- *Corresponding Author:
- L. Chen
Anhui Suzhou, Anhui Health Vocational College, Anhui, China
E-mail: xutie889@163.com
| This article was originally published in a special issue, |
| "Clinical and Experimental Studies on Drug and Intervention Repurposing in China" |
| Indian J Pharm Sci 2019:81(4)spl issue1;181-185 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
In order to explore the nursing utility and relevant mechanism of boric acid in promoting wound healing of diabetic mice, a total of 36 healthy male mice were randomly divided into the control group (group A) and the experimental group (group B) to construct the diabetic mouse model. Each mouse model was built with 3 different wounds and the changes in expressions of Tgf-β1 and collagen I in the wound tissue were detected using quantitative real-time polymerase chain reaction. The results showed the body weight of diabetic mice in group B was significantly lower than that of the control mice in group A, while the plasma glucose increased significantly. In addition, in group A mice both saline and boric acid could increase the expression levels of Tgf-β1 in wound tissues and the drug combination of povidone-iodine, hydrogen peroxide and boric acid could increase the expression levels of collagen I in wound tissue, thereby promoting the wound healing, while in group B mice, boric acid alone could increase the expression levels of Tgf-β1 and collagen I in wound tissue and promoted wound healing. Therefore, boric acid solution could promote wound healing in diabetic mice and the mechanism could be associated with the increased expression of Tgf-β1 and Collagen I. Despite the deficiencies in the research process, it has provided certain basis and ideas for subsequent research.
Keywords
Boric acid, diabetes mellitus, wound healing, Tgf-β1, collagen I
Diabetes mellitus (DM) is a familiar disease from metabolism disorder characterized by hyperglycaemia[1]. Hyperglycaemia is caused by insulin secretion deficiency or insulin action defects. Clinically, DM can be divided into type I and II. The most typical clinical manifestations of type I DM are polydipsia, polyuria, polyphagia, and weight loss. Patients with type II DM are often obese before the incidence of the disease, and gradually lose their weights after the incidence of the disease, with the feelings of fatigue and weakness[2]. If the plasma glucose of diabetic patients is not well-controlled, various chronic damages and dysfunctions of tissues and organs would occur, such as eyes, hearts, kidneys, blood vessels, and nerves[3]. In addition, diabetic foot (DF) is one of the most common complications of DM. The wounds of DF are difficult to heal due to a series of causes such as the obstruction of blood circulation in diabetic patients, the growth of bacteria, the decrease of auto-resistance, and the destruction of body fluid equilibrium[4]. DF can induce lesions in the feet of diabetic patients with different degrees of infections, ulcers, and gangrene; it limits the movement of diabetic patients and leads to a permanent disability of the patients, which seriously affects the physical and mental health and quality of life of the patients[5].
The basic processes of wound healing include acute inflammation stage, cell proliferation stage, scar formation stage, and epidermal and other tissue regeneration stage; based on the injured degrees of the wounds, wound healing can also be divided into the primary healing stage, the secondary healing stage, and the tertiary healing stage[6]. In the acute inflammatory stage and the cell proliferation stage, the wounds of diabetic patients have different degrees of repair disorders, which are healed slowly and recurrently, resulting in the formation of refractory wounds[7]. The boric acid solution is a clear, colourless, and odourless liquid[8]. Clinically, the boric acid solution is used as a topical antibacterial drug due to its effects of antiinflammatory, antiswelling, antibacterial, astringent, and wound-cleansing[9]. It is suitable for redness swelling or erosion with secondary infections, and acute dermatitis and eczema with a large amount of methods[10].
In summary, the current treatment methods for diabetic refractory wounds still have limitations and the pathogenesis of the disease has not yet been conclusive. Therefore, healthy male mice were taken as research subjects to explore the nursing utility and relevant mechanism of boric acid in promoting the wound healing in diabetic mice, which provided certain references for the clinical treatments of diabetic refractory wounds.
A total of 36 healthy male C57BL/6 mice, aged 6-8 w and weighed approximately 18-25 g, were used in this investigation. The animal experiments were conducted in accordance with the International Animal Protection and Management Regulations and were approved by the respective ethics committee.
The 36 male mice were randomly divided into 2 groups of 18 mice in each, the control group (group A) and the experiment group (group B). Each group was randomly divided into 3 subgroups with 6 mice in each subgroup, which were respectively recorded as 4, 8, and 16 d. After fasting for 16 h, blood samples were collected from the caudal veins in mice in the group B and plasma glucose levels were detected using a plasma glucose analyser (J&J, USA); the mice were then given streptozotocin (STZ, Shanghai Qiyi Biological Technology, China) through intraperitoneal injections at a dose of 70 mg/ml for 4 consecutive days; the plasma glucose levels of mice were determined weekly, once the plasma glucose levels exceeded 16.7 mmol/l, the diabetic mouse model was developed. Mice in group A received citrate buffer instead of STZ.
