbauer parameters (stoichiometrically pure and chemically stable) [23]. Various methods of α-Fe2O3 nanoparticles synthesis such calcinating precursor ferric nitrate non-ahydrate at 450° in air and multistep dehydration of ferrihydrite under specific conditions have been reported but with drawbacks of aggregation and phase impurity[24]. Thus, the present method of preparation of LB also provides specific physicochemical properties as discussed further. The final colour of the product; negligible losses on drying and ignition; absence of SO4+ ions as well as passing through mesh of 53 μm indicated complete incineration in our method and further confirmed by advanced techniques. Other reports on LB prepared with Triphala decoction have shown Fe2O3 phase, calculated particle size in nano range by XRD[6] and elemental composition as Fe (~52 %), Ca (~2.5 %) with particle size upto 1.8 μm[7]. In another study[8], LB prepared in muffled furnace at 650° when analysed by X-Ray Fluorescence technique revealed Fe up-to 70 %, but its oxide status was not reported. However, SEM showed aggregates of irregularly shaped and variedly sized particles (100-500 nm).
In the present study, the size and surface morphology determined with SEM and TEM revealed batchto- batch consistency. TEM confirmed that this method of LB formation yielded powder containing nano- to micro- sized particle agglomerates. It also depicted nearly spherical shaped particles comparable with the reported study on α-Fe2O3 [25]. However, the irregularities in the shape of particles may be attributed to the grinding process.
The particle size range (0.25-1 μm) and negative ZP (-14 mV) of LB, both are unique for this method. ZP ranging from 5-20 is considered fairly stable. Moreover, negatively charged higher sized nano-particles (150 nm) accumulating more efficiently in tumours, are recognized by scavenger receptors facilitating uptake by the reticulo-endothelial system and pharmaco-kinetically cleared faster[26]. It could be expounded that Bhasma particles colloid in aqueous medium and make a suspension of clustered negatively charged hydrophobic particles as in the present study, possibly due to the repeated incineration. Detailed ZP analysis on LB can further help to understand the ionic properties that are responsible for its efficacy.
Further, the VSM study showed magnetic behaviour but the mean Ms value (3.4 emu/g) was much less as compared to the previous reports (58.6 and 65.2 emu/g) on porous α-Fe2O3 nanostructures synthesized by rapid combustion[27]. The mean Mr value was also in accordance with some reports (0.13 and 0.73 emu/g)[28] whereas the Hc value was either higher or lower[29]. Magnetic property depends on size, shape, crystallinity, surface structure and porosity of the material. From XRD studies, the phase content of LB was identified as hematite. Therefore, the differences in the magnetization and coercivity values could be due to the difference in the particle size. Though hematite is a very weak ferromagnetic material, a relatively larger magnetization observed in our case could be attributed to the incineration at higher temperatures[30]. In addition, the effect of anions (Cl-, SO2 4-, NO3 -) acting as structure and surface directing agents in the synthesis of different shaped and larger particle sized red coloured α-Fe2O3 nanoparticles might throw light on the optical and magnetic properties of LB as reported in chemical synthesis of α-Fe2O3 [31]. Generally, Fe2O3 has less magnetization and coercivity as compared to Fe3O4 [32] indicating ferromagnetic and superparamagnetic natures, respectively; irrespective of the method of preparation of iron oxides.
It is also possible that LB may contain minor amounts of ferromagnetic iron oxides like magnetite (Fe3O4) or maghemite (γ-Fe2O3) that can be ruled out by phonon confinement effects using Raman or Mössbauer Spectroscopy[33]. These were not detected in the present XRD studies because they might be present in the samples probably beyond the detection limit of XRD. The XRD pattern of raw iron turnings showed metallic Fe without any oxides while LB demonstrated α-Fe2O3 without metallic iron/other oxides or sulphide/ sulphate forms of Fe indicating purity. This is also in accordance with the recent XRD studies on α-Fe2O3 wherein higher crystallinity and greater particle size was observed at higher annealing temperature (700°)[30].
