- *Corresponding Author:
- A. D. Khan
Department of Pharmacy, Ram-Eesh Institute of Vocational and Technical Education, Greater Noida, Uttar Pradesh 201310, India
E-mail: azhardk@gmail.com
| Date of Received | 04 February 2021 |
| Date of Revision | 14 July 2021 |
| Date of Acceptance | 03 March 2022 |
| Indian J Pharm Sci 2022;84(2):247-260 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Wound healing process is a natural phenomenon but it is found to be delayed due to microbial infection. In addition, the formation of a biofilm further makes the condition more complex. The condition may become worse and may lead to necrosis and gangrene. The treatment may involve systemic antibiotics which may be required in high dose and also suffer the problem of antibiotic resistance. Topical antibiotics or antiseptics are successfully used but they are found to have various adverse effects although they have no issue of any resistance. Since early times mankind has a significant belief on natural substances for curing many ailments. Similarly, various plant parts have been used traditionally for treating wounds and also studied for wound healing activities. These phytoextracts can be used to formulate into various dosage forms to be applied topically on the wound area. These phytoconstituents work by complex mechanisms with minimal side effects. Apart from the conventional dosage forms, novel drug delivery systems are being developed for incorporating these herbal constituents and delivering them to the wound area in a more targeted and sustained manner. This review deals with the various herbal topical dosage forms with special focus on novel drug delivery systems for wound healing.
Keywords
Wound healing, herbal, novel drug delivery, phytoconstituents, chronic wounds
Numerous drug delivery systems have been developed to minimize the drug degradation process to minimize harmful side-effects and to enhance the drug bioavailability. Various new drug delivery systems have been employed like liposomes, phytosomes, microspheres, nanoparticles, nano-emulsions and nanofibers scaffolds. These drug delivery systems utilize various polymers or carriers to release the drug in a targeted and controlled way. By targeting the drug is directed to the site of interest. The main reasons for the popularity of herbal medicines are their safety, effectiveness and better result with minimal side effects[1]. Phytopharmaceuticals are compounds which employ natural compounds derived from plants in place of synthetic chemicals. As they are easily metabolized in the body therefore, they produce fewer side effects and are absorbed in a better thus are very effective as a treatment. Pharmaceuticals obtained from chemical compounds show more adverse side effects. The human body rejects these chemical compounds from synthetic source in the form of adverse effects which are usually and sometimes may be lethal. These phytoconstituents if isolated as a single and purified compound can be easily incorporated in novel drug delivery system[2]. Wound is process which involves multiple mechanisms, the use of systemic antibiotics has declined due to drug resistance. The topically used antibiotics and antiseptics are also associated with adverse effects. Due to increase in antibiotic resistance and other adverse effects, a dramatic shift has been seen to study herbal drugs as a potential approach for wound healing. But these drugs when used as conventional dosage forms limit their effectiveness and decreased bioavailability. So, there is need of such a drug delivery system which may control the pharmacokinetics, pharmacodynamics, non-specific toxicity and effectiveness. These new strategies are called as Novel Drug Delivery Systems (NDDS), work by various interdisciplinary disciplines which are a combina-tion of polymer science, pharmaceutics, bio-conjugate chemistry and molecular biology[3]. This NDDS solve the problems which are associated with conventional delivery systems of herbal drugs. They also eliminate various disadvantages associated with the conventional therapy. The main aim of any NDDS for wound healing is the quick healing of the skin so that it may regain its functional and aesthetic value as well as reduce pain and inflammation[4]. The deep understanding of the pathophysiology of wounds has resulted in the development of various novel drug delivery systems which may not only provide physical protection to the wound but also maintain adequate moisture in the wound area[5].
Wound
The skin is the largest organ of the human body. Its main function is to protect the body from external factors and it is called as first line of defense[6]. The two other functions which are associated with the mechanism of defense are sensation and regulation. Skin is involved in protecting us from unwanted pressure and impact. It also minimizes the influence of temperature and radiation. It also protects us from potential microorganism. The extensive network of nerve cells also helps us to detect changes in temperature, touch and pain. The integrity of skin is very pivotal for a healthy body[7]. A wound is a damage of living tissue or disruption in the epithelial cells of the top most layer of the skin. A wound may bring about the opening and break down of the skin and it may also cause disturbance to the anatomical and physiological functions of the skin[8]. Wound healing is a natural phenomenon which takes place by replacing the damaged cells and skin layers. This process involves various complex mechanisms.
