- *Corresponding Author:
- Peizhong Li
Department of Otorhinolaryngology, The Affiliated Huaian No.1 People’s Hospital of Nanjing Medical University, Huaian, Jiangsu 223300
China
E-mail: lpzent310@163.com
| This article was originally published in a special issue, “Recent Developments in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(4) Spl Issue “162-170” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Long noncoding RNAs are involved in the pathogenesis of nasopharyngeal carcinoma. This study was designed to investigate the clinical roles of long noncoding RNAs somatostatin receptor 5 antisense RNA 1 and tubulin alpha 4B in prediction of nasopharyngeal carcinoma prognosis. Reverse transcriptionquantitative polymerase chain reaction was performed to detect gene expression levels. The relationship between aberrant expressions of somatostatin receptor 5 antisense RNA 1, tubulin alpha 4B, 5 y overall survival and relapse-free survival rates were evaluated by Kaplan-Meier analysis. The clinical effects of somatostatin receptor 5 antisense RNA 1 and tubulin alpha 4B in nasopharyngeal carcinoma cell viability and apoptosis were further verified and evaluated by the in vitro experiments. We found that expression levels of somatostatin receptor 5 antisense RNA 1 and tubulin alpha 4B were significantly decreased in serum of nasopharyngeal carcinoma patients and nasopharyngeal carcinoma cell lines. Somatostatin receptor 5 antisense RNA 1 and tubulin alpha 4B expression levels were associated with the node stage, clinical stage and grade of nasopharyngeal carcinoma. In addition, nasopharyngeal carcinoma patients with lower somatostatin receptor 5 antisense RNA 1 and tubulin alpha 4B expression presented shorter 5 y overall survival and relapse-free survival rates than those with higher expression. After receiving chemoradiotherapy, the expression levels of somatostatin receptor 5 antisense RNA 1 and tubulin alpha 4B were significantly increased. In vitro experimental results further verified that somatostatin receptor 5 antisense RNA 1 and tubulin alpha 4B overexpression strongly promote nasopharyngeal carcinoma cell apoptosis. To sum up, tumor-suppressors long noncoding RNAs somatostatin receptor 5 antisense RNA 1 and tubulin alpha 4B might be potential indicators for nasopharyngeal carcinoma prognosis and treatment.
Keywords
Nasopharyngeal carcinoma, long noncoding RNAs, somatostatin receptor 5 antisense RNA 1, tubulin alpha 4B, prognosis
Nasopharyngeal Carcinoma (NPC) is a malignant tumor occurring at the top and lateral wall of the nasopharynx[1]. As one of the most common malignancies in China, the incidence ranks first among all the otorhinolaryngology malignant tumors[2-4]. According to global cancer statistics, there are 133 354 new cases in the nasopharynx site and 80 008 NPCrelated deaths around the world in 2020[5]. There are 64 165 estimated new cases and 36 315 estimated deaths of NPC in China in 2022[6]. NPC is mainly treated by chemo-radiotherapy and the residual lesions could be removed by surgical resection. Up to now, biopsy and imaging are the traditional methods for detecting NPC[7]. However, clinical outcomes are poor due to the distant metastasis and recurrence[8,9]. Hence, it is urgent to discover novel indicators that were related to NPC prognosis to accurately recognize and monitor therapeutic response in time.
Long Non-Coding RNAs (lncRNAs) are at a length of more than 200 nt and may regulate gene expression at the post-transcriptional level[10]. Emerging studies indicated that lncRNAs were abnormally expressed in various cancers, including NPC. It reported that lncRNA TUC338 was remarkably increased in lung cancer tissues compared with the paracarcinoma tissues[11]. LncRNA Urothelial Carcinoma-Associated 1 (UCA1), HOXA Distal Transcript Antisense RNA (HOTTIP), and High Mobility Group AT-Hook 1 Pseudogene 4 (HMGA1P4) were found to be dysregulated in gastric cancer samples through integrative analysis of Gene Expression Omnibus (GEO) and The Cancer Genome Atlas (TCGA) data[12]. 137 different lncRNAs were proved to be aberrantly expressed in NPC samples as well[13]. Furthermore, some lncRNAs were directly correlated with NPC development and prognosis. For instance, lncRNA Nuclear Paraspeckle Assembly Transcript 1 (NEAT1), Small Nucleolar RNA Host Gene 12 (SNHG12) and RP11-624L4.1 were down regulated in NPC and related to prognosis[14-16]. Somatostatin Receptor 5 Antisense RNA 1 (SSTR5-AS1) and Tubulin Alpha 4B (TUBA4B) were aberrantly expressed functioning as tumor suppressors or oncogenes in lung cancer[17,18], gastric cancer[19,20], colorectal cancer[21], epithelial ovarian cancer[22], laryngeal squamous cell carcinoma[23] and breast cancer[24]. However, the potential values of SSTR5-AS1 and TUBA4B in the clinical aspect remain largely unknown.
