- *Corresponding Author:
- Sarasija Suresh
Department of Pharmaceutical Technology (Formulation)
E-mail: sarasija_s@hotmail.com
| Date of Submission | 18 September 2013 |
| Date of Revision | 12 August 2014 |
| Date of Acceptance | 17 August 2014 |
| Indian J Pharm Sci 2014;76(5):415-422 |
Abstract
Curcumin-diclofenac conjugate as been synthesized by esterification of phenolic group of curcumin with the acid moiety of diclofenac, and characterized by mass spectrometry, NMR, FTIR, DSC, thermogravimetric analysis and X-ray diffraction analysis. The relative solubility of curcumin-diclofenac conjugate, curcumin and diclofenac; stability of curcumin-diclofenac conjugate in intestinal extract; permeability study of curcumin-diclofenac conjugate using the everted rat intestinal sac method; stability of curcumin-diclofenac conjugate in gastrointestinal fluids and in vitro efficacy have been evaluated. In vivo bioavailability of curcumin-diclofenac conjugate and curcumin in Sprague-Dawley rats, and antiarthritic activity of curcumin-diclofenac conjugate, curcumin and diclofenac in modified streptococcal cell wall-induced arthritis model in Balb/c mice to mimic rheumatoid arthritis in humans have also been studied. In all of the above studies, curcumin-diclofenac conjugate exhibited enhanced stability as compared to curcumin; its activity was twice that of diclofenac in inhibiting thermal protein denaturation taken as a measure of in vitro antiinflammatory activity; it enhanced the bioavailability of curcumin by more than five folds, and significantly (P<0.01) alleviated the symptoms of arthritis in streptococcal cell wall-induced arthritis model as compared to both diclofenac and curcumin.
Keywords
Curcumin, diclofenac, oral bioavailability, rheumatoid arthritis, drug conjugate
Curcumin, chemically diferuloyl methane, is a yellow polyphenol isolated from the rhizomes of turmeric (Curcuma longa). Although it has been intensively studied and has a plethora of therapeutic benefits [1-3], the exact mechanism of its activity is not fully understood to date. Its potent antioxidant and antiinflammatory activities have been reported to be responsible for its therapeutic activity [4,5]. Despite its various known pharmacological properties, curcumin has not yet been approved as a therapeutic agent, mainly because of its poor oral bioavailability (1% or lower) [6]. Poor solubility, permeability, aqueous stability along with rapid presystemic metabolism has been attributed to the poor bioavailability of curcumin. Preclinical studies have demonstrated that avid sulfation, glucuronidation, and reduction of curcumin occur in the gastrointestinal tracts of rats and humans [7,8].
Curcumin exhibits keto-enol tautomerism, and there are three sites namely, two phenolic and one active methylene group available for attachment of drugs/ ligands to synthesize novel conjugates. Although, curcumin conjugates with different amino acids have been reported to exhibit good solubility and permeability along with enhanced anticancer activity [9], but conjugates of curcumin with antiinflammatory drug molecules has not been reported to date. For this investigation, diclofenac was selected as the drug to synthesize curcumin-diclofenac conjugate (CDC) because of its high permeability, which could potentially increase the permeability of curcumin. Additionally, conjugation with diclofenac could provide stability to the curcumin molecule in the gastrointestinal tract because of steric hindrance in CDC, and may thereby prevent its intestinal metabolism.
Rheumatoid arthritis (RA) is a chronic autoimmune inflammatory disease where both cell-mediated and humoral immune reactions participate in its pathogenesis [10]. Diclofenac has been used as the firstline treatment for arthritis, especially for RA as it ameliorates the symptoms of pain by inhibiting the inflammatory pathway catalyzed by cyclooxygenase 2 (COX-2) without halting its progression. Curcumin is a potent antiinflammatory agent which has the potential to halt RA as it is known to directly interact with COX-2 and lipoxygenase (LOX), the primary mediators of inflammation. In addition, curcumin is reported to indirectly down-regulate the various transcription factors responsible for inflammation, particularly (NF)-κB. Further, curcumin suppresses proinflammatory mediators and cytokines and downregulates (NF)-κB; and suppresses the genetic targets of (NF)-κB implicated in RA and thus regulates humoral and cellular immune responses and delays the occurrence oralleviates the severity of RA [11,12]. CDC has the potential to decrease or mitigate the side effects of long term non steroidal antiinflammatory drugs (NSAID) treatment because of the inherent gastro-protective actions of curcumin.