The chemical status of finished product was also confirmed by decomposition pattern obtained in TGA (fig. 7). The first mass loss (<1 %) occurring up-to 200° may be attributed to the moisture and residual sulphur while the second loss (~1 %) observed up-to 500° may be due to the elimination of carbon residues. Finally, the third mass loss at temperatures 600°-900° (~2 %) may indicate phase transformation of iron oxides. Thus, the total loss in the product and the pattern of TGA is in accordance with synthesized α-Fe2O3 [30]. Moreover, the elemental Fe in the present preparation (60 %-65 %) analysed by titration and spectrophotometric methods is comparable with the Pharmacopoeia. Heavy metals were within permissible limits[10] while trace elements viz., Na, K, Mg, Zn, Ca and Cu were detected.
There are various reports on α-Fe2O3 regarding therapeutic uses such as anti-bacterial, anti-cancer and anti-tumour due to its good bio-compatibility, bioavailability, biodegradability, ease of synthesis and differential magnetic behaviour[34]. Iron Oxide Nano-Particles (IONP’s) have also been studied as targeted drug delivery system with anticancer drugs, silica coated IONP’s as alternative immunosuppressants, anti-interleukin- 1β monoclonal antibody functionalized IONP’s as anticonvulsants and neuroprotectives, antifungals and antibiotics[35] as well as specific targeting to red blood cells and cancer cells[34] and cytotoxic to liver cancer cells[36]. Yakrut Plihari Lauha containing LB as major ingredient when administered along with Picrorrhiza kurroa showed symptomatic relief in liver disorders including liver cancers patients[37]. Moreover, iron deficiency anaemia as well as inhibition of iron absorption due to specific dietetics and disease conditions has been recognised as an important health issue. Furthermore, the large dose of iron becomes toxic due to formation of free radicals in circulation, causing corrosion in the gastrointestinal tract, thus penetrating the cells resulting in damage to important organs. Therapeutically, iron oxide in high doses has been found similar to that of ferrous sulphate (FeSO4), but the latter has several side effects like black stools[38]. Animal experiments using iron deficit diet and phlebotomy induced anaemia has proved that LB is more bioavailable and useful in increasing the hemoglobin than FeSO4, probably due to the particle size and solubility of ferric ions more soluble in neutral pH[39,40]. Therefore, extensive bioavailability studies of oxide form compared to salt counterparts needs to be conducted since the proper absorption, metabolism and its recycling is essential for good health condition.
α-Fe2O3 is reported to be a stable n-type semiconductor used for biomedical application due to its haematinic, anti-tumour, anti-cancer and anti-bacterial activities[33]. LB prepared in the present study having chemical nature of α-Fe2O3 fulfils the standards required to be therapeutically used in anaemia and malignant conditions.
Thus, in the present study LB (α-Fe2O3) was manufactured from raw iron by traditional method of incineration. Final product assessed by quality control measures for standard operating procedure generated here can be followed for consistency. Such prepared LB has irregularly shaped agglomerates, particle size from 250-1000 nm, negative zeta potential and magnetic behaviour. Incinerated iron, one of the potential ingredients of various Ayurvedic herbo-metallic mineral formulations, if prepared by these guidelines, can be further studied to understand the mechanistic aspects of its varied activities.
Funding:
This work was financially supported by the Tata Trusts (Grant number Health-BSDT-20151007).
Acknowledgements:
Authors acknowledge Anagha Desai and Shyam Deshmukh from Indian Drug Research Association and Laboratory, Pune, India as well as Pattayil Joy and Suresh Bhat from CSIR-National Chemical Laboratory, Pune, India for carrying out part of the analysis of raw material and finished product. Shyam Shitole is acknowledged for helping out in preparing the figures.
Conflict of interest:
The authors declare no conflict of interest.
References
- Gupta KLV, Gupta P, Patgiri BJ, Prajapati PK. Critical review on the Pharmaceutical vistas of Lauha kalpas (iron formulations). J Ayurveda Integr Med 2012;3(1):21-8.
[Crossref] [Google Scholar] [PubMed]
- Bhatacharya V, Sharma S, Gupta A. Rasaratna samucchaya. Motilal Banarasidas 1996.
- The ayurved formulary of India. 2003.
