- *Corresponding Author:
- H. T. Ma
Department of Thoracic Surgery, The First Affiliated Hospital of Soochow University, China
E-mail: haitaomasoochow@163.com
| This article was originally published in a special issue, “Current Trends in Pharmaceutical and Biomedical Sciences” |
| Indian J Pharm Sci 2022:84(5) Spl Issue “59-66” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Esophageal cancer is a common cancer among people in the world. The molecular mechanism of complement C1q tumor necrosis factor related protein 6 in esophageal cancer is still unclear. This article aimed to clarify the role of complement C1q tumor necrosis factor related protein 6 in esophageal cancer. The expression of complement C1q tumor necrosis factor related protein 6 in esophageal cancer tissues was detected using quantitative real-time polymerase chain reaction and Western blot. Furthermore, short hairpin ribonucleic acid was used to knockdown the expression of complement C1q tumor necrosis factor related protein 6 in esophageal cancer cell lines. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide, wound healing assays and transwell assays were used to determine the role of complement C1q tumor necrosis factor related protein 6 in esophageal cancer cells. In our research, we found that the expression of complement C1q tumor necrosis factor related protein 6 was up-regulated in esophageal cancer tissues. The increased expression of complement C1q tumor necrosis factor related protein 6 was significantly correlated with tumor stage and lymph node metastasis. The proliferation and migration of esophageal cancer cells were inhibited after complement C1q tumor necrosis factor related protein 6-short hairpin ribonucleic acid transfection in vitro. These results suggested that complement C1q tumor necrosis factor related protein 6 serves as an oncogene and promotes cell proliferation and migration in esophageal cancer. In addition, complement C1q tumor necrosis factor related protein 6 may be a potential therapeutic target for esophageal cancer patients.
Keywords
C1q tumor necrosis factor related protein 6, esophageal cancer, short hairpin ribonucleic acid, proliferation, migration, quantitative real-time polymerase chain reaction polymerase chain reaction
Esophageal Cancer (EC) is the eighth most common cancer worldwide and has the sixth highest cancer mortality[1-3]. Esophageal Squamous Cell Carcinoma (ESCC) has become the most frequent subtype of EC[4,5]. Despite with the great advances in treatment, the 5 y survival rate of EC is remaining at 10 %-20 % related to proliferation and invasion of tumor cells[6]. The molecular mechanisms of EC remain unclear to date. Thus, it is important for us to investigate the molecular mechanisms underlying EC tumorigenesis.
C1q is associated with the tumor necrosis factor superfamily in several biological processes, including inflammation, apoptosis and cell differentiation. C1q/Tumor Necrosis Factor-Associated Protein (CTRP protein) has been found to play a role in carcinogenesis and cancer progression[7]. The C1q/TNF-related protein family is consisted of 16 CTRP members. Among them, CTRP3, CTRP4 and Complement C1q Tumor Necrosis Factor Related Protein 6 (C1QTNF6) have been found to be associated with tumor deterioration. All CTRP members are secreted proteins that are widely expressed in various tissues and cell types[8-12]. CTRP4 was shown to be a tumor-promoting inflammatory modulator and to promote tumor cell survival and reduce drug-induced apoptosis[7]. These findings strongly suggest that CTRP4 is a potential therapeutic target. CTRP8 has been reported to be involved in brain cancer[13]. It has also been shown to enhance motility and stromal invasion by glioblastoma cells[14]. CTRP8-induced migration of human glioma cells revealed inhibition of small competitive peptides derived from C1QTNF6[15]. Western blotting showed that C1QTNF6 was highly expressed in human hepatocellular carcinoma tissues. High expression of C1QTNF6 can activate Protein Kinase B (Akt) signaling pathway, increase tumor angiogenesis and reduce Human Liver Carcinoma Cells (HepG2) necrosis[16]. C1QTNF6-interference inhibits Extracellular Signal-Regulated Kinase 1/2 (ERK1/2) signaling in mouse embryonic fibroblast cell line (3T3-L1) adipocytes[17].