In view of the above, synthesis of curcumin diclofenac via a chemical bond to form CDC appears to be a novel approach to create new drug-drug conjugates with complementary therapeutic activities to obtain substantially enhanced activities of either one or both e.g. in this case CDC would be highly beneficial for the treatment of RA. We report a novel drug-drug conjugate approach, adopted to increase the bioavailability of curcumin hence its enhanced antiinflammatory activity.
Materials and Methods
Diclofenac sodium was a gift sample from Indswift Pvt. Ltd. Panchkula, India. Curcumin, dicyclohexylcarbodiimide, 1,4-dioxane, triethylamine and 4-(dimethylamino)pyridine (DMAP) were obtained from Sigma-Aldrich, Mumbai, India, and 1-ethyl-(3- dimethylaminopropyl) carbodiimide hydrochloride (EDAC) was obtained from HiMedia Laboratories Pvt. Ltd. Mumbai, India. Di-tert-butylpyrocarbonate (t-BOC) was procured from Spectrochem Pvt. Ltd. Mumbai, India. Streptococcus mutans strain (MTCC No. 890) was obtained from Institute of Microbial Technology (IMTech), Chandigarh, India. The animal experiments were performed in accordance with Institutional Animal Ethics Committee (IAEC) following NIPER guidelines (108/1999/CPCSEA). The animals for the studies were provided by the central animal facility (CAF), NIPER, Mohali, India. Solvents were purchased from Fisher Scientific India Pvt. Ltd. Mumbai, India and used as such without any further purification.
Synthesis of CDC
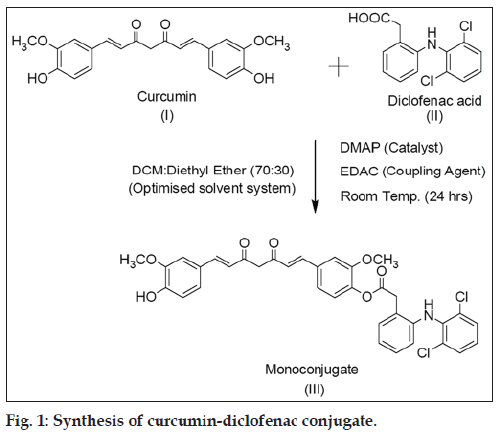
To a solution of curcumin (5 g, 13.6 mmol) in dichloromethane:ethyl ether (70:30, 500 ml) an equimolar concentration of DMAP was added, and the resultant mixture was stirred for 30 min. To the above mixture, diclofenac (4.83 g, 16.3 mmol) and EDAC (20.4 mmol) was added, and it was stirred for another 24 h. The progress of reaction was monitored by thin layer chromatography (TLC) using hexane:ethyl acetate (55:45) mixture as an eluent. After completion of the reaction, the reaction mixture was washed with 5% HCl and concentrated. The crude product was purified by column chromatography using silica gel (60-120 mesh, Merck) as the stationary phase and hexane:ethyl acetate (55:45) mixture as the mobile phase. The fractions containing the monoconjugated were collected, concentrated and dried to obtain CDC in an isolated yield of 60%. 1H NMR (CDCl3): δ 3.75 (s, 3H), 3.94 (s, 3H), 4.09 (s, 2H), 5.87 (d, 2H), 6.47 (d, 2H, J=16.4 Hz), 6.58 (d, 1H, J=8.04 Hz), 6.73 (s, 1H), 6.98 (m, 3H), 7.06 (m, 3H), 7.15 (m, 3H), 7.34 (m, 3H), 7.57 (d, 1H, J=7.2 Hz), 7.61 9d, 1H, J=6.92 Hz).
Analysis and characterization of CDC
CDC mass was confirmed in a mass spectrometer used in LCQ (positive mode) mode (Finnigan MAT system with Xcaliber software system). 1H NMR obtained in CDCl3 using ZH079807 Bruker 400 UltraShieldTM spectrophotometer (with Bruker B-ACS120 Autosampler spectrophotometer and Topspin 2.7 software) gave peaks in accord with CDC structure, which was further confirmed by Infrared spectroscopy (IR) using Perkin Elmer Synthesis Monitoring System FTIR (with Spectrum-1 software). Comparative and solid-state studies were also performed using differential scanning calorimetry (DSC), thermogravimetric analysis (TGA) and X-ray diffractometry (XRD) techniques. DSC behaviour was analyzed in Pyris™ Diamond DSC (Perkin Elmer) having STARe software. TGA was performed in GA/SDTA851 (Mettler Toledo) with Pyris Manager Software system. XRD was studied in AXS D8 (Bruker) with solid-state detector and XRD commander experiment software with Eva XRD evaluation software, and the temperature of instrument was maintained at 18-22° while sample temperature was at room temperature.