Complications associated with wound healing:
The non-healing or the chronic wounds have a significant effect on millions of people every year and also remarkably contribute to their morbidity and mortality. These wounds lead to economic burden, low quality of life and increased risk of death for those who experience these non-healing wounds[9]. A study was conducted in 2014 by a US Medicare company which revealed a data that around 15 % or 8.2 million Medicare beneficiaries are affected by chronic nonhealing wounds and related complications. An annual cost between 28.1 billion dollars and 31.7 billion dollars was estimated by this study for curing these wounds[10]. According to a study in India the prevalence of acute and chronic wounds was 10.55 and 4.48 per 1000 of the population respectively[11]. The most common complications associated with wound healing are infection, osteomyelitis, tissue necrosis, gangrene, oedema and periwound oedema[12-15].
Treatment Strategies for Wound Healing
Antimicrobial agents:
As infection is a significant cause of delay in wound healing the main strategy for curing chronic wounds is the use of aseptic techniques and use of antimicrobial agents[16]. Antimicrobial agents especially systemic antibiotics are now being less used due to increased antimicrobial resistance cause by antimicrobial abuse[17]. Therefore, topical antimicrobials are gaining importance in the treatment for wound healing[18]. Topical antimicrobial agents can be antiseptics and antibiotics. They can be directly applied to the wound site at high concentration. Topical antiseptics have multiple mechanisms of action and have broad spectrum of antimicrobial activity. They hardly suffer from any resistance. Topical antibiotics have a narrow spectrum but are found less cytotoxic than antiseptics. But these synthetic topical agents have various local and systemic adverse effects reported but still fewer than systemic agents. The local effects include pain, rash cytotoxic effects on the cells involved in wound healing process. These effects occur at the application site. The systemic effects may include toxicity to liver, kidney and other organs[19]. Some of the reported adverse effects of topical antimicrobials and antiseptics are mentioned in Table 1[20-31].
| S.No | Antimicrobial agent | Adverse effects | Reference |
|---|---|---|---|
| Antiseptics | |||
| 1 | Chlorohexidine | Cytotoxicity | [20] |
| 2 | Silver nitrate | Stains black, effects mitochondrial function | [21] |
| 3 | Silver nanoparticles | Toxicity to brain, lungs kidney and reproductive system | [22] |
| 4 | Povidone iodine | Cytotoxicity, hyperthyroidism or hypothyroidism | [23,24] |
| Antibiotics | |||
| 5 | Bacitrain | Allergic contact reaction, hypersensitivity | [25] |
| 6 | Mupirocin | Cytotoxicity, burning and itching | [26] |
| 7 | Neomycin | Renal and auditory toxicity, hypersensitivity | [27,28] |
| 8 | Silver sulfadiazine | Cytotoxicity, renal and silver toxicity | [29] |
| 9 | Mafenide | Pain, allergic responses and pulmonary complication | [30,31] |