In our study, we found that C1QTNF6 was significantly higher in tumor compared to normal tissue. In addition, its biological effects on EC cancer cell lines were also identified. This article aimed to explore the relationship between C1QTNF6 and EC.
Materials and Methods
Human tissue samples:
50 pairs of EC tissues and matched adjacent normal tissues were obtained from EC patients who underwent surgical resection at the First Affiliated Hospital of Soochow University from January 2017 to September 2020. All participants received no other treatment prior to surgery and signed written informed consent. Our study was approved by the Ethical Committee of the First Affiliated Hospital of Soochow University.
Cell culture:
Two EC cell lines i.e., Epithelial cell lines (TE-1) and human Esophageal Carcinoma cell lines (ECa-109) are typical representatives of esophageal adenocarcinoma and squamous cell carcinoma, so we used these two cell lines for our study. Two EC cell lines (TE-1 and ECa109) were incubated in Dulbecco’s Modified Eagle Medium (DMEM) with 10 % fetal bovine serum (Thermo Fisher Scientific) at 37° in a 5 % Carbon dioxide (CO2) atmosphere. After the confluence of cells reached 40 %-50 %, the cells would be used for transfection.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR):
Ribonucleic Acid (RNA) extraction from the cell lines and human tissues was performed with a TRIzol kit (Invitrogen, United States of America (USA)). The isolated RNA was measured at 260/280 nm using spectrophotometry (Thermo, San Jose, California, USA). All the RNA samples were stored at 80°. SYBR Premix Ex Taq II (Stratagene, USA) was used for qRT-PCR on ABI 7500 fast RT-PCR system (Applied, Biosystems). The primers were as follows. C1QTNF6-Forward: 5'-GAAAGGGTCTTTGTGAACCTTGA-3', C1QTNF6-Reverse, 5'‑CTGCGCGTACAGGATGACAG-3'; Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH)-Forward: 5'-TGACTTCAACAGCGACACCCA-3', GAPDH-Reverse, 5'-CACCCTGTTGCTGTAGCCAAA-3'. GAPDH was amplified as an internal control. The relative gene expression was calculated by a 2-∆∆CT method.
Short hairpin Ribonucleic Acid (shRNA) establishment:
The siRNA is expressed instantaneously in the cell, while the shRNA is stabilized by virus-mediated transduction. In order, for the knockdown of C1QTNF6, a shRNA vector targeting the C1QTNF6 gene was established by Shanghai GeneChem Co., Ltd. The targeting sequences of C1QTNF6 were cloned into the lentiviral vector pGVX115-Green Fluorescent Protein (GFP) to obtain pGVX115-shC1QTNF6. The pGVX115-GFP was served as a control and was titled pGVX115-shCtrl.
Western blot:
Total protein was extracted with Radioimmunoprecipitation Assay (RIPA) buffer from the cultured cells and the protein concentration was detected by a Bicinchoninic Acid (BCA) protein assay kit (Bio-Rad, Italy). Proteins were isolated by 10 % Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE), moved to Polyvinylidene Fluoride (PVDF) membranes and immunoblotted with the following primary antibodies: C1QTNF6 (1:500, SantaCruz, USA) and GAPDH (1:1000, SantaCruz, USA) overnight at 4°. After washing three times, the membranes were incubated with secondary antibody for 2 h at 37°. Protein bands were visualized using Enhanced Chemiluminescence (ECL) reagents (Pierce, Rockford, Illinois, USA).
Cell proliferation and wound healing assays:
Cell proliferation was measured using 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyl-2H-Tetrazolium Bromide (MTT) assay. Cells were seeded in 96-well plates for 24 h. 20 µl of MTT solution (MedChemExpress, Monmouth Junction, New Jersey, USA) was added to each well at 1, 2, 3, 4 and 5 d after transfection. The absorbance values at 490 nm were detected using a microplate absorbance reader (MRXII, Dynex Technologies, Chantilly, Virginia, USA). Each experiment was performed in triplicate.