- Sarkar PK, Prajapati PK, Choudhary AK, De S, Ravishakar B. A comparative pharmaceutico-pharmaco-clinical study of Lauha bhasma and Mandura bhasmaw.s.r. to its panduhara effect. AYU 2007;28(1):11-6.
- Sharma S, Shastri K. Rasatarangini. Motilal Banarasidas 1979.
- Singh N, Reddy KRC. Pharmaceutical study of Lauha bhasma. AYU 2010;31(3):387-90.
[Crossref] [Google Scholar] [PubMed]
- Simpi P, Sarashetti RS, Sangolgi B. Standardisation of Lauha Bhasmaprepared with different methods. Paryeshana Int J Ayu Res 2017;1(5):49-55.
- Thakur RK, Gupta LN, Kumar N. Standard manufacturing procedure of Teekshna lauha bhasma. J Ayurveda Integr Med 2016;7(2):100-8.
[Crossref] [Google Scholar] [PubMed]
- Chavan S, Tayade S, Gupta V, Deshmukh V, Sardeshmukh S. Pharmaceutical standardization and physicochemical characterization of traditional ayurvedic marine drug: Incinerated conch shell (Shankha Bhasma). Mar Drugs 2018;16(11):450.
[Crossref] [Google Scholar] [PubMed]
- The ayurvedic pharmacopoeia of India. 1986.
- Pharmacopoeial standards for ayurvedic formulations. 1987.
- Different types of cast iron. Calmet; 2021.
- Prakash B. Use of metals in ayurvedic medicine. Indian J Hist Sci 1997;32.
- Balaji K. Deciphering the science and molecular targets of Lauha bhasma-an iron based erbo metallic preparation. SASTRA University 2015.
- Krishnamachary B, Rajendran N, Pemiah B, Krishnaswamy S, Krishnan UM, Sethuraman S, et al. Scientific validation of the different purification steps involved in the preparation of an Indian ayurvedic medicine, Lauha bhasma. J Ethnopharmacol 2012;142(1):98-104.
[Crossref] [Google Scholar] [PubMed]
- Donald I. Handbook of stainless steels. 1997.
- Geisser P. In vitrostudies on interactions of iron salts and complexes with food-stuffs and medicaments. Arzneimittelforschung 1990;40(7):754-60.
[Google Scholar] [PubMed]
- Rajendran N, Pemiah B, Sekar RK, Krishnan UM, Sethuraman S, Krishnaswamy S. Role of gallic acid in the preparation of an iron-based indian traditional medicine-Lauha bhasma. Int J Pharm Pharm Sci 2012;4(4):45-8.
- Cottis RA. Stress corrosion cracking, guides to good practice in corrosion control. National Physical Laboratory 2000.
- Morales G, Campillo G, Vélez E, Osorio J, Urquijo J, Velásquez AA. Green synthesis of magnetic nanoparticles using leaf extracts of Aloe vera and Kalanchoe daigremontiana to remove divalent mercury from natural waters. J Phys Conf Ser 2019;1247:012021:
- Chandrababu P, Cheriyan S, Raghavan R. Aloe vera leaf extract-assisted facile green synthesis of amorphous Fe2O3 for catalytic thermal decomposition of ammonium perchlorate. J Therm Anal Calorim 2019;139(1):1-12.
- Dunn JG, De GC, O'Connor BH. The effect of experimental variables on the mechanism of the oxidation of pyrite. Part 2. Oxidation of particles of size 90-125 um. Thermochim Acta 1989;155:135-1-49.
- Kaur M, Sharma S, Sharma JP. Mossbauer spectroscopic analysis of iron containing allopathic, homeopathic and ayurvedic pharmaceutical compounds. Indian J Pharm Biol Res 2014;2(3):96-104.
- Nene A, Yu X, Kaithal P, Luo H, Somani P, Ramakrishna S. Magnetic Iron Oxide Nanoparticle (IONP) synthesis to applications: Present and future. Materials 2020;13(20):4644.
[Crossref] [Google Scholar] [PubMed]
- Lassoued A, Dkhil B, Gadri A, Salah A. Control of the shape and size of iron oxide (α-Fe2O3) nanoparticles synthesized through the chemical precipitation method. Results Phys 2017;7:3007-15.