Table 1: Reported Adverse Effects of Some Topical Antimicrobials/ Antiseptics
Herbal alternatives for wound healing:
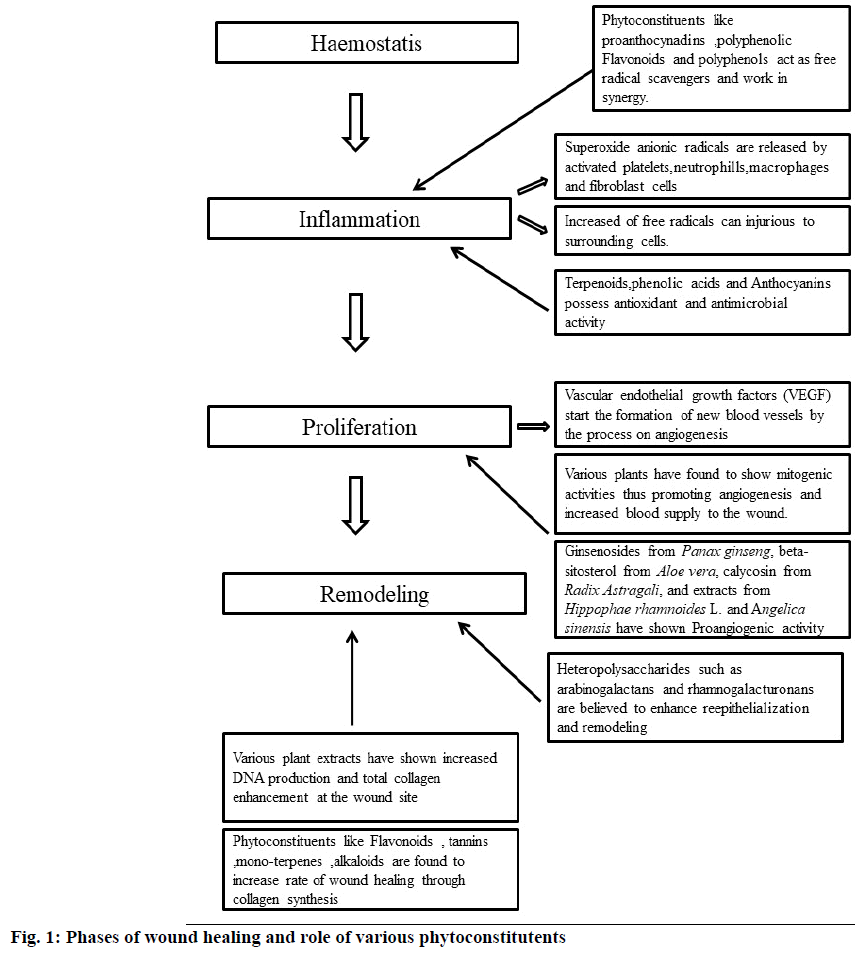
Nature has been a source of various alternative medicines in the form of phytoconstituents for curing different diseases. Many plants have been used traditionally for treating wounds due to their huge potential to influence the wound healing process[32]. The extracts from these plants along with the isolated compounds have been found to heal the wound by multiple and connected mechanisms which work by the concept of synergism and improve the overall healing process[33]. The phytoconstituents responsible for wound healing activity possess antimicrobial activity, antioxidant and free radical scavengers, enhanced mitogenic activity, promoting angiogenesis and collagen production, increased Deoxyribonucleic Acid (DNA) synthesis[34]. The phytoextracts obtained from the plant materials have been analysed and it was found the various phytoconstituents present in these plants are alkaloids, glycosides, carbohydrates, terpenoids, diterpenes, sesquiterpenes, phytosterols, phenolic compounds and different kinds of proteins, tannins, flavonoids, saponins, lignins, alkaloids and essential oils[35-37]. Medicinal plants heal the process of wound healing by aiding blood clotting, confronting against infection and thus speeding the wound healing process. The mechanism of wound healing and the role of various phytoconstituents have been mentioned in fig. 1[38,39]. So, it can be said that plants and phytoconstituents obtained from plants improve treatment and manage the process of wound healing[40]. Some of the plants with reported wound healing activity have been listed in Table 2[41-54].
| S.No | Plant name | Part used | Reference |
|---|---|---|---|
| 1 | Allamanda cathartica | Leaves | [41] |
| 2 | Lawsonia alba | Leaves | [42] |
| 3 | Glycine max | Seeds | [43] |
| 4 | Annona squamosa | Leaves | [44] |
| 5 | Laurus nobilis | Leaves | [41] |
| 6 | Aegle marmelos | Whole plant | [45] |
| 7 | Nelumbo nucifera | Rhizomes | [46] |
| 8 | Aloe vera | Leaves | [47] |
| 9 | Embelia ribes | Leaves | [48] |
| 10 | Morinda citrifolia | Fruit | [49] |
| 11 | Bambusa vulgaris | Leaves | [50] |
| 12 | Moringa oleifera | Leaves | [51] |
| 13 | Elephantopus scaber | Leaves | [52] |
| 14 | Hemigraphis colorata | Leaves | [53] |
| 15 | Flaveria trinervia | Leaves | [54] |
Table 2: List of Some Plants with Reported Wound Healing Activity
Herbal Drug Delivery Systems for Wound Healing
Conventional drug delivery systems:
Topical delivery of herbal drugs is a type of conventional dosage form. The various examples of such type are creams, ointments and gel etc. They can be easily prepared but they have certain disadvantages like low bioavailability and inadequate retention[55]. Some of the recent reported works on conventional topical drug delivery systems of herbal drugs for wound healing have been summarized in Table 3[56-72].