HPLC method development
HPLC methods for quantitative analysis of curcumin, diclofenac and CDC were developed and validated. For curcumin and CDC, the analysis was performed in a Shimadzu (Kyoto, Japan) HPLC system with LC-10AS pump and RF-10 AXL fluorescence detector at excitation wavelength of 420 nm and emission wavelength of 530 nm. Phenomenex Luna® C18 100Å LC column (250×4.6 mm of 5 μm pore size), acetonitrile and water mixture (adjusted to pH 3.4 with acetic acid) in a 95:5 (v/v) ratio as the mobile phase, flow rate of 1 ml/min, injection volume of 10 μl and column temperature at 25° were the other parameters employed in the analysis. Diclofenac was estimated with SPD-M20A PDA detector at λmax of 280 nm with the mobile phase composed of a mixture of ACN and water (adjusted to pH 3.4 with acetic acid) in a ratio of 55:45 (v/v). Other parameters were unaltered and were akin to the analysis of curcumin and CDC. Above methods were validated as per ICH guidelines for linearity, precision and accuracy.
Relative solubility study
The relative solubility of CDC, curcumin and diclofenac were determined by adding excess solute to two ml of isopropyl myristate (IPM) in a screwcapped bottle which was shaken in a shaker water bath at 37° and 100 rpm for 24 h. The samples were then centrifuged at 20 000 rpm for 10 min. The supernatant layer was diluted with methanol and analyzed by HPLC to determine the solute concentration. Further, the supernatant was mixed with an equal volume of water, followed by vigorous shaking and the mixture was allowed to settle. The CDC concentration in IPM layer was quantified, and the partition coefficient (K) was calculated by substituting the obtained values in Eqns. 1 and 2, K=Aa/(Ab–Aa)(VWater/VIPM) and Swater=SIPM/K, where, K the partition co-efficient in IPM-water, Aa area under the curve after partitioning, Ab area under the curve before partitioning, Vwater volume of water, VIPM volume of IPM taken for the study, Swater and SIPM is CDC solubility in water and SIPM is CDC solubility in IPM. All of the above analyses were performed in triplicates.
Enzymatic hydrolysis study
CDC stability was studied in the presence of small intestinal tissue extract [13]. Sprague-Dawley (SD) rats of 180-200 g and 6-7 w old were fasted overnight with free access to water. Small intestinal segments (about 30 cm from the end of the stomach) were collected, rinsed with ice-cold solution of 0.9% NaCl supplemented with 3% glucose, and homogenized in tissue homogenizer (Ultra-Turrax, IKA). The homogenate was centrifugedat 22 000 rpm for 30 min at 4° and the supernatant was collected. An aliquot of the supernatant (5 ml) was incubated with CDC solution (45 μg) at 37° and sampled periodically and analyzed by HPLC for CDC and curcumin content. All analyses were performed in triplicates.
In vitro permeability study
The permeability of CDC was determined by adopting the everted rat sac method. An indigenously designed permeability apparatus was developed for this study. The apparatus consisted of two cylindrical glass tubes; one (110×16 mm) attached via J-shaped tubing to the second one (50×16 mm) and connected at the top with provision for attaching intestinal segments at the base. The whole setup was placed into a beaker with constant aeration and agitation. The lumen of everted intestinal sac represents the serosa while beaker represents the mucosal compartment [14].
Small intestinal segments 5-6 cm distal to the ileocaecal junction were collected, washed with modified Krebs-Henseleit buffer, and carefully evertedand mounted on the permeability apparatus. The apparatus was then dipped in a solution of 170 μM CDC in modified Krebs-Henseleit buffer while a plain buffer was added into the lumen. Samples were withdrawn from the lumen at specific time intervals and amount of CDC quantified by HPLC [15].
Curcumin was detected in the lumen while CDC was undetectable, and the permeability of curcumin was calculated by using Eqn. 3, Papp=(dQ/dt)/(C0.A), where, dQ/dt cumulative transport rate defined by the slope obtained after linear regression of cumulative amount transported as a function of time, C0 initial concentration on the donor side (beaker), A=surface area of the intestinal sac.
Stability study
The stability of CDC (2 μg/ml solution) in pH 1.2, pH 7.4 buffers, simulated gastric fluid (SGF) and intestinal fluids (SIF) were investigated. The solutions were stirred at 37° and sampled at 0, 15, 30, 45, 60, 90, 120, 180, 240 and 300 min and CDC concentration quantified by HPLC.