In the 4th w after the diabetic model was developed, all mice were anesthetized through intraperitoneal injections of 3 ml/kg chloral hydrate (1 %, Baoji GK Bio-Technology, China). Taking the spine as the median, a total of 3 full thickness skin defect wounds in the diameter of approximately 0.8 cm were made on each mouse using a puncher. These 3 wounds were marked a, b, and c. Wound a was treated with saline (Tianjin Taize Biological Technology, China) once a day, wound b was treated with boric acid solution (4 %, Nanjing Sbjbio, China) once a day and wound c was treated with povidone-iodine (0.5 %, Shenzhen Simeiquan Biotechnology, China) and hydrogen peroxide (3 %, Xi’an Web Lion Chemical, China) once a day. On the 4th, 8th, and 16th d after wound formation, the wounds were harvested as specimens.
Approximately 100 mg of mouse wound tissue was placed in a glass grinder, triturated and homogenized with Trizol (400 μl, Jiaozuo LFFBio, China). The homogenate was collected in a EP tube, the lid was closed and the tube was shaken vigorously and allowed to stand for 5 min at room temperature. Afterwards, trichloromethane (200 μl, Sinopharm Chemical Reagent, China) was added, capped, shaken vigorously and the tube was allowed to stand for 5 min at room temperature and centrifuged on a high-speed centrifuge (Allegra X-22, Beckman Coulter, USA) at 14 000 rpm, 4° for 15 min. The upper solution in the tube was pipetted and transferred into another EP tube, equal volume of isopropanol (Tianjin Guangcheng Chemical Reagent, China) was added, shaken vigorously, allowed to stand at -20° for 30 min and centrifuged (14 000 rpm, 4°) for 15 min. The supernatant was discarded, 75 % ethanol (100 μl, Tianjin Guangcheng Chemical Reagent, China), was added, mixed evenly and centrifuged again (14 000 rpm, 4°) for 15 min. The supernatant was discarded. After the precipitate was spread and dried, diethylpyrocarbonate (DEPC) water (50 μl, Shanghai Huyu Biological Technology, China) was added, mixed evenly, and stored at -80°.
The template for the reverse transcription reaction was 1 μg of tissue total RNA template extracted through the Trizol method. The RT reaction solution was formulated by 1 μl of 5×gDNA Eraser buffer and 0.5 μl of gDNA Eraser and was supplemented to a total volume of 5 μl by DEPC water. The mixture was evenly mixed, and the reverse transcription reaction was carried out at 42° for 2 min by using a Q-PCR instrument (Stepone Plus, Applied Biosystems, USA). Then, the reaction solution was continued in the reaction system formulated by 2 μl of 5×buffer, 0.5 μl of enzyme, and 0.5 μl of primer mix, and was supplemented to a total volume of 10 μl by DEPC water. The mixture was evenly mixed, and the reverse transcription reaction was carried out at 37° for 15 min and 85° for 5 s by using a Q-PCR instrument After the reverse transcription reaction was terminated, the final product was diluted to 100 μl with DEPC water and stored at 20°. Primer Bank was used to design the primer sequences of TGF-β1, collagen I, and internal reference 18s; the PCR reaction solution was formulated by 5 μl of 2×SYBR Green qPCR mix (High Rox), 0.4 μl of PCR forward primer (5 μM), 0.4 μl of PCR reverse primer (5 μM), and 2 μl of cDNA template, and was supplemented to a total volume of 10 μl by UP water. The reaction was carried out once at 94° for 3 min. Then, the pre-denaturation was performed at 94° for 15 s, the PCR reaction was carried out at 94 for 15 s and the reaction was then carried out at 72° for 20 s; the reaction was repeated for 40 cycles, and the dissociation curve was plotted. The test results were calculated by the relative quantitative method, and the relative value of the gene expression was calculated by the 2-ΔΔCT method (the control gene was 18s gene). ΔCt value = Ct value of the target gene-Ct value of the control gene; ΔΔCt value = ΔCt value of the sample to be tested-ΔCt value of the control sample. 2-ΔΔCt = the relative expression multiple Δ of the sample to be tested compared with the control sample.
SPSS 22.0 statistics software was used for statistical analysis of the data; all quantitative data were submitted to normal distribution test and homogeneity test of variance and were expressed as the mean number±standard deviation. The one-way ANOVA was used for comparison between groups; if the normal distribution and variance were consistent, the LSD method was used; otherwise, the SNK-q test was used. Pearson correlation analysis was used to analyse the correlations between two parameters, p<0.05 indicated the statistical significance of the difference.