| S.No | Plant(s) | Part used | Extract | Dosage form | Wound model | Reference |
|---|---|---|---|---|---|---|
| 1 | Hibiscus sabdariffa | Calyx | Methanol | Cream | Excision | [56] |
| 2 | Clinacanthus nutans | Leaves | Aqueous | Polyherbal cream | Excision | [57] |
| Elephantopus scaber | ethanol | Incision | ||||
| 3 | Vitex negundo | Leaves | Aqueous | Polyherbal cream | Excision | [58] |
| Emblica officinalis | Bark | |||||
| Tridax procumbens | Stem | |||||
| 5 | Oliveria decumbens Pelargonium graveolens | Flowering Aerial | Oils | Polyherbal cream | Diabetic | [59] |
| 6 | Tridax procumbens | Leaves | Aqueous juice | Ointment | Excision | [60] |
| 7 | Malva sylvestris | Leaves | Aqueous | Polyherbal ointment | Burn | [61] |
| Solanum nigrum | Leaves | Aqueous | ||||
| Rosa damascena | Petals | Oily | ||||
| 8 | Lawsonia inermis | Leaves | - | Polyherbal ointment | Excision | [62] |
| Punica granatum | Fruits | Dead space | ||||
| Commiphora myrrha | Resins | |||||
| 9 | Bidens pilosa | Leaves | Ethanol | Polyherbal ointment | Fresh wound | [63] |
| Aloe barbadensis | ||||||
| 10 | Psorolea corylifalia | Leaves | Ethanol | Polyherbal ointment | Excision | [64] |
| Achryanthes aspera | ||||||
| 11 | Aloe vera | Leaves | - | Polyherbal paste | Excision | [65] |
| Commiphor amyrrha Boswellia carteri | Oleo gum resin | |||||
| 12 | Pothos scandens | Leaves | Ethanol | Gel | Burn | [66] |
| 13 | Ziziphus nummularia | Leaves | Ethanol | Gel | Excision | [67] |
| 14 | Aloe vera | leaves | Juice | gel | Excision | [68] |
| 16 | Lawsoniainermis | Leaves | - | Gel | Excision | [69] |
| 17 | Pistaciaatlantica | Fruit powder | n-hexane | Gel | Incision | [70] |
| 18 | Aegle marmelos, | Leaves | Methanol | Gel | Excision | [71] |
| Mucuna pruriens | seeds | Ointment | ||||
| 19 | Alchemilla vulgaris | Whole plant | Aqueous ethanol, Propylene glycol |
Gel | - | [72] |
Table 3: Conventional Herbal Dosage Forms for Wound Healing
NDDS:
As the conventional dosage forms for wound healing do not release the drug to a particular site in a required amount for a prolonged period, there is requirement of novel topical delivery system for chronic wounds. These novel formulations are found to have remarkable advantages over conventional formulations of herbal extracts like enhancement of solubility and bioavailability, non-toxicity, enhancement of pharmacological activity, high stability, improved tissue macrophages distribution, sustained delivery and protection from physical and chemical degradation. As already discussed above that the acute wounds heal in a timely manner where as there are problems associated with chronic wounds. Therefore, chronic wounds require a longer and more rigorous treatment. A high concentration in the localized area and prolonged delivery can be achieved by developing novel drug delivery systems for wound healing which can be given by topical route. In this way the high systemic exposure of antibiotics can also be avoided. Encapsulation of drugs can provide sustained effect and also maintain the safety of drugs by the preventing the contact of the drug with skin at once[73].
A delivery system for wound healing should perform the functions including maintaining its activity by protecting itself from proteolysis in the wound area, available at a localized level by avoiding getting diluted in the wound fluid and getting absorbed and distributed systematically, releasing the drug within the wound area at a proper rate and duration[74].