In vitro efficacy study
The solutions (2.7 μM) of diclofenac, curcumin and CDC were separately prepared containing 15% of methylated PEG (M.W. 550). Each solution was added to three ml of bovine serum albumin solution (10 %) and incubated at 27±1° for 15 min. The solution mixtures were heated to 70° for 10 min to effectuate denaturation. The solutions were cooled, and turbidity was determined spectrophotometrically at 660 nm. The extent of inhibition (%) was calculated by comparing with negative control and substituting in Eqn. 4, % inhibition=(A0–AT)/A0)×100, where, A0 is the absorbance of albumin solution without the added drug component, AT is the absorbance of albumin in presence of the drug component.
Bioanalytical method development
HPLC method for bioanalysis was developed as described earlier under development of HPLC method of analysis. A calibration curve was obtained in the range of 2.5-1000 ng/ml (r2 of 0.996). Briefly, an aliquot (180 μl) of plasma was mixed with 50 μl of internal standard solution (rhodamine 6G, 600 ng/ ml) and 20 μl of curcumin solution. The solution was vortexed for 60 s, 200 μl of acetonitrile was added and vortexed further for 2 min. The mixture was centrifuged at 10 000 rpm for 7 min. An aliquot of the supernatant (50 μl) was analyzed by HPLC. The mobile phase was composed of a mixture of ACN:THF and water in a ratio 42:41:17, respectively. The flow rate was maintained at 1 ml/min, and detection wavelengths of 420 nm and 530 nm.
Estimation of pharmacokinetic parameters
The bioavailability of CDC and curcumin were estimated in Sprague-Dawley rats. The animals were distributed randomly into two groups containing six animals each. Curcumin (650 mg/ kg) and CDC (150 mg/kg) suspensions in 0.5% CMC were administered by oral gavage [16]. Blood samples (~0.5 ml) were withdrawn at different time intervals from the retro-orbital plexus under mild ether anaesthesia into heparinised micro centrifuge tubes (containing 50 μl of 1000 U of heparin). The blood samples were centrifuged at 10 000 rpm for 7 min at 15° to separate plasma. Curcumin was extracted from plasma as described earlier. Briefly, internal standard 50 μl (600 ng/ml of rhodamine G) was added to 180 μl of plasma and vortexed for 60 sec. ACN (220 μl) was added, and the sample was vortexed further for 2 min and centrifuged for 5 min at 10 000 rpm. The amount of curcumin present in the supernatant was quantified by HPLC.
Pharmacodynamic study
Balb/c mice of body weight in the range of 15-20 g were selected in order to develop an animal model of antigen-induced arthritis. Arthritis was induced by intravenous injection of modified Streptococcus mutans cell wall extract (SCW) pretreated with proteolytic enzymes [17-19]. SCW was prepared from freeze-dried Streptococcus mutans, which was revived on Brain Heart Infusion Agar by incubating (Innova®44 shaker) for 48 h at 37°. The revived culture was subcultured thrice, sequentially, followed by production of a large batch (Innova®44 Shaker) under the same conditions. The cells were harvested as a pellet after centrifugation at 10 000 rpm for 20 min. The pellet was washed with double distilled water, and resuspended 1 g (wet weight) in 15 ml of water. The cell suspension formed was subjected to vigorous agitation in a DYNO® Mill (Multilab, WAB, Germany) containing glass beads (0.1 mm in diameter) rotated at 1000 rpm. The disrupted cell wall suspension was diluted and centrifuged at 10 000 g for 20 min to obtain the crude cell wall fraction. The cell wall fraction was successively washed with NaCl (1M) and water and digested with pepsin (2000 U/g), washed thoroughly with water and lyophilized.
For determining antirheumatic activity of diclofenac, curcumin and CDC, the animals were distributed randomly into six groups each consisting of six animals. Group 1: control; Group 2: oral diclofenac 50 mg/kg/day; Group 3: oral curcumin 62.50 mg/kg/day; Group 4: oral diclofenac 50 mg/kg+oral curcumin 62.50 mg/kg/day; Group 5: oral CDC 107.1 mg/kg/day; Group 6: i.v. CDC 1 mg/kg/day.
The administration of drug was started seven days prior to the induction of arthritis and continued until completion of the study. The duration of study was fourteen days. Arthritis was induced by injecting modified SCW extract intravenously (dose of 10 mg/kg) on the eight day of the study. The animals were observed for changes in joint thickness, tail thickness, body weight and mobility while the test samples were continued to be administered until the fourteenth day.