- Honary S, Foruhe Z. Effect of zeta potential on the properties of nano-drug delivery systems-a review (part 2). Trop J Pharm Res 2013;12(2):265-73.
- Manikandan A, Judith JV, Kennedy LJ. Structural, optical and magnetic properties of porous-Fe2O3 nanostructures prepared by rapid combustion method. J Nanosci Nanotechnol 2013;13(4):2986-92.
[Crossref] [Google Scholar] [PubMed]
- Tadic M, Citakovic N, Panjan M, Stanojevic B, Markovic D, Jovanovic D. Synthesis, morphology and microstructure of pomegranate-like hematite (α-Fe2O3) superstructure with high coercivity. J Alloy Compd 2012;543:118-124.
- Rufus A, Sreeju N, Philip D. Synthesis of biogenic hematite (α-Fe2O3) nanoparticles for antibacterial and nanofluid applications. RSC Adv 2016;96:1-27.
- Sharma P, Dhiman S, Kumari S, Rawat P, Srivastava C, Sato H, et al. Revisiting the physiochemical properties of Hematite (α-Fe2O3) nanoparticle and exploring its bio-environmental application. Mater Res Express 2019;6(9):095072.
- Liu J, Yang H, Xue X. Preparation of different shaped α-Fe2O3 nanoparticles with large particle of iron oxide red. Cryst Eng Comm 2019;21(7):1097-101.
- Sahadevan J, Sojiya R, Padmanathan N, Kulathuraan K, Shalini MG, Sivaprakash P, et al. Magnetic property of Fe2O3 and Fe3O4 nanoparticle prepared by solvothermal process. Mater Today: Proc 2022;58(3):895-7.
- Jubb AM, Allen HC. Vibrational spectroscopic characterization of hematite, maghemite, and magnetite thin films produced by vapor deposition. ACS App Mater Interfaces 2010;2(10):2804-12.
- Sangaiya P, Jayaprakash R. A review on iron oxide nanoparticles and their biomedical applications. J Supercond Nov Magn 2018;31(1):3397-413.
- Lunin AV, Lizunova AA, Mochalova EN, Yakovtseva MN, Cherkasov VR, Nikitin MP, et al. Hematite nanoparticles from unexpected reaction of ferrihydrite with concentrated acids for biomedical applications. Molecules 2020;25(8):1984.
[Crossref] [Google Scholar] [PubMed]
- Kumar R, Vithiya K, Biswanath M, Sen S. Biosynthesis of hematite nanoparticles and its cytotoxic effect on HepG2 cancer cells. Int J Biol Macromol 2015;74:376-81.
[Crossref] [Google Scholar] [PubMed]
- Sardeshmukh B, Deshmukh V. Assessment of ayurvedic treatment in liver disorders with yakrutplihodari Lauha . J Sanskrit Samhita Siddhanta 2017;2(3):11-8.
- Kheiri R, Koohi MK, Sadeghi-Hashjin G, Nouri H, Khezli N, Hassan MA, et al. Comparison of the effects of iron oxide, as a new form of iron supplement, and ferrous sulfate on the blood levels of iron and total iron-binding globulin in the rabbit. Iran J Med Sci 2017;42(1):79-84.
[Google Scholar] [PubMed]
- Verma PRP, Prasad CM. Standardization and bioavailability of ayurvedic drug Lauha bhasma-part-II comparative bioavailability studies. Anc Sci Life 1995;15(2):140-4.
[Google Scholar] [PubMed]
- Pandit S, Biswas TK, Debnath PK, Saha AV, Chowdhury U, Shaw BP, et al. Chemical and pharmacological evaluation of different ayurvedic preparations of iron. J Ethnopharmacol 1999;65(2):149-56.