Cell migration was performed using wound healing assays. For wound healing assays, cells were plated into six-well plates with each experimental group. A liner wound was made by scraping a non-opening Pasteur pipette across the confluent cell layer. Cells were washed twice to remove detached debris. Then, size of wounds was observed and measured at indicated times. Each experiment was performed in triplicate.
Transwell assay:
After transfection for 72 h, cells were inoculated into the 6-well transwell upper chamber with a density of 25 000 cells/well. The transwell assay was conducted based on the manufacturer’s instructions. The transwell chamber was then placed in a humidified incubator with 5 % CO2 at 37° for 48 h. The lower chamber was stained with hematoxylin and photographed.
Statistical analysis:
All statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) 17.0 (IBM Corporation, Armonk, New York, USA). Data were expressed as mean±Standard Deviation (SD). Statistical differences were analyzed by Student’s t-test or one-way Analysis of Variance (ANOVA), p<0.05 was defined statistically significant.
Results and Discussion
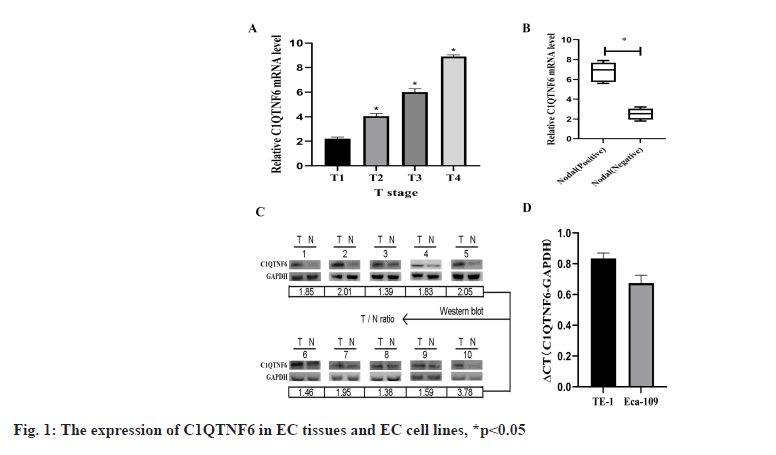
C1QTNF6 is up-regulated in EC tissues were shown in fig. 1. Western blot and qRT-PCR were used to detect the expression levels of C1QTNF6 in 50 paired EC tissues and adjacent normal tissues. With the increasing of Tumor (T) stage, C1QTNF6 expression level increased continuously (p<0.05; fig. 1A). According to the results of chi-square test, the expression of C1QTNF6 was significantly up-regulated in positive lymph node compared with negative lymph node (p<0.05; fig. 1B). Then, we selected 10 of these patients and found higher levels of C1QTNF6 expression level than in adjacent tissues (p<0.05; fig. 1C). To assess whether C1QTNF6 expression is related with the development of EC, the relationship between C1QTNF6 and clinicopathological characteristics of EC patients was conducted. As displayed in Table 1, we divided the patients into high-expression group and low-expression group according to the median value in EC tissues and we found C1QTNF6 expression was significantly correlated with differentiation (p=0.039), lymph node metastasis (p=0.031) and T stage (p=0.022). However, no significant correlation was identified between the expression of C1QTNF6 and other characteristics such as sex, age and histology (all p>0.05). Univariate and multivariate Cox analysis of clinical parameters associated with overall survival was shown in Table 2, p<0.05.