The main purpose of this study was to discover the clinical roles and value of SSTR5-AS1 and TUBA4B in NPC. Reverse Transcription-quantitative Polymerase Chain Reaction (RT-qPCR) analysis was performed to determine the expression of SSTR5-AS1 and TUBA4B in NPC tissues and cell lines. Receiving Cell Counting Kit-8 (CCK-8) and Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) staining assays were utilized for determination of NPC cell proliferation. Kaplan-Meier analysis was conducted to reveal the 5 y overall survival and diseasefree survival of NPC patients with aberrant SSTR5- AS1 or TUBA4B expression levels.
Materials and Methods
Study population:
A total of 121 participants (30 healthy controls and 91 NPC patients) were recruited from Affiliated Huaian No.1 People’s Hospital between December 2012 and November 2014 (Table 1 and Table 2). Before our study, all the NPC patients did not receive any chemotherapy, radiotherapy or adjuvant therapy. All the NPC patients were diagnosed by biopsy. The serum samples (10 ml each) were collected and isolated by centrifugation at 12 000 g at 4° for 5 min. Then, the samples were preserved at -80°. The present study was approved by the Ethics Committee of Affiliated Huaian No.1 People’s Hospital and written consent was received from each participant in this study.
| Variable | Cases (n) | lncRNA TUBA4B expression | p value | |
|---|---|---|---|---|
| Low (n) | High (n) | |||
| Gender | ||||
| Female | 41 | 19 | 22 | 0.2677 |
| Male | 50 | 29 | 21 | |
| Age | ||||
| ≥60 | 39 | 22 | 17 | 0.5444 |
| <60 | 52 | 26 | 26 | |
| T stage | ||||
| T1+T2 | 48 | 27 | 21 | 0.4795 |
| T3+T4 | 43 | 21 | 22 | |
| N stage | ||||
| N0-N1 | 49 | 18 | 31 | 0.001 |
| N2-N3 | 42 | 30 | 12 | |
| M stage | ||||
| M0 | 47 | 25 | 22 | 0.9301 |
| M1 | 44 | 23 | 21 | |
| Clinical stage | ||||
| I-II | 52 | 20 | 32 | 0.0016 |
| III-IV | 39 | 28 | 11 | |
| EBV infection | ||||
| Yes | 82 | 42 | 40 | 0.3782 |
| No | 9 | 6 | 3 | |
| Grade | ||||
| G1-G2 | 55 | 21 | 34 | 0.0006 |
| G3 | 36 | 27 | 9 | |
| Lymph node metastasis | ||||
| Yes | 37 | 22 | 15 | 0.2884 |
| No | 54 | 26 | 28 | |
Table 1: The Association between Serum Sstr5-As1 Expression and Npc Clinopathological Characteristics
| Variable | Cases (n) | lncRNA TUBA4B expression | p value | |
|---|---|---|---|---|
| Low (n) | High (n) | |||
| Gender | ||||
| Female | 41 | 19 | 22 | 0.2677 |
| Male | 50 | 29 | 21 | |
| Age | ||||
| ≥ 60 | 39 | 22 | 17 | 0.5444 |
| < 60 | 52 | 26 | 26 | |
| T stage | ||||
| T1+T2 | 48 | 27 | 21 | 0.4795 |
| T3+T4 | 43 | 21 | 22 | |
| N stage | ||||
| N0-N1 | 49 | 18 | 31 | 0.001 |
| N2-N3 | 42 | 30 | 12 | |
| M stage | ||||
| M0 | 47 | 25 | 22 | 0.9301 |
| M1 | 44 | 23 | 21 | |
| Clinical stage | ||||
| I-II | 52 | 20 | 32 | 0.0016 |
| III-IV | 39 | 28 | 11 | |
| EBV infection | ||||
| Yes | 82 | 42 | 40 | 0.3782 |
| No | 9 | 6 | 3 | |
| Grade | ||||
| G1-G2 | 55 | 21 | 34 | 0.0006 |
| G3 | 36 | 27 | 9 | |
| Lymph node metastasis | ||||
| Yes | 37 | 22 | 15 | 0.2884 |
| No | 54 | 26 | 28 | |
Table 2: The Association between Serum Lncrna Tuba4b Expression and Npc Clinopathological Characteristics
RT-qPCR:
RT-qPCR assays were applied to distinguish expression levels of SSTR5-AS1 and TUBA4B in NPC patients at admission and after chemo-radiotherapy treatment. Total Ribonucleic Acid (RNA) was extracted from samples using QIAamp RNA Blood Kit (Qiagen, Hilden, Germany) under the manufacturer’s protocol. Then, complementary Deoxyribonucleic Acid (cDNA) was synthesized using reverse transcription kit (Applied Biosystems, Carlsbad, California, United States of America) and amplified using SYBR® PrimeScript RT-PCR kit (Takara Biotechonology Co., Ltd, Dalian, China) on the 7500 real-time PCR systems (Applied Biosystems, Carlsbad, California, United States of America). PCR conditions were described as follows; initial denaturation at 94° for 10 min, 40 cycles at 94° for 10 s, 62° for 20 s and 72° for 15 s. The expression of SSTR5-AS1 and TUBA4B were normalized to Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) expression and analyzed by 2–ΔΔCt method[25]. The primers sequences were as follows; SSTR5-AS1 (forward): 5’-GGCAGCCGGAATCTGGAACT-3’, SSTR5-AS1 (reverse): 5’-CAGTGCGCAGCAATGATTAG-3’, TUBA4B (forward): 5’0CCCACAGGCTTTAAGGTTGA-3’, TUB4AB (reverse): 5’-AGGCCATAGTGATGGCTGTC-3’, GAPDH (forward): 5’-CAATGACCCCTTCATTGAC-3’ and GAPDH (reverse): 5’-GACAAGCTTCCCGTTCTC-3’.