The above approaches can also be utilized for developing novel drug delivery system for herbal dsize and high surface-to-volume ratio which prugs as well. As there is a rich source of information available about the traditional uses of herbal drugs in various serious diseases including wound healing. With the development of different modern techniques for standardization, extraction and isolation of various phytoconstituents, it is the need of the hour to apply novel drug delivery to these herbal drugs to increase their efficacy. Various Herbal novel drug delivery systems for wound healing have been discussed below.
Hydrogel:
Hydrogels are becoming popular due to their distinctive properties e.g. high content of water, flexibility, softness and compatibility to biological systems. Hydrogels are hydrophilic in nature with a three-dimensional network and are capable of imbibing large amounts of water or biological fluids and have significant resemblance to the biological tissue. They can be used to deliver various compounds to the body. Chen et al.[75] prepared a solid lipid nanoparticle enriched hydrogel for the topical delivery of astragaloside IV by solvent evaporation method. The effect of this hydrogel was studied for wound healing and anti-scar formation. They used scratch test for in vitro studies and excision model was used for determining various stages of wound repairing. The hydrogel was found to be an excellent method for topical delivery of astragaloside IV with good wound healing and anti-scar activity. Beyranvand et al.[76] studied the effect of alginate hydrogel of encapsulated extract of Satureja khuzistanica on wound healing in rats. Alginate solution was prepared by heating 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) and sodium chloride and further adding sodium alginate followed stirring and adjusting the pH to 7.4. This alginate solution is added in calcium chloride solution to gel alginate beads. The extract of Satureja khuzistanica was diluted with ethanol and kept with alginate hydrogel for encapsulation. The animals were treated with both the Satureja khuzistanica extract and encapsulated Satureja khuzistanica alginate hydrogel for 22 d. The encapsulated Satureja khuzistanica alginate hydrogel dressing was found to be more effective.
Wang et al.[77] isolated a polysaccharide fraction from Periplaneta americana herbal residue. This residue was found to have a potential wound healing effect. For improving the topical delivery, a hydrogel of the polysaccharide fraction was formulated using Carbopol® 940 (Cross linked polyacrylic acid polymer) and carboxymethyl cellulose. Different ratios of polymers were used and the formulation was optimized. The gel was confirmed for cytotoxicity using 3T3 fibroblast proliferation assay. The hydrogel proved to be safe as it contained a natural substance and it accelerated the wound healing in diabetic rat model. Mittal et al.[78] prepared a polymeric hybrid hydrogel of dimethyl aminoethyl acrylate and hyaluronic acid and it was impregnated with herbal extract of Didymocarpus pedicellatus. These hydrogels were evaluated of swelling property. The swelling ratio was observed to be 1600 % in both types of gels. Wistar rats full- thickness excision model was used to study the wound healing activity. It was found that this gel can be used as an effective treatment for curing wounds. As hyaluronic acid has significant role in wound healing mechanism, this hydrogel was found to be better as compared to the standard market treatment.
Microspheres:
The technique of microencapsulation is used to prepare microspheres in which a thin coating is applied to small solid particles, liquid droplets and dispersions. They are formed in the size range of 1-1000 μm. They are found to be compatible for various dosage forms due to their swelling and bio-adhesion characteristics. Microspheres are found to control drug release rate and can be used for treating chronic wounds[79]. Basha et al.[80] developed a topical formulation for treating wounds and bacterial infection of skin. Microspheres of Lawsone loaded with chitosan were prepared by the method of emulsification and cross linking. These prepared microspheres were incorporated into chitosan scaffolds by emulsification and freeze-drying method. Lawsone scaffolds were evaluated for wound healing ability using excision wound model in albino rats. The anti-bacterial activity was determined by agar disc diffusion method. The results obtained were found to be satisfactory.
Wound dressing can also be formulated as a film but in order to achieve a sustained release of the herbal drug, Poly-Lactic-co-Glycolic Acid (PLGA) microspheres were prepared. Liu et al.[81] embedded curcumin encapsulated PLGA microspheres in the chitosan/ aloe membrane by using high-power ultrasonic emulsification and a tape-casting process. It was found that as the release of curcumin was in a controlled manner in the form of microspheres, the aloe/chitosan film promoted the tissue regeneration in full-thickness skin wound model in Wistar rats.