| Characteristics | All patients | C1QTNF6 low expression | C1QTNF6 high expression | p |
|---|---|---|---|---|
| n | 50 | 24 | 26 | |
| Age (y) | 0.866 | |||
| <60 | 22 | 10 | 12 | |
| ≥60 | 28 | 13 | 9 | |
| Sex | 0.897 | |||
| Male | 27 | 14 | 13 | |
| Female | 23 | 12 | 11 | |
| Histology | 0.772 | |||
| Adenocarcinoma | 7 | 3 | 4 | |
| Squamous cell carcinoma | 43 | 20 | 23 | |
| Differentiation | 0.039 | |||
| Well+moderate | 12 | 4 | 8 | |
| Poor | 38 | 14 | 24 | |
| Lymph node metastasis | 0.031 | |||
| Positive | 34 | 12 | 22 | |
| Negative | 16 | 5 | 11 | |
| T stage | 0.022 | |||
| ≤II | 14 | 4 | 10 | |
| >II | 36 | 12 | 24 |
Table 1: Relationship between C1QTNF6 and Clinicopathological characteristics of EC Patients.
| Parameters | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95 % CI | p | HR | 95 % CI | p | |
| C1QTNF6 | 3.528 | 1.849-5.114 | 0.012* | 3.382 | 1.738-4.993 | 0.009* |
| Age (y) | 1.721 | 0.664-2.804 | 0.201 | 1.806 | 0.773-2.975 | 0.197 |
| Sex | 1.016 | 0.464-1.682 | 0.593 | 1.031 | 0.453-1.691 | 0.709 |
| Histology | 2.173 | 0.559-3.975 | 0.404 | 2.022 | 0.528-3.593 | 0.516 |
| Differentiation | 1.773 | 0.491-2.921 | 1.076 | 1.742 | 0.515-3.041 | 1.011 |
| Lymph node metastasis | 1.128 | 0.516-1.783 | 0.825 | 1.298 | 0.583-1.884 | 0.818 |
| T stage | 0.936 | 0.482-1.577 | 0.116 | 0.885 | 0.407-1.764 | 0.183 |
Note: HR: Hazard Ratio; CI: Confidence Intervals, *p<0.05.
Table 2: Univariate and Multivariate COX analysis of Clinical Parameters associated with overall Survival.
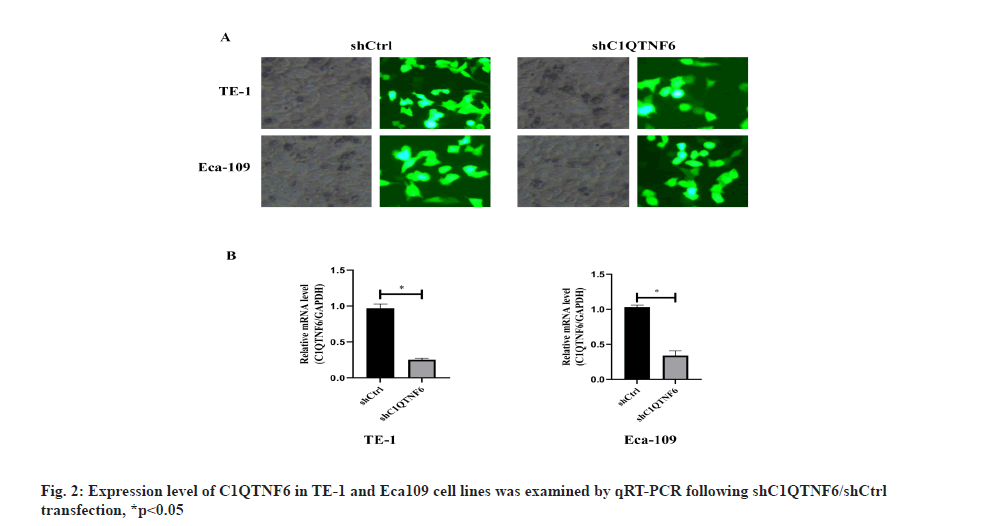
Knockdown expression of C1QTNF6 by shRNA lentivirus transfection into TE-1 and Eca-109 cell lines was shown here. Endogenous C1QTNF6 expression in TE-1 and Eca-109 cell lines was detected by qRT-PCR. The assay showed that C1QTNF6 was largely expressed both in TE-1 and Eca-109 cell lines, showing that TE-1 and 177 Eca-109 were fit for knocking down analysis (fig. 1D). In order to examine the function of C1QTNF6 in TE-1 and Eca-109 cell lines, lentiviruses carrying C1QTNF6-shRNA and shCtrl were used to infect TE-1 and Eca-109 cell lines. Transfection efficacy was examined by GFP (fig. 2A). The transfection efficiency was assessed by qRT-PCR and the results suggested that C1QTNF6 messenger RNA (mRNA) expression level was significantly reduced in the C1QTNF6-shRNA group compared with the shCtrl group (fig. 2B).