Cell culture:
Nasopharyngeal epithelial cell line NP69 (#SCC197) was purchased from Merck (Germany) and cultured in Keratinocyte Serum-Free Medium (KSFM) (Gibco, New York, United States of America) without serum growth factor. NPC cell lines 5-8F, CNE-1 and CNE-2 were purchased from Mingzhou Bio (Ningbo, China) and cultured in Roswell Park Memorial Institute (RPMI) 1640 medium containing 10 % Fetal Bovine Serum (FBS) (Gibco, New York, United States of America). All cell lines were placed in a humidified incubator with 5 % Carbon dioxide (CO2) at 37°.
Plasmids construction and cell transfection:
Overexpressing plasmid pcDNA3.1-SSTR5-AS1, pcDNA3.1-TUBA4B and pcDNA3.1 empty plasmids were designed and obtained from GenePharma (Shanghai, China). The transfection was conducted strictly, followed the protocol of Lipofectamine™ 2000 (Thermo Fisher Scientific, Shanghai, China) at 37°, 5 % CO2. The transfected cells were harvested and collected at 24~48 h after transfection.
CCK-8 assay:
At 48 h after transfection, CNE-1 cells were harvested and inoculated in 96-well plates with the density of 3×104 cells/well. At 0, 12, 24 and 48 h, 10 μl CCK-8 reagents (Dojindo, Japan) was added to each well and cultured for 2 h in the dark. Finally, the Optical Density (OD) value was tested at 450 nm using a micro plate reader (#E0226, Beyotime, Shanghai, China).
TUNEL staining assay:
After transfection, 100 μl cell suspensions at the density of 5×107 cells/ml were prepared. Samples were washed with Phosphate Buffered Saline (PBS) and then fixed using 4 % paraformaldehyde. TUNEL assay was conducted with a TUNEL Assay Kit (ab66110; Abcam, Shanghai, China) under the manufacturer’s protocol. TUNEL-positive cells were considered as apoptotic cells. The fluorescence images were obtained using a fluorescence microscope (EVOS M5000; Thermo Fisher Scientific, Shanghai, China). Five randomly chosen fields were analyzed for each group.
Statistical analysis:
Statistical Package for Social Sciences (SPSS) 21.0 (SPSS Inc., Chicago, Illinois) and GraphPad Prism 6.0 (GraphPad software Inc., La Jolla, California) were employed to analyze all the data in this study. Unpaired t-test was used to analyze the differences of SSTR5- AS1 and TUBA4B in NPC and healthy controls. Chisquare test followed by Tukey’s post hoc test was applied to analyze the association of SSTR5-AS1, TUBA4B expression and clinic pathological characteristics of NPC patients. Kaplan-Meier analysis with the longrank test was performed to determine the associations between SSTR5-AS1, TUBA4B expression and 5 y overall survival and relapse-free survival rates. p<0.05 indicates statistical significance.