Results and Discussion
CDC was synthesized by conjugation of curcumin with diclofenac free base at room temperature (fig. 1) in an isolated yield of 60% after purification. The proton NMR was in accord with the structure of mono-conjugated compound (see experimental section). The mass spectrum showed base peak corresponding to CDC at m/z ratio of 647.91, with positive mode of ionization but very little fragmentation was observed. The main fragment was at m/z value of 369.46 corresponding to curcumin. The proton NMR data was in addition supported by COSY and HSQC analysis (data not shown). The IR analysis, furthermore, confirmed the structure as it showed peaks corresponding to C=O stretch at 1747.34 cm-1, N-H stretch at 3358.17 cm-1, aliphatic C-H stretch at 2931.90 cm-1, C=C stretch at 1588.14 cm-1 and C-Cl bending vibrations at 745.10 cm-1. Powder XRD (P-XRD) patterns for curcumin and diclofenac supported the presence of crystalline structures (showing presence of defined peaks) for both compounds while a hollow pattern was observed for CDC indicating an amorphous structure.
Standard calibration curves constructed with seven concentrations of curcumin (in the range of 1-300 ng/ml), eight concentrations of CDC (in the range of 1-1000 ng/ml) and eight concentrations of diclofenac (within the range of 0.1-100 μg/ml) gave good linearity with correlation coefficients in the range 0.989 to 0.999 for all the above compounds. The other validation parameters obtained according to ICH guidelines are listed in Table 1.
| Parameters | Specifications | Bioanalytical Curcumin | ||
|---|---|---|---|---|
| Curcumin | Conjugate | Diclofenac | ||
| Linearity range | 1-300 ng/ml | 1-1000 ng/ml | 0.1-100 µg/ml | 2.5-1000 ng/ml |
| R2 | 0.9999 | 1.0000 | 0.9998 | 0.9963 ± 0.0013 |
| Slope | 44752.2 ± 228.07 | 9879.58 ± 126.84 | 25245.66 ± 308.20 | 0.00686 ± 0.0001 |
| Intercept | 13297.48 ± 12475.62 | 16563.32 ± 6139.71 | 5145.28 ± 2244.11 | 0.11698 ± 0.006 |
| LOD | 0.92 ng/ml | 2.05 ng/ml | 0.29 µg/ml | 2.79 ng/ml |
| LOQ | 2.79 ng/ml | 6.22 ng/ml | 0.89 µg/ml | 8.44 ng/ml |
Table 1: Development Of In Vitro And Bioanalytical Method
The descending order of relative solubility at pH 3 was: curcumin>CDC>diclofenac. The data is given in Table 2. Ester prodrugs and ester linked conjugates are known to undergo hydrolysis during thermodynamic solubility studies [20]; therefore, relative solubility of curcumin and CDC was studied at pH 3, at which curcumin is found to be most stable [21]. The decreased solubility of CDC when compared to curcumin can be attributed to an increase in its molecular size and loss of the hydrophilic carboxylic acid moiety.
| Drug | Curcumin | Conjugate | Diclofenac |
|---|---|---|---|
| Water | 3.034 ± 0.51 mg/ml | 1.277 ± 0.44 mg/ml | 0.598 ± 0.06 mg/ml |
| solubility | |||
| IPM | 3.894 ± 0.17 mg/ml | 13.76 ± 0.31 mg/ml | 4.210 ± 0.05 mg/ml |
| solubility | |||
| Partition | 1.304 ± 0.18 | 11.528 ± 3.28 | 7.110 0.94 |
| coefficient |
Table 2: Relative Solubility and partition Coefficient of curcumin, diclofenac and cdc
The effect of small intestinal tissue extract on CDC was studied. The concentration of CDC decreased exponentially with time, and the plot of percentage of CDC concentration remaining Vs. time (in min) is presented in Table 3. CDC concentration decreased rapidly with less than 15% remaining at 15 min. This reduction may be attributed to hydrolysis of CDC by the enzymes present in the intestinal extract to liberate curcumin and diclofenac molecules. The potential site of hydrolysis of CDC may be present either on the surface or within the intestinal membrane. Hydrolysis of CDC could have resulted in a uniform concentration gradient of curcumin between the intestinal lumen and cells resulting in enhanced bioavailability of curcumin.
| Time (h) | Amount (%) |
|---|---|
| 0 | 100 |
| 1 | 36.7 ± 3.07 |
| 15 | 13.1 ± 1.64 |
| 30 | 11.7 ± 0.67 |
| 45 | 11.5 ± 0.62 |
| 60 | 10.9 ± 1.30 |
| 90 | 10.4 ± 0.67 |
| 120 | 9.5 ± 0.67 |
The cumulative rate of curcumin transported was calculated from the slope of the linear regression line obtained when curcumin was plotted against a function of time. The rate obtained was 0.19 μM/min. The permeability coefficient obtained was ‘2.66±0.43×10-6 cm/sec, for curcumin and was comparable to its reported permeability of ‘2.93±0.94×10-6 cm/sec, in Caco-2 cell line.