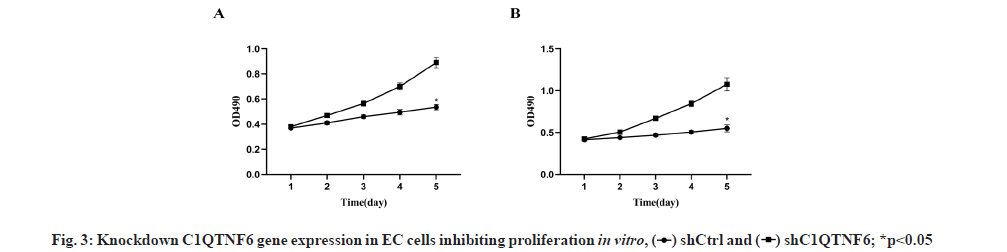
Knockdown of C1QTNF6 inhibited cell proliferation of TE-1 and Eca-109 cell lines in vitro was shown in fig. 3. To decipher the effect of C1QTNF6 on growth of EC cells, MTT assay was used. Cell proliferation of TE-1 cell lines following shC1QTNF6/shCtrl infection was detected by MTT assay from d 1 to d 5 was shown in fig. 3A. Cell proliferation of Eca-109 cell lines following shC1QTNF6/shCtrl infection was detected by MTT assay from d 1 to d 5 was shown in fig. 3B. As shown in fig. 3A and fig. 3B, compared with shCtrl group, the cell proliferation ability of C1QTNF6-shRNA group was significantly decreased between d 1 to d 5 in TE-1 and Eca-109 cell lines (p<0.05).
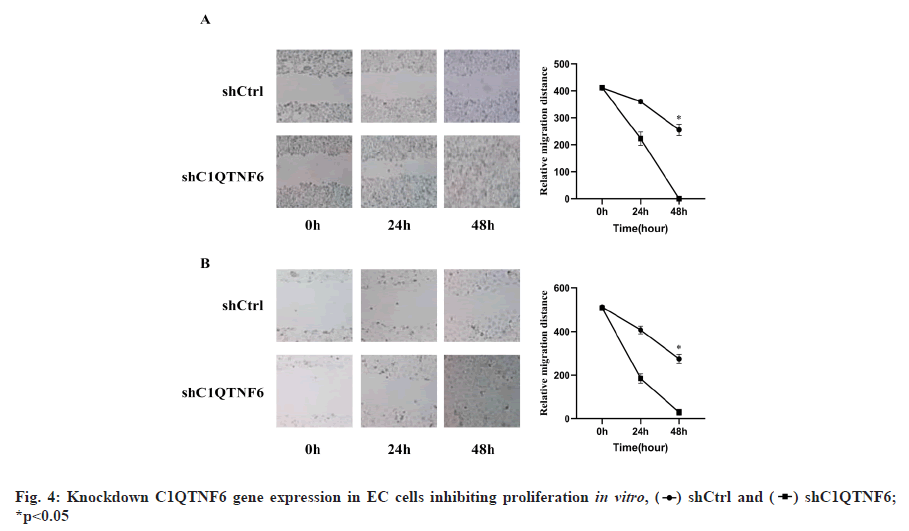
Knockdown of C1QTNF6 gene expression in EC cells inhibiting proliferation in vitro was shown in fig. 4. Cell proliferation was also evaluated using wound healing assays. Cell distance of TE-1 cell lines following shC1QTNF6/shCtrl infection was detected by wound healing assay from 0 h to 48 h was shown in fig. 4A. Cell distance of Eca-109 cell lines following shC1QTNF6/shCtrl infection was detected by wound healing assay from 0 h to 48 hours was shown in fig. 4B. As demonstrated in fig. 4A and fig. 4B, compared with the shCtrl group, the distance between cells was significantly increased after C1QTNF6-shRNA transfection into TE-1 and Eca-109 cell lines (p<0.05).