Results and Discussion
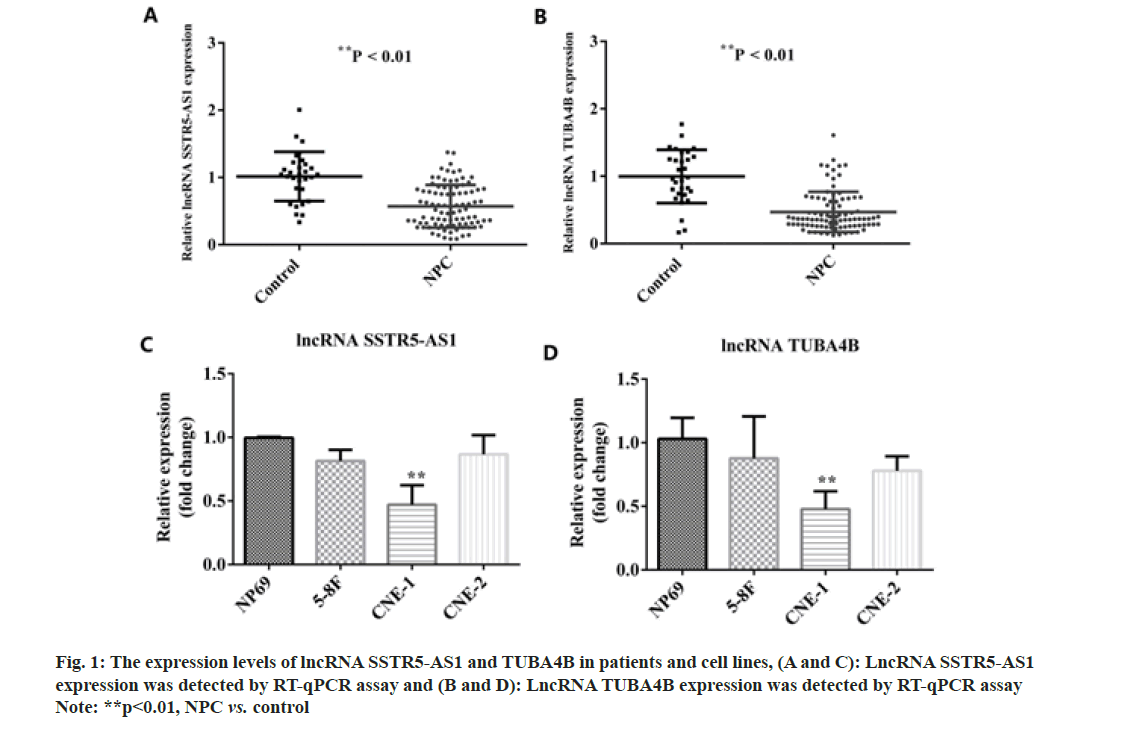
We first detected the expression levels of SSTR5- AS1 and TUBA4B in patients with NPC and healthy controls using RT-qPCR assay. From the results in fig. 1A and fig. 1B, the expression levels of SSTR5-AS1 and TUBA4B were significantly decreased in NPC patients as compared with healthy controls (p<0.01). SSTR5-AS1 and TUBA4B expression levels were decreased in 5-8F, CNE-1 and CNE-2 NPC cells lines compared with nasopharyngeal epithelial cell line NP69. Meanwhile, the decrease was most obvious in CNE-1 cell line, which was chosen for the following experiments as shown in fig. 1C and fig. 1D.
As shown in Table 1, SSTR5-AS1 expression was correlated with Node (N) stage (p=0.0207), clinical stage (p=0.0087) and grade (p<0.0001) and has no significant association with gender (p=0.3907), age (p=0.9514), T stage (p=0.4212), M stage (p=0.5710), Epstein Barr Virus (EBV) infection (p=0.1664) or lymph node metastasis (p=0.2321). Meanwhile, as shown in Table 2, lncRNA, TUBA4B expression was correlated with N stage (p=0.0010), clinical stage (p=0.0016) and grade (p=0.0006) and has no significant association with gender (p=0.2677), age (p=0.5444), T stage (p=0.4795), M stage (p=0.9301), EBV infection (p=0.3782) or lymph node metastasis (p=0.2884).
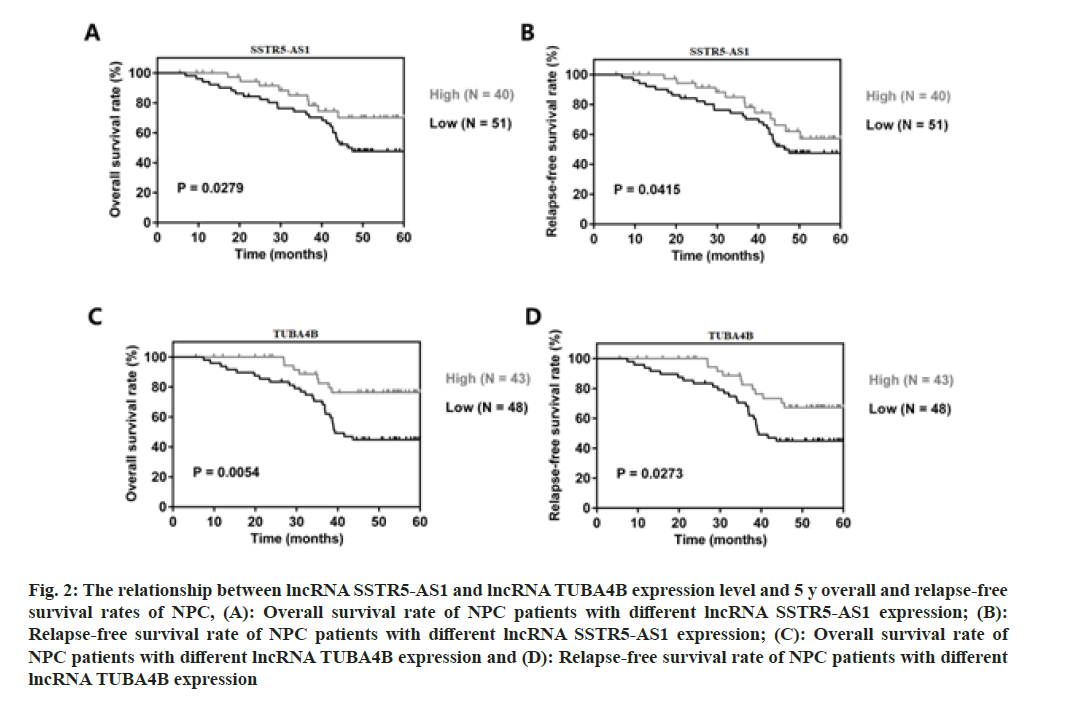