In vitro permeability study concluded that permeability of curcumin per se has not been altered in CDC. However, increased stability of CDC in the gastrointestinal tract followed by its subsequent hydrolysis, possibly by intestinal esterases present in the intestinal lining, to release the curcumin molecules has the potential to enhance the bioavailability of curcumin.
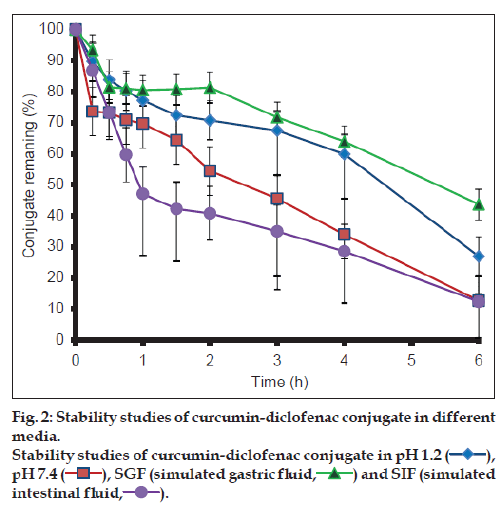
The results from the stability studies are shown in fig. 2. CDC exhibited greater stability at pH 1.2 and in SGF in comparison to basic medium. It was observed more than 90% of curcumin degraded within one hour in the basic conditions, while only 20% of CDC degraded under the similar conditions.
CDC was found to be more stable in acidic environment than in basic environment (fig. 2) as compared to curcumin. Although esters are labile to hydrolysis both in the acidic and basic medium, CDC appears to undergo predominantly base hydrolysis. This can be attributed to lower stability of curcumin in the basic medium as compared to acidic medium. Enhanced stability of curcumin in the basic medium may thus be attributed to steric hindrance by diclofenac molecule in the conjugate.
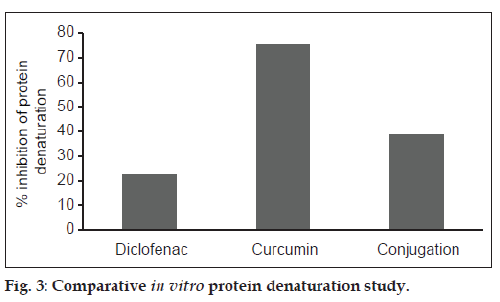
The maximum inhibition of thermal protein denaturation followed the order: Curcumin>CDC>diclofenac, and is shown in fig. 3. Protein denaturation study was adopted as a simple in vitro method for comparing the in vitro efficacy of CDC with diclofenac and curcumin. Denaturation of proteins is a well known cause of inflammation and arthritis and many antiinflammatory drugs have been known to inhibit thermally-induced protein denaturation. The inhibition of protein denaturation is reported to be proportional to the antiinflammatory action of the drug. The maximum inhibition of thermal protein denaturation of curcumin may be due to its high affinity for albumin binding. Although, CDC exhibited less inhibition of thermal protein denaturation in comparison to curcumin, the extent of its inhibition was observed to be twice that of diclofenac.
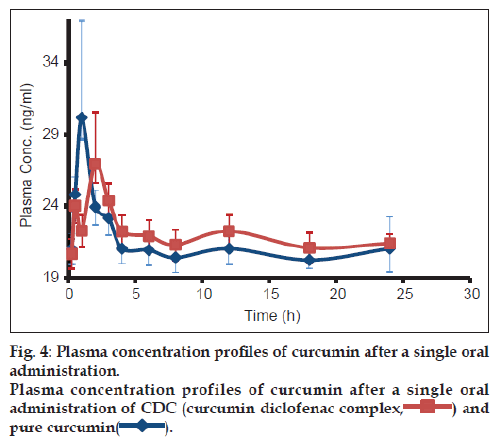
The plasma concentration vs. time profiles in rats, of curcumin following single oral administration of CDC (150 mg/kg) and CRM (650 mg/kg), are shown in fig. 4. The pharmacokinetic parameters were estimated by one compartmental analysis of the experimental data. The peak plasma concentration (Cmax) of curcumin obtained was 31.64±2.6 ng/ml. The mean values of AUC0-∞ and half-life (t1/2) of curcumin were 6.67±4.17 mg/ml/h and 212.6±137.5 h, respectively. CDC exhibited Cmax of 28.93±1.49 ng/ml, with AUC0-∞ and half-life (t1/2) of 4.49±0.16 mg/ml/h and 132.62±51 h, respectively. On comparing the AUC0-∞ values of both curcumin and CDC ~ five times enhancement in bioavailability of curcumin was observed in CDC.