Knockdown of C1QTNF6 inhibited cell migration of TE-1 and Eca-109 cell lines in vitro was shown in fig. 5. Cell migration of TE-1 cell lines following shC1QTNF6/shCtrl infection was detected by transwell assay after 72 h was shown in fig. 5A. Cell migration of Eca-109 cell lines following shC1QTNF6/shCtrl infection was detected by transwell assay after 72 h was shown in fig. 5B. Cell counts of TE-1 and Eca-109 cell lines following shC1QTNF6/shCtrl infection were investigated by transwell assay after 72 h was shown in fig. 5C. As displayed in fig. 5A-fig. 5C, compared with the shCtrl group, the cell counts of cell migration was significantly increased after C1QTNF6-shRNA transfection into TE-1 and Eca-109 cell lines (p<0.05).
EC occurrence is a complex process, including vascular production, cell adhesion, motility, Extracellular Matrix (ECM) degradation and angiogenesis[18-22]. EC patients diagnosed at an advanced stage usually have a poor prognosis[6]. Therefore, to improve the clinical outcomes of EC patients, it is crucial for us to identify novel biomarkers. With the development of technique, sequencing analysis has been applied to understand its molecular mechanisms comprehensively[19]. In our research, 50 pairs of EC tumors and adjacent non-cancer tissues were conducted with RNA sequencing and we discovered that C1QTNF6 is differentially expressed. At present, C1QTNF6 up-regulation was found in gastric cancer[23]. Previous studies have confirmed that down-regulation of C1QTNF6 mRNA promoted the invasiveness of human breast cancer cell line (Michigan Cancer Foundation-7 (MCF-7)). In the above studies, C1QTNF6 was found to be directly targeted by microRNA-29b (miR-29b), which may be regarded as a tumor suppressor or promoter, depending on the cell or tissue type. However, little is known about the function of C1QTNF6 in EC. The goal of this article was to search the biological role of C1QTNF6 in EC cells and to clarify its function in the occurrence of EC.
In this study, C1QTNF6 was significantly up regulated in EC tissues. Moreover, up-regulation of C1QTNF6 was significantly correlated with differentiation, lymph node metastasis and T stage, which suggested that C1QTNF6 may be correlated with the prognosis of EC patients and may be related with the progression of EC. In addition, C1QTNF6 was largely expressed in TE-1 and Eca-109 cell lines, C1QTNF6 shRNA could be constructed and transfected into TE-1 and Eca-109 cell lines.
In conclusion, these results displayed that down-regulation of C1QTNF6 expression, which could inhibit cell proliferation and migration ability in vitro. However, there are some limitations in our study. The sample size is restricted, which may influence the results. Therefore, more experiments are needed to elucidate the specific molecular mechanism of C1QTNF6 in EC in the future. Nevertheless, we should conduct more in vivo experiments to validate the oncogene effects of C1QTNF6. As a result, this article indicates that C1QTNF6 has significant biological implications in EC. Therefore, C1QTNF6 might serve as an oncogenic role in EC and be a potential target for treatment of EC.
Acknowledgements:
Haitao Ma designed this study. Haifeng Xia, Fei Lu, Xiaojun Yu and Yu Feng performed experiments and analyzed data. This paper was written by Haifeng Xia.