We further verified the associations between SSTR5- AS1, TUBA4B and the survival rate and relapse-free survival rates in NPC patients using Kaplan-Meier analysis. From the data, NPC patients with lower SSTR5-AS1 or TUBA4B expression presented shorter 5 y survival rate and relapse-free survival rates than those with higher SSTR5-AS1 or TUBA4B expression (fig. 2A, p=0.0279; fig. 2B, p=0.0415; fig. 2C, p=0.0054 and fig. 2D, p=0.0273). High or low SSTR5- AS1 or TUBA4B expression was defined based on their averaged expression in NPC patients. Hence, lower SSTR5-AS1 and TUBA4B expression was associated with poor prognosis in NPC.
Fig. 2: The relationship between lncRNA SSTR5-AS1 and lncRNA TUBA4B expression level and 5 y overall and relapse-free survival rates of NPC, (A): Overall survival rate of NPC patients with different lncRNA SSTR5-AS1 expression; (B): Relapse-free survival rate of NPC patients with different lncRNA SSTR5-AS1 expression; (C): Overall survival rate of NPC patients with different lncRNA TUBA4B expression and (D): Relapse-free survival rate of NPC patients with different lncRNA TUBA4B expression
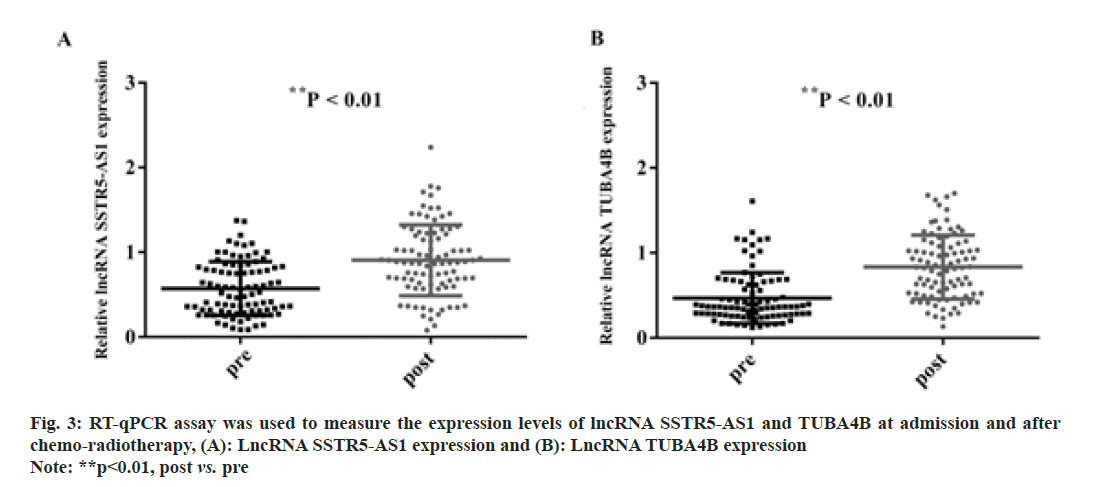
Furthermore, we detected the expression of SSTR5- AS1 and TUBA4B in the serum of NPC patients before and after chemo-radiotherapy. As presented in fig. 3A (p<0.01), the expression of SSTR5-AS1 was significantly increased after receiving chemoradiotherapy (p<0.01). In addition, the expression of lncRNA TUBA4B was significantly increased as well (fig. 3B; p<0.01). Hence, SSTR5-AS1 and TUBA4B expression may be potential indicators for monitoring NPC treatment response.