Inflammation and loss of mobility are considered as the markers of arthritis and were observed within two hours of i.v. injection of modified SCW extract. Peak symptoms were observed on day ten when all investigations of the study were conducted. Arthritis was graded using in-house modified Pearson and Wood’s method [22]. The parameters fixed were thickness of the four ankle joints (fore limbs and hind limbs) along with the thickness of the tail. The maximum swelling of the joint was given a score of 4 and minimum swelling was given a score of 1. All animals were scored from 5 to 20 score points to indicate the severity of arthritis. Thus, the data was indicative of the comparative measure of severity of arthritis in different groups (Table 4).
| Group | Treatment | Total scorea (mean ± SD) | Difference in weightb (mean ± SD) | Mobilityc | Appetited |
|---|---|---|---|---|---|
| I | Control | 19.17 ± 0.75 | 0.55 ± 0.15 | Very poor | Poor |
| II | Curcumin | 18.33 ± 1.21 | 0.3 ± 0.15 | Very poor | Poor |
| III | Diclofenac | 14.33 ± 1.03 | 0.17 ± 0.18 | Moderate | Normal |
| IV | Curcumin and diclofenac | 15.33 ± 2.25 | 0.17 ± 0.12 | Moderate | Normal |
| V | CDC (oral) | 8.00 ± 2.19 | −0.017 ± 0.09** | Good | Normal |
| VI | CDC (IV) | 7.00 ± 2.12 | 0.04 ± 0.18** | Good | Normal |
Table 4: Evaluation Of Antiarthritic Activity Of Curcumin, Diclofenacand Cdc In Balb/C Mice
Animals in the control group exhibited maximum symptoms of arthritis, along with animals in Group 2 (curcumin administered) exhibiting symptoms similar to the control group potentially indicating the inefficacy of curcumin alone in alleviating symptoms of arthritis. In Group 3 (diclofenac administered), the symptoms of arthritis were significantly less severe when compared to the control group and results in Group 4 (curcumin and diclofenac-physical mixture administered) were also comparable to Group 3. In Groups D and E (CDC administered), the severity of signs of arthritis were significantly reduced as compared to all other groups. This was further confirmed by the comparison of other parameters such as mobility, quantity of food consumed and effect of treatment on body weights of the animals - various parameters used as measure of arthritis severity in all groups from Day 8 to 13.
The dose of CDC administered in the bioavailability study was based on the equivalent dose of diclofenac at 70 mg/kg. The amount of curcumin present in CDC (80 mg/kg) was considerably less than pure curcumin (650 mg/kg). However, AUC of CDC was significantly enhanced. This enhancement in bioavailability of CDC can be attributed to: (a) higher stability of CDC in the GIT, (b) affinity of CDC for esterases present in intestinal lining and (c) a possible decrease in the presystemic metabolism of curcumin before absorption in GIT.
The severity of arthritis symptoms, including inflammation and loss of mobility, were least prevalent in CDC administered group. The reduction in body weight was observed in all the groups tested by administration of the antigen, followed by the weight gain after treatment. Maximum weight gain was observed within the group administered with CDC indicative of recovery after the initial weight loss. Thus, the enhanced activity of CDC can be attributed to the synergistic activities of curcumin and diclofenac and the increase in bioavailability of curcumin.
Conjugation as an important strategy has been adopted resulting in a large number of drug molecules with enhanced solubility, stability, permeability, bioavailability, organoleptic properties and in vivo performance of small and large molecules. In our study, drug-drug conjugate was adopted as a novel approach to synthesize CDC to increase the bioavailability of curcumin, enhance the therapeutic benefit of diclofenac and in addition, the inherent beneficial effects of curcumin in the GIT has the potential to alleviate the side effects and severity of arthritis treatment. The enhanced bioavailability of curcumin in CDC was by over five folds can be attributed to the improved stability of CDC, and affinity of CDC to the intestine bound enzymes. CDC also exhibited better antiinflammatory activity in modified SCW model of arthritis in Balb/c mice. Thus, conjugation of CRM with drug molecules could be a novel strategy for improvement of its bioavailability and for potentiating its numerous pharmacological activities.