The results of body weights and plasma glucose of mice before and after induction of diabetes were shown in Table 1. It could be seen that before the intraperitoneal injections STZ, compared to the group A, the body weights and plasma glucose levels in the group B mice were not significantly different; after the intraperitoneal injections STZ, compared to the group A mice, the body weights of group B mice decreased significantly (p<0.05). In addition, the plasma glucose levels of mice in group B increased significantly (p<0.05).
| Groups | Body weights before (g) | Body weights after (g) | Plasma glucose before (mM) | Plasma glucose after (mM) |
|---|---|---|---|---|
| Group A | 21.57±1.02 | 21.86±1.09 | 7.45±0.71 | 7.36±0.66 |
| Group B | 21.94±1.18 | 17.65±0.97 | 7.14±0.52 | 20.69±3.88 |
Table 1: Body weights and plasma glucose of mice before and after model development
The results of wound healing rates of mice were shown in Table 2. It could be seen that the wound healing rates of wound a at different time phases in the group B were significantly lower than that of the group A (p<0.05); the wound healing rates of wound b at different time phases in the group B were significantly higher than those in the group A (p<0.05); the wound healing rates of wound c at different time phases in the group B were significantly lower than those in the group A (p<0.05). Among mice of group A on the 4th and 8th d after wound formation, the wound healing rates of wound a were higher than those of wounds b and c; on the 16th d after wound formation, the wound healing rate of wound c was higher than that of wounds a and b, which suggested that within 8 d of wound formation, saline treatment could promote the wound healing; after 8 d of wound formation, povidone-iodine and hydrogen peroxide could improve the wound healing rate of mice. In the experiment group, the wound healing rates of wound b at different time phases were higher than those of wounds a and c, which suggested that boric acid could improve the wound healing rates of diabetic mice.
| Wound healing rates | Wound formation (d) | Group A | Group B |
|---|---|---|---|
| Healing rates of wound a (%) | 4 | 26.37±1.02 | 20.75±0.95 |
| 8 | 67.84±1.87 | 34.17±1.54 | |
| 16 | 91.05±2.39 | 78.69±2.03 | |
| Healing rates of wound b (%) | 4 | 23.61±1.56 | 25.01±1.89 |
| 8 | 53.86±2.76 | 58.56±2.88 | |
| 16 | 87.19±4.13 | 92.38±4.26 | |
| Healing rates of wound c (%) | 4 | 11.37±0.54 | 10.21±0.43 |
| 8 | 37.74±1.39 | 36.02±1.35 | |
| 16 | 93.59±2.86 | 79.55±2.32 |
Table 2: Comparisons of wound healing rates of mice
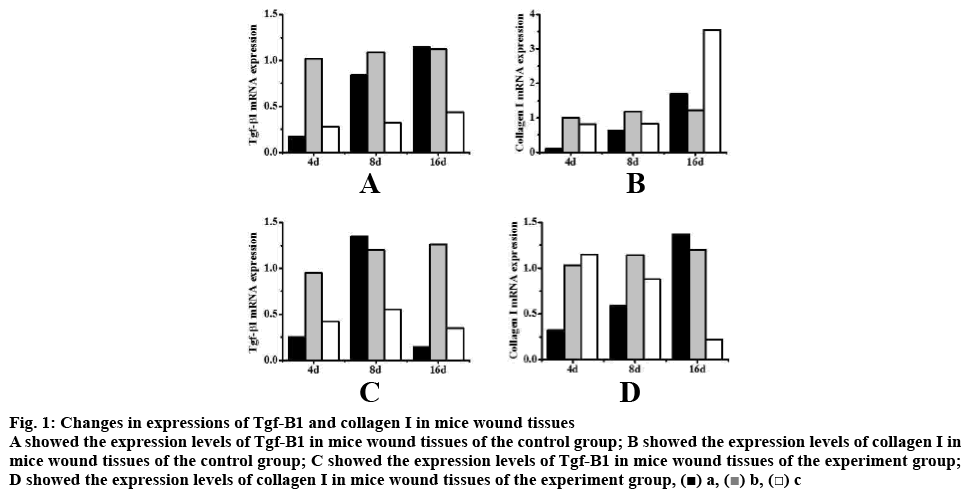
Among mice in the group A, on the 4th d of wound formation, the expression levels of Tgf-β1 in wound b were significantly higher than those in wounds a and c (p<0.05); on the 8th and 16th d of wound formation, the expression levels of Tgf-β1 in wounds a and b were significantly higher than those in wound c (p<0.05). Thus suggesting that both saline and boric acid could increase the expression levels of Tgf-β1 in wound tissues of healthy mice and promote wound healing. On the 4th d of wound formation, the expression levels of collagen I in wounds b and c were significantly higher than those in wound a (p<0.05); while on the 8th and 16th d of wound formation, the differences in expression levels of collagen I in wounds a, b, and c were not statistically significant; however, on the 16th d, the expression levels of collagen I in wound c were relatively higher. Thus, it was apparent that the combination of povidone-iodine, hydrogen peroxide and boric acid could increase the expression levels of collagen I in wound tissues of healthy mice and promote the wound healing.