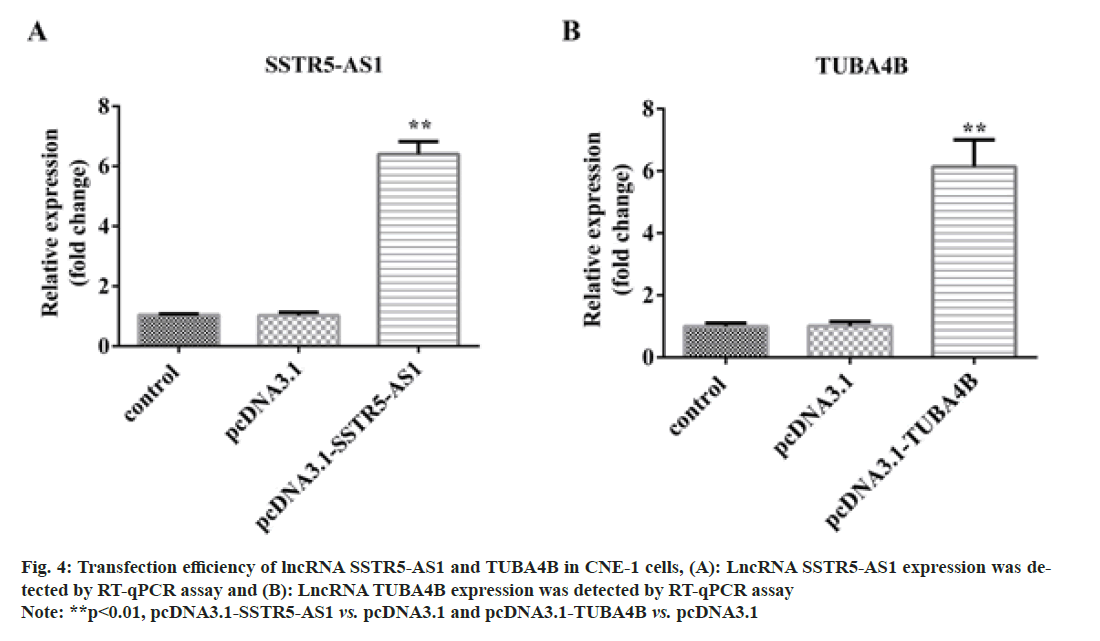
After transfection with pcDNA3.1, pcDNA3.1-SSTR5- AS1 and pcDNA3.1-TUBA4B, the transfection efficacy was confirmed by RT-qPCR assays. As shown in fig. 4A and fig. 4B, the expression of pcDNA3.1- SSTR5-AS1 and pcDNA3.1-TUBA4B were increased after transfection, suggesting the transfection was successful. Then, the CCK-8 (fig. 5A and fig. 5B) and TUNEL staining (fig. 5C and fig. 5D) assays illustrated that overexpressing of SSTR5-AS1 and TUBA4B could remarkably suppress CNE-1 cell viability but promote apoptosis in vitro.
Fig. 5: CCK-8 and TUNEL staining assays were performed to determine the effects of SSTR5-AS1 and TUBA4B on NPC cell
viability, (A and B): Effects of SSTR5-AS1 on cell viability and apoptosis were assessed by a CCK-8 kit and a TUNEL kit and
(C and D): Effects of TUBA4B on cell viability and apoptosis
Note: **p< 0.01, pcDNA3.1-SSTR5-AS1 vs. pcDNA3.1 and pcDNA3.1-TUBA4B vs. pcDNA3.1 (A) pcDNA3.1-SSTR5-AS1;
pcDNA3.1-SSTR5-AS1; pcDNA3.1;
pcDNA3.1; Control; (B)
Control; (B) pcDNA3.1-TUBA4B; (C)
pcDNA3.1-TUBA4B; (C) pcDNA3.1-SSTR5-AS1 and (D)
pcDNA3.1-SSTR5-AS1 and (D) pcDNA3.1-TUBA4B
pcDNA3.1-TUBA4B
SSTR5-AS1 and TUBA4B have been recently found to be involved in tumor genesis and development in various human cancers. Previous study had reported that lncRNA TUBA4B expression was markedly decreased in lung cancer tissues or cells and may inhibit lung cancer cell progression and metastasis[18]. Downregulated lncRNA TUBA4B and up-regulated SSTR5- AS1 were found in gastric cancer and modulated tumor cell malignant behaviors in vitro[19,20]. Other studies revealed that SSTR5-AS1 and TUBA4B functioned as potential anti-tumor factors in colorectal cancer[21], epithelial ovarian cancer[22], laryngeal squamous cell carcinoma[23] and breast cancer[24]. SSTR5-AS1 and TUBA4B were found to be down-regulated in NPC patients or cells, indicating that SSTR5-AS1 and TUBA4B may be potential therapeutic targets for NPC clinical treatment[13,26]. Consistent with previous finding, SSTR5-AS1 and TUBA4B were significantly decreased in serum samples of NPC patients as compared with healthy controls, as well as in NPC cell lines in the present study.