[Crossref] [Google Scholar] [PubMed]
 after quenching in specific purification (in decoction of Triphala)
after quenching in specific purification (in decoction of Triphala)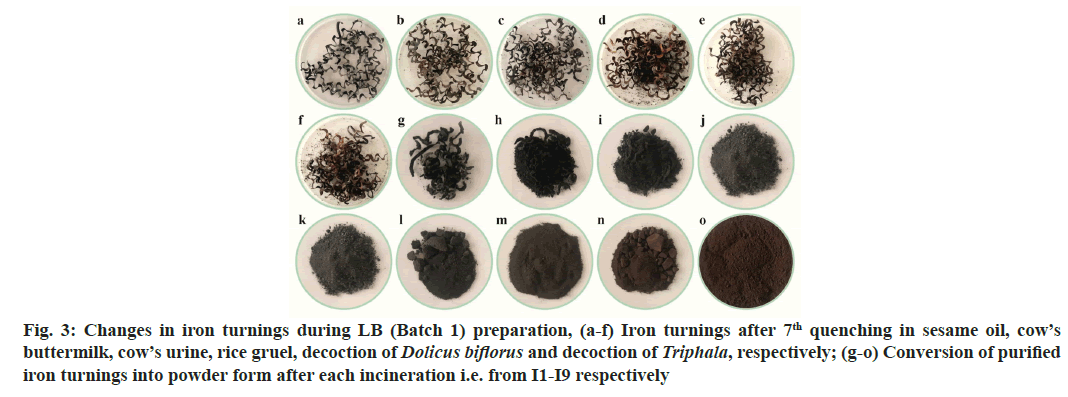
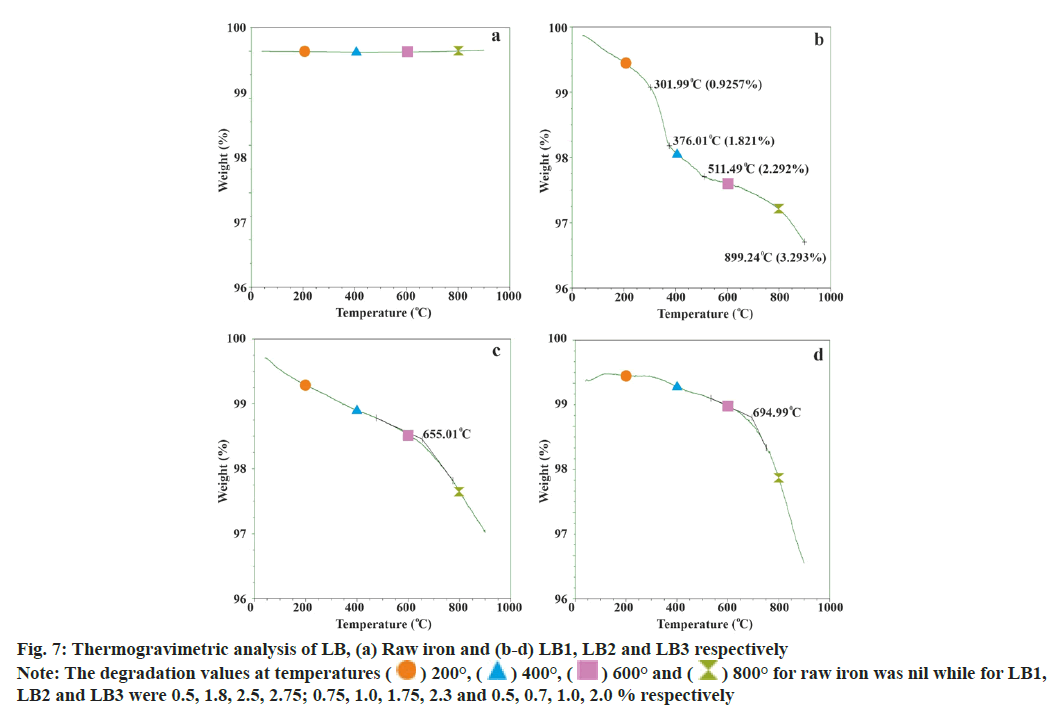
 800° for raw iron was nil while for LB1, LB2 and LB3 were 0.5, 1.8, 2.5, 2.75; 0.75, 1.0, 1.75, 2.3 and 0.5, 0.7, 1.0, 2.0 % respectively
800° for raw iron was nil while for LB1, LB2 and LB3 were 0.5, 1.8, 2.5, 2.75; 0.75, 1.0, 1.75, 2.3 and 0.5, 0.7, 1.0, 2.0 % respectively