Polymeric, gold and silver nanoparticles:
Some free drugs have problems of low aqueous solubility, narrow bio distribution, rapid degradation and fast clearance. Such drugs can be encapsulated into nanoparticles[82]. These nanoparticles have small size and high surface-to-volume ratio which provide a facility of intracellular ingress and passage across the skin barrier which is ideal requirement for topical drug delivery[83]. Chereddy et al.[84] prepared PLGA- encapsulated curcumin nanoparticles for wound healing. The nanoparticles exhibited two type of drug release pattern. The two mechanisms were found to be diffusion and degradation or erosion. The wound healing potential was evaluated using the splinted mouse full-thickness excision model. The animal group treated with PLGA curcumin nanoparticles was found to be recovered almost completely by the 10th d whereas the groups which received curcumin only and PLGA nanoparticles only showed around 75 % recovery. Leu et al.[85] studied the effect of Gold Nanoparticles (AuNPs) with antioxidant Epigallocatechin Gallate (EGCG) and A-Lipoic Acid (ALA) in wound healing in mice. The preparation when applied topically showed a satisfactory effect. The mixture of AuNPs, EGCG and ALA was found to enhance the wound healing in vivo in mice through its anti-inflammatory and antioxidant activity. Medina et al.[86] prepared chitosan-sodium tripolyphosphate nanoparticles loaded with Arrabidaea chica standardized extract. The Antiulcerogenic activity of extract loaded nanoparticles was also evaluated using an acute gastric ulcer experimental model which was induced by ethanol and indomethacin. It was found that nanoparticles loaded with Arrabidaea chica extract lessened the ulcerative lesion index at lower doses as compared to the free extract. This suggests that encapsulation of the extract in chitosan nanoparticles leads to dose reduction for a gastro protective effect. Sharma et al.[87] studied the wound healing potential of aqueous alcoholic extract of seeds of Madhuca longifolia. Several flavonoids were isolated from the extract. They prepared flavonoids loaded gold, silver and Au-Ag bimetallic nanoparticles. A statistically significant (p<0.001) enhancement in the wound healing efficacy was observed in swiss albino mice model. Among the prepared nanoparticles, silver nanoparticles showed an extraordinary enhancement in the wound healing efficiency of 80.33 % attaining very close the level of reference drug Placentrex of 84.02 %.
Liposomes:
Liposomes are bilayer phospholipid vesicles which are capable of incorporating both hydrophilic and lipophilic drugs. Liposomes have been studied for topical delivery of numerous synthetic and herbal compounds. These vesicles can play a vital role in enhancing the solubility and permeability of drugs. Cui et al.[88] used a Chinese herb formula Danggui Buxue decoction for studying its effect on wound healing. For this purpose, Danggui Buxue loaded liposomes were prepared using thin-film dispersion-ultrasonic method and then incorporated into a thermo sensitive gel. It was evaluated for wound healing using the excision wound model. The results showed the significant improvement in wound healing which may be due the various mediators and signaling pathways. This gel provided a sustained delivery for the Chinese extract and proved to be valuable approach for enhanced wound healing.
Jangde et al.[89] prepared quercetin loaded liposomes for wound healing. Liposomes were prepared using thin-film hydration technique by dissolving quercetin, phospholipids and cholesterol in 25 ml of a chloroform-methanol mixture in a molar ratio of 4:1. By using response surface methodology this liposomal formulation was optimized. It was observed that the liposomes provided a sustained release of quercetin in the wound area. Castangia et al.[90] prepared bio-nano vesicles containing polyphenols such as quercetin and curcumin. These bio-nanovesicles were evaluated against 12-O-tetradecanoylphorbol 13-Acetate (TPA) induced skin inflammation and ulceration. These polyphenols are excellent free radical scavengers and also show significant anti-inflammatory activity. After treatment with polyphenolic bio-nanovesicles it was found that skin lesions formation was prevented. Also, the biochemical processes which are involved epithelial loss and skin damage during the TPA treatment were also nullified. The liposome loaded with quercetin and curcumin and the penetration enhancer containing vesicles exhibited an excellent ability compared to the herbal drug dispersion to decrease myelo-peroxidase activity in damaged tissues.