Among mice in the group B on the 4th and 16th d of wound formation, the expression levels of Tgf-β1 in wound b were significantly higher than those in wounds a and c (p<0.05); on the 8th d of wound formation, the expression levels of Tgf-β1 in wounds a and b were significantly higher than those in wound c (p<0.05). On the 4th d of wound formation, the expression levels of collagen I in wounds b and c were significantly higher than those in wound a (p<0.05); on the 8th d of wound formation, the differences in expression levels of collagen I in wounds a, b, and c were not statistically significant (p>0.05); on the 16th d, the expression levels of collagen I in wounds a and b were higher than those in wound c, and the differences were statistically significant (p<0.05). Thus, it was suggested that the boric acid could increase the expression levels of Tgf-β1 and collagen I in wound tissues of diabetic mice and promote the wound healing (fig. 1).
Figure 1: Changes in expressions of Tgf-Β1 and collagen I in mice wound tissues
A showed the expression levels of Tgf-Β1 in mice wound tissues of the control group; B showed the expression levels of collagen I in
mice wound tissues of the control group; C showed the expression levels of Tgf-Β1 in mice wound tissues of the experiment group;
D showed the expression levels of collagen I in mice wound tissues of the experiment group, 
In order to explore the nursing utility and relevant mechanism of boric acid in promoting wound healing of diabetic mice, a total of 36 healthy male mice were selected and were randomly divided into group A and group B to construct the diabetic mouse model. Each mouse model was inflicted with 3 different wounds and the changes in expressions of Tgf-β1 and collagen I in the wound tissues of mice were detected by qRT-PCR. The results showed that in diabetic mice, compared with group A, the body weights of mice decreased significantly and the plasma glucose increased significantly. In addition, in mice in group A, both saline and boric acid could increase the expression levels of Tgf-β1 in the wound tissue, and the combination of povidone-iodine, hydrogen peroxide, and boric acid could increase the expression levels of collagen I in the wound tissue, thereby promoting the wound healing; in mice of group B, boric acid alone could increase the expression levels of Tgf-β1 and collagen I in wound tissues of diabetic mice to promote the wound healing.
Therefore, boric acid solution could promote the wound healing of diabetic mice, and its mechanism could be associated with the expression increase of Tgf-β1 and collagen I. However, certain deficiencies were found in the research process; for example, the data collection of samples was relatively less that caused the result being biased to a certain extent. Therefore, the data capacity would be further increased in the subsequent works to make the obtained results more valuable.
References
- Napoli N, Chandran M, Pierroz DD, Abrahamsen B, Schwartz AV, Ferrari SL, et al. Mechanisms of diabetes mellitus-induced bone fragility. Nat Rev Endocrinol 2017;13(4):208.
- Zheng Y, Ley SH, Hu FB. Global etiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol 2018;14(2):88.
- Shan K, Liu C, Liu BH, Chen X, Dong R, Liu X, et al. Circular noncoding RNA HIPK3 mediates retinal vascular dysfunction in diabetes mellitus. Circulation 2017;136(17):1629-42.
- Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med 2017;376(24):2367-75.
- MacLeod AS. Bad Staph in the Wound Environment of Diabetic Foot Ulcers. Cell Host Microbe 2019;25(5):638-40.
- Zhang P, Lu J, Jing Y, Tang S, Zhu D, Bi Y. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis. Ann Intern Med 2017;49(2):106-16.
- Jeffcoate WJ, Vileikyte L, Boyko EJ, Armstrong DG, Boulton AJM. Current challenges and opportunities in the prevention and management of diabetic foot ulcers. Diabetes Care 2018;41(4):645-52.
- Ge X, Li J, Zhang C. Liquid superlubricity of polyethylene glycol aqueous solution achieved with boric acid additive. Langmuir 2018;34(12):3578-87.
- Vaghetto R, Childs M, Jones P. Experimental observations of boric acid precipitation scenarios. Nucl Eng Des 2017;312:422-8.
- Graff A, Barrez E, Baranek P. Complexation of nickel ions by boric acid or (poly) borates. J Solution Chem 2017;46(1):25-43.