References
- Epstein J, Sanderson IR, MacDonald TT. Curcumin as a therapeutic agent: The evidence from in vitro, animal and human studies. Br J Nutr 2010;103:1545-57.
- Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as ‘‘Curcumin’’: From kitchen to clinic. Biochem Pharmacol 2008;75:787-809.
- Duvoix A, Blasius R, Delhalle S, Schnekenburger M, Morceau F, Henry E, et al.Chemopreventive and therapeutic effects of curcumin. Cancer Lett 2005;223:181-90.
- Jayaprakasha G, JaganmohanRao L, Sakariah K. Antioxidant activities of curcumin, demethoxycurcumin and bisdemethoxycurcumin. Food Chem 2006;98:720-4.
- Kohli K, Ali J, Ansari M, Raheman Z. Curcumin: A natural antiinflammatory agent. Indian J Pharmacol 2005;37:141.
- Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: Problems and promises. MolPharmaceut 2007;4:807-18.
- Ireson CR, Jones DJ, Orr S, Coughtrie MW, Boocock DJ, Williams ML, et al. Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine. Cancer Epiderm Biomar 2002;11:105-11.
- Vareed SK, Kakarala M, Ruffin MT, Crowell JA, Normolle DP, Djuric Z, et al. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol Biomarkers Prev 2008;17:1411-7.
- Mishra S, Narain U, Mishra R, Misra K. Design, development and synthesis of mixed bioconjugates of piperic acid–glycine, curcumin–glycine/alanine and curcumin–glycine–piperic acid and their antibacterial and antifungal properties. Bioorg Med Chem 2005;13:1477-86.
- Venkatesha SH, Yu H, Rajaiah R, Tong L, Moudgil KD. Celastrus-derived celastrol suppresses autoimmune arthritis by modulating antigen-induced cellular and humoral effector responses. J Biol Chem 2011;286:15138-46.
- Moon DO, Kim MO, Choi YH, Park YM, Kim GY. Curcumin attenuates inflammatory response in IL-1β-induced human synovial fibroblasts and collagen-induced arthritis in mouse model. Int Immunopharmacol 2010;10:605-10.
- Venkatesha SH, Berman BM, Moudgil KD. Herbal medicinal products target defined biochemical and molecular mediators of inflammatory autoimmune arthritis. Bioorg Med Chem 2011;19:21-9.
- Masaki K, Taketani M, Imai T. First-pass hydrolysis of a propranolol ester derivative in rat small intestine. Drug Metab Dispos 2006;34:398-404.
- Dixit P, Jain DK, Dumbwani J. Standardization of an ex vivo method for determination of intestinal permeability of drugs using everted rat intestine apparatus. J Pharmacol Toxicol Methods 2012;65:13-7.
- Wahlang B, Pawar YB, Bansal AK. Identification of permeability-related hurdles in oral delivery of curcumin using the Caco-2 cell model. Eur J Pharm Biopharm 2011;77:275-82.
- Sintara K, Thong-Ngam D, Patumraj S, Klaikeaw N, Chatsuwan T. Curcumin suppresses gastric NF-κB activation and macromolecular leakage in Helicobacter pylori-infected rats. World J Gastroenterol 2010;16:4039.
- Li X, Bradford BU, Dalldorf F, Goyert SM, Stimpson SA, Thurman RG, et al. CD14 mediates the innate immune responses to arthritopathogenic peptidoglycan-polysaccharide complexes of Gram-positive bacterial cell walls. Arthritis Res Ther 2004;6:R273-81.
- Kannan K, Ortmann RA, Kimpel D. Animal models of rheumatoid arthritis and their relevance to human disease. Pathophysiol 2005;12:167-81.
- Koga T, Kakimoto K, Hirofuji T, Kotani S, Ohkuni H, Watanabe K, et al. Acute joint inflammation in mice after systemic injection of thecell wall, its peptidoglycan, and chemically defined peptidoglycan subunits from various bacteria. Infect Immun 1985;50:27-34.
- Majumdar S, Mueller-Spaeth M, Sloan KB. Prodrugs of Theophylline Incorporating Ethyleneoxy Groups in the Promoiety: Synthesis, Characterization, and Transdermal Delivery. AAPS Pharmscitech 2012;13:853-62.
- Pfeiffer E, Höhle S, Solyom AM, Metzler M. Studies on the stability of turmeric constituents. J Food Eng 2003;56:257-9.
- Pearson CM, Wood FD. Studies of polyarthritis and other lesions induced in rats by injection of mycobacterial adjuvant. I. General clinical and pathologic characteristics and some modifying factors. Arthritis Rheum 1959;2:440-59.