SSTR5-AS1 and TUBA4B have been reported to function as potential biomarkers in various tumors and higher SSTR5-AS1 was associated with poorer survival rate in patients with gallbladder carcinoma[27]. LncRNA, TUBA4B expression was closely correlated with the pathological grade, FIGO stage and lymph node metastasis in epithelial ovarian cancer[22], while lower TUBA4B expression was associated with a poorer survival rate in patients with lung cancer[17]. In the present study, we investigated the relationship of SSTR5-AS1, TUBA4B and clinicopathological characteristics in NPC. As shown, SSTR5-AS1 and TUBA4B were correlated with the N stage, clinical stage and grade. However, there were no significant correlations among gender, age, Tumor (T) stage,
Metastasis (M) stage, EBV infection or lymph node metastasis. Furthermore, the 5 y survival rate and relapse-free survival rate were followed up. From the results, the 5 y survival rates of NPC patients with lower expression of SSTR5-AS1 or TUBA4B were remarkably lower than that of higher SSTR5-AS1 or TUBA4B expression. Consistent with 5 y survival rates, the relapse-free survival results demonstrated the similar trends. Moreover, the expression levels of SSTR5-AS1 and TUBA4B were markedly increased after chemo-radiotherapy as compared at admission. In vitro experiments demonstrated the similar decreased expression of SSTR5-AS1 and TUBA4B in NPC cell lines. Meanwhile, up-regulation of SSTR5-AS1 and TUBA4B could prominently suppress NPC cell proliferation and promote apoptosis.
However, the present study lacks an in vitro assay to confirm the clinical tumor-suppressive roles of SSTR5- AS1 and TUBA4B in NPC. An RNA-fluorescence in situ hybridization assay was needed to reveal the subcellular location of SSTR5-AS1 and TUBA4B in NPC. Moreover, more assays are required for assessing the effects of SSTR5-AS1 and TUBA4B in NPC cell motility.
In conclusion, our findings elucidated the clinical roles of SSTR5-AS1 and TUBA4B in NPC for the first time. The results confirmed that the tumor-suppressive and prognostic roles of SSTR5-AS1 and TUBA4B in NPC, providing new therapeutic targets for NPC clinical treatment.
Acknowledgments:
We thank the technical support from Affiliated Huaian No.1 People’s Hospital.
Conflict of interests:
The authors have declared no conflict of interest.
References
- Kang Y, He W, Ren C, Qiao J, Guo Q, Hu J, et al. Advances in targeted therapy mainly based on signal pathways for nasopharyngeal carcinoma. Signal Trans Target Ther 2020;5(1):1-20.
[Crossref] [Google Scholar] [PubMed]
- Su L, She L, Shen L. The current role of adjuvant chemotherapy in locally advanced nasopharyngeal carcinoma. Front Oncol 2021;10:585046.
[Crossref] [Google Scholar] [PubMed]
- Liang SB, Chen LS, Chen HY, Yang XL, Wang DH, Cui CY, et al. Prognostic influence of prevertebral space involvement in nasopharyngeal carcinoma: A retrospective study. Radiother Oncol 2021;156:113-9.
[Crossref] [Google Scholar] [PubMed]
- Faisal HH, Kubo N, Nuryadi E, Prihartono J, Atmakusuma TD, Rachmadi L, et al. Patterns of care and outcome analysis of nasopharyngeal carcinoma: An indonesian single institution study. Asian Pac J Cancer Prev 2020;21(5):1481.
[Crossref] [Google Scholar] [PubMed]
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71(3):209-49.
[Crossref] [Google Scholar] [PubMed]
- Xia C, Dong X, Li H, Cao M, Sun D, He S, et al. Cancer statistics in China and United States, 2022: Profiles, trends and determinants. Chin Med J 2022;135(5):584-90.
[Crossref] [Google Scholar] [PubMed]
- He Y, Zhang L, Cheng G, Yuan R, Zhuang Y, Zhang D, et al. Up regulation of circulating miR-21 is associated with poor prognosis of nasopharyngeal carcinoma. Int J Clin Exp Pathol 2017;10(7):7362-8.
[Google Scholar] [PubMed]
- Zhou Y, Xia L, Lin J, Wang H, Oyang L, Tan S, et al. Exosomes in nasopharyngeal carcinoma. J Cancer 2018;9(5):767-7.
[Crossref] [Google Scholar] [PubMed]
- Zhou Z, Zhang L, Xie B, Wang X, Yang X, Ding N, et al. FOXC2 promotes chemoresistance in nasopharyngeal carcinomas via induction of epithelial mesenchymal transition. Cancer Lett 2015;363(2):137-45.
[Crossref] [Google Scholar] [PubMed]
- Fang Y, Fullwood MJ. Roles, functions and mechanisms of long non-coding RNAs in cancer. Genom Proteom Bioinform 2016;14(1):42-54.
[Crossref] [Google Scholar] [PubMed]
- Zhang YX, Yuan J, Gao ZM, Zhang ZG. LncRNA TUC338 promotes invasion of lung cancer by activating MAPK pathway. Eur Rev Med Pharmacol Sci 2018;22(2):443-9.