Conflict of interests:
The authors declared no conflict of interest.
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61(2):69-90.
[Crossref] [Google scholar] [PubMed]
- Wu Q, Wu Z, Bao C, Li W, He H, Sun Y, et al. Cancer stem cells in esophageal squamous cell cancer. Oncol Lett 2019;18(5):5022-32.
- Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends-An update. Cancer Epidemiol Biomarkers Prev 2016;25(1):16-27.
[Crossref] [Google scholar] [PubMed]
- Reichenbach ZW, Murray MG, Saxena R, Farkas D, Karassik EG, Klochkova A, et al. Clinical and translational advances in esophageal squamous cell carcinoma. Adv Cancer Res 2019;144:95-135.
[Crossref] [Google scholar] [PubMed]
- Han B, Li W, Sun Y, Zhou L, Xu Y, Zhao X. A prolyl-hydroxylase inhibitor, ethyl-3, 4-dihydroxybenzoate, induces cell autophagy and apoptosis in esophageal squamous cell carcinoma cells via up-regulation of BNIP3 and N-myc downstream-regulated gene-1. PLoS One 2014;9(9):e107204.
[Crossref] [Google scholar] [PubMed]
- Wang Y, Hu Y, Guo J, Wang L. miR-148a-3p suppresses the proliferation and invasion of esophageal cancer by targeting DNMT1. Genet Test Mol Biomarkers 2019;23(2):98-104.
[Crossref] [Google scholar] [PubMed]
- Chavali VR, Khan NW, Cukras CA, Bartsch DU, Jablonski MM, Ayyagari R. A CTRP5 gene S163R mutation knock-in mouse model for late-onset retinal degeneration. Hum Mol Genet 2011;20(10):2000-14.
[Crossref] [Google scholar] [PubMed]
- Hofmann C, Chen N, Obermeier F, Paul G, Büchler C, Kopp A, et al. C1q/TNF-related protein-3 (CTRP-3) is secreted by visceral adipose tissue and exerts anti-inflammatory and antifibrotic effects in primary human colonic fibroblasts. Inflamm Bowel Dis 2011;17(12):2462-71.
[Crossref] [Google scholar] [PubMed]
- Thanasupawat T, Glogowska A, Burg M, Wong GW, Hoang-Vu C, Hombach-Klonisch S, et al. RXFP1 is targeted by complement C1q tumor necrosis factor-related factor 8 in brain cancer. Front Endocrinol 2015;6:127.
[Crossref] [Google scholar] [PubMed]
- Klonisch T, Glogowska A, Thanasupawat T, Burg M, Krcek J, Pitz M, et al. Structural commonality of C1q TNF‐related proteins and their potential to activate relaxin/insulin‐like family peptide receptor 1 signalling pathways in cancer cells. Br J Pharmacol 2017;174(10):1025-33.
[Crossref] [Google scholar] [PubMed]
- Glogowska A, Kunanuvat U, Stetefeld J, Patel TR, Thanasupawat T, Krcek J, et al. C1q‐tumour necrosis factor‐related protein 8 (CTRP8) is a novel interaction partner of relaxin receptor RXFP1 in human brain cancer cells. J Pathol 2013;231(4):466-79.
[Crossref] [Google scholar] [PubMed]
- Takeuchi T, Adachi Y, Nagayama T. Expression of a secretory protein C1qTNF6, a C1qTNF family member, in hepatocellular carcinoma. Anal Cell Pathol 2011;34(3):113-21.
[Crossref] [Google scholar] [PubMed]
- Wu WJ, Mo DL, Zhao CZ, Zhao C, Chen YS, Pang WJ, et al. Knockdown of CTRP6 inhibits adipogenesis via lipogenic marker genes and Erk1/2 signalling pathway. Cell Biol Int 2015;39(5):554-62.