[Crossref] [Google Scholar] [PubMed]
- Zhang X, Zhang W, Jiang Y, Liu K, Ran L, Song F. Identification of functional lncRNAs in gastric cancer by integrative analysis of GEO and TCGA data. J Cell Biochem 2019;120(10):17898-911.
[Crossref] [Google Scholar] [PubMed]
- Wen X, Tang X, Li Y, Ren X, He Q, Yang X, et al. Microarray expression profiling of long non-coding RNAs involved in nasopharyngeal carcinoma metastasis. Int J Mol Sci 2016;17(11):1956.
[Crossref] [Google Scholar] [PubMed]
- Liu Z, Wu K, Wu J, Tian D, Chen Y, Yang Z, et al. NEAT1 is a potential prognostic biomarker for patients with nasopharyngeal carcinoma. J Cell Biochem 2019;120(6):9831-8.
[Crossref] [Google Scholar] [PubMed]
- Liu ZB, Tang C, Jin X, Liu SH, Pi W. Increased expression of lncRNA SNHG12 predicts a poor prognosis of nasopharyngeal carcinoma and regulates cell proliferation and metastasis by modulating Notch signal pathway. Cancer Biomark 2018;23(4):603-13.
[Crossref] [Google Scholar] [PubMed]
- Zhou L, Liu R, Liang X, Zhang S, Bi W, Yang M, et al. lncRNA RP11-624L4.1 is associated with unfavorable prognosis and promotes proliferation via the CDK4/6-Cyclin D1-Rb-E2F1 pathway in NPC. Mol Ther Nucl Acids 2020;22:1025-39.
- Zhang T, Wu DM, Deng SH, Han R, Liu T, Li J, et al. Integrated analysis reveals that long non-coding RNA TUBA4B can be used as a prognostic biomarker in various cancers. Cell Physiol Biochem 2018;49(2):530-44.
[Crossref] [Google Scholar] [PubMed]
- Chen J, Hu L, Wang J, Zhang F, Chen J, Xu G, et al. Low expression LncRNA TUBA4B is a poor predictor of prognosis and regulates cell proliferation in non-small cell lung cancer. Pathol Oncol Res 2017;23(2):265-70.
[Crossref] [Google Scholar] [PubMed]
- Cheng Y, Di J, Wu J, Shi HT, Zou BC, Zhang Y, et al. Diagnostic and prognostic significance of long noncoding RNA SSTR5-AS1 in patients with gastric cancer. Eur Rev Med Pharmacol Sci 2020;24(10):5385-90.
[Crossref] [Google Scholar] [PubMed]
- Guo J, Li Y, Duan H, Yuan L. LncRNA TUBA4B functions as a competitive endogenous RNA to inhibit gastric cancer progression by elevating PTEN via sponging miR-214 and miR-216a/b. Cancer Cell Int 2019;19(1):156.
[Crossref] [Google Scholar] [PubMed]
- Zhou YG, Sun F, Zhou YF. Low expression of lncRNA TUBA4B promotes proliferation and inhibits apoptosis of colorectal cancer cells via regulating P15 and P16 expressions. Eur Rev Med Pharmacol Sci 2020;24(6):3023-9.
[Crossref] [Google Scholar] [PubMed]
- Zhu FF, Zheng FY, Wang HO, Zheng JJ, Zhang Q. Down regulation of lncRNA TUBA4B is associated with poor prognosis for epithelial ovarian cancer. Pathol Oncol Res 2018;24(2):419-25.
[Crossref] [Google Scholar] [PubMed]
- Wang B, Zhao L, Chi W, Cao H, Cui W, Meng W. Aberrant methylation-mediated downregulation of lncRNA SSTR5-AS1 promotes progression and metastasis of laryngeal squamous cell carcinoma. Epigenetics Chromatin 2019;12(1):1-7.
- Liu AX, Yang F, Huang L, Zhang LY, Zhang JR, Zheng RN. Long non-coding RNA Tubulin Alpha 4B (TUBA4B) inhibited breast cancer proliferation and invasion by directly targeting miR-19. Eur Rev Med Pharmacol Sci 2019;23(2):708-15.
[Crossref] [Google Scholar] [PubMed]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001;25(4):402-8.
[Crossref] [Google Scholar] [PubMed]
- Zhang W, Wang L, Zheng F, Zou R, Xie C, Guo Q, et al. Long noncoding RNA expression signatures of metastatic nasopharyngeal carcinoma and their prognostic value. Biomed Res Int 2015;2015:618924.
[Crossref] [Google Scholar] [PubMed]
- Xue Z, Yang B, Xu Q, Zhu X, Qin G. Long non-coding RNA SSTR5-AS1 facilitates gemcitabine resistance via stabilizing NONO in gallbladder carcinoma. Biochem Biophy Res Commun 2020;522(4):952-9.
[Crossref] [Google Scholar] [PubMed]