[Crossref] [Google scholar] [PubMed]
- Murayama MA, Kakuta S, Inoue A, Umeda N, Yonezawa T, Maruhashi T, et al. CTRP6 is an endogenous complement regulator that can effectively treat induced arthritis. Nat Commun 2015;6(1):1-2.
[Crossref] [Google scholar] [PubMed]
- Fan RH, Zhu XM, Sun YW, Peng HZ, Wu HL, Gao WJ. CTRP6 inhibits fibrogenesis in TGF-β1-stimulated human dermal fibroblasts. Biochem Biophys Res Commun 2016;475(4):356-60.
[Crossref] [Google scholar] [PubMed]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001;25(4):402-8.
[Crossref] [Google scholar] [PubMed]
- Chen Z, He T, Zhao K, Xing C. Anti-metastatic activity of fangchinoline in human gastric cancer AGS cells. Oncol Lett 2017;13(2):655-60.
[Crossref] [Google scholar] [PubMed]
- Wilson SH, Bailey AM, Nourse CR, Mattei MG, Byrne JA. Identification of MAL2, a novel member of the mal proteolipid family, though interactions with TPD52-like proteins in the yeast two-hybrid system. Genomics 2001;76(1-3):81-8.
[Crossref] [Google scholar] [PubMed]
- Heyn H, Carmona FJ, Gomez A, Ferreira HJ, Bell JT, Sayols S, et al. DNA methylation profiling in breast cancer discordant identical twins identifies DOK7 as novel epigenetic biomarker. Carcinogenesis 2013;34(1):102-8.
[Crossref] [Google scholar] [PubMed]
- Zhao L, Liu Y, Tong D, Qin Y, Yang J, Xue M, et al. MeCP2 promotes gastric cancer progression through regulating FOXF1/Wnt5a/β-catenin and MYOD1/caspase-3 signaling pathways. EBioMedicine 2017;16:87-100.
[Crossref] [Google scholar] [PubMed]
- Shen B, Sun D. Natural diterpenoid isoferritin a (IsoA) inhibits glioma cell growth and metastasis viaregulating of TGFβ-induced EMT signal pathway. Med Sci Monit 2018;24:3815-23.
[Crossref] [Google scholar] [PubMed]
- Wu X, Liu W, Liu X, Ai Q, Yu J. Overexpression of MCPH1 inhibits the migration and invasion of lung cancer cells. Onco Targets Ther 2018;11:3111-7.
[Crossref] [Google scholar] [PubMed]
- Qu HX, Cui L, Meng XY, Wang ZJ, Cui YX, Yu YP, et al. C1QTNF6 is overexpressed in gastric carcinoma and contributes to the proliferation and migration of gastric carcinoma cells. Int J Mol Med 2019;43(1):621-9.
[Crossref] [Google scholar] [PubMed]
 .
.
 *p<0.05.
*p<0.05.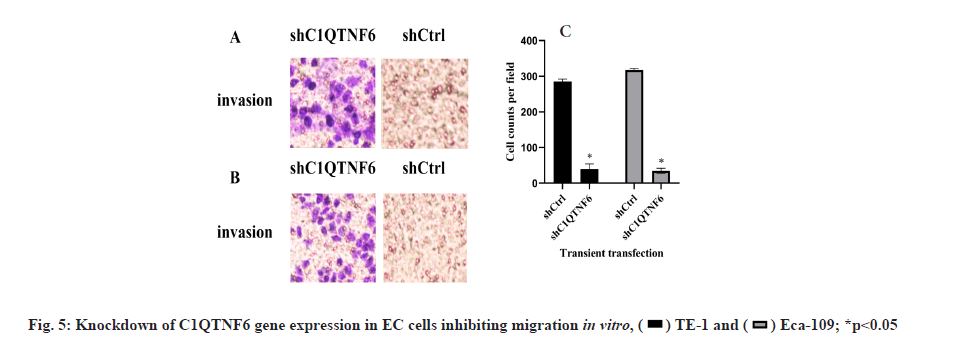
 .
.