Nanoemulsions, microemulsions, self-nanoemulsifying drug delivery systems, self-microemulsifying drug delivery systems:
Nanoemulsion and microemulsion are sometimes misunderstood on the basis of their droplet size. As far as the terminology is considered the nanoemulsion means 10-9 while the microemulsion fall in the micrometer range i.e. 10-6. But despite of this the size ranges may overlap. Microemulsions are in the size range of 10-100 nm whereas nanoemulsions are in the range of 100-500 nm[91]. Microemulsions are more stable thermodynamically and clear in appearance whereas nanoemulsions are more stable kinetically and they are opalescent to milky white in colour[92]. They are widely accepted as promising vehicle for drugs used for wound healing. The reasons may be their small droplet size, high stability, extended shelf-life, simple formulation process, high surface area and enhances efficiency of solubilization. Nanoemulsions enhance the solubility of both hydrophilic and hydrophobic drugs and can modify the release of drugs in sustained manner. Therefore, their use has become significant for the delivery of herbal based wound healing formulations[93]. Apart from these new self-emulsifying lipid carriers are becoming popular for enhancing solubility and bioavailability of poorly soluble drugs. These lipid carriers have been classified on the type of and amount of excipients and their morphology. There are four classes Type I containing only oily phase, Type II self-dispersing carriers called as Self-Emulsifying Drug Delivery Systems (SEDDS), Type IIIA called as Self Nano-Emulsifying Drug Delivery System (SNEDDS) and Type IIIB Self Micro-Emulsifying Drug Delivery System (SMEDDS) and Type IV are made of surfactants. Type IIIA forms oil in water nanoemulsion with a droplet size in the range of 100-250 nm whereas Type IIIB forms oil in water microemulsion with droplet size 10-100 nm range. These type IIIB when diluted small droplets are formed and the system appears to be transparent[94].
Ghosh et al.[95] developed Cinnamon oil microemulsion using a non-ionic surfactant Tween®20 (Polysorbate 20) and water. Oil and surfactant were taken in the ratio of 1:4. The microemulsion was formed with a droplet diameter of about 5.79 nm and was found to be kinetically stable. This microemulsion was found to exhibit antibacterial activity and triggered the process of wound healing in wistar rats. Shakeel et al.[96] prepared a SNEDDS using the Piper cubeba Oil (PO) by utilizing the low energy emulsification technique. The prepared SNEDDS were characterized for thermodynamic stability and self-emulsification power. The excipients used for preparation of SNEDDS were Sefsol-218 (Propylene glycol caprylate) as the oil phase, Triton X100TM (Octylphenol ethylene oxide condensate1) as the surfactant, Transcutol®-HP (Diethyl glycol monoethyl ether) as cosurfactant and deionized water as aqueous phase. It was concluded that the use of surfactants Transcutol®-HP along with surfactant Triton X100™ lead to the formation of a stable SNEDDS. It was found that SNEDDS of PO showed better wound healing as compared to PO alone. However, the healing was comparable to the standard Gentamycin.
Oliveria decumbens developed a wound healing dressing based on Propolis (EPP AF®) containing a self- microemulsifying formulation which was incorporated in biocellulose membranes. Various formulations were prepared by varying the proportion of Capmul® MCM EP (medium chain mono- and diglycerides,) as surfactant. Polyethylene glycol 400 (PEG 400), as cosurfactant, and the oil phase, which was composed of a mixture of propolis (EPP-AF®) soft extract and C. cassia essential oil. The optimized self-microemulsifying formulation was incorporated in the biocellulose membrane. The results showed that the developed wound dressing can be used successfully to treat chronic infected wounds[97].
Phytosomes:
Phytoconstituents have been used extensively for curing many ailments. In order to enhance their bioavailability and targeted delivery they can be complexed with phospholipids. The complexes between phytoconstituents and phospholipids are called as phytosomes. Phospholipids are integral part of our cell membrane so they are biocompatible and safe for use. Phytosomes provide high entrapment efficiency and can be successfully used for topical, delivery of phytoconstituents for wound healing[98,99]. Few of the phyto-extracts are poorly soluble in aqueous substances and their permeation across biomembrane is also limited. Therefore, these extracts can be formulated as phytosomes. Demir et al.[100] developed phytosomes of Calendula officinalis extract. These phytosomal formulation was loaded with Au nanoparticles thus utilizing the wound healing and antioxidant properties of gold nanoparticles. The Au loaded phytosomes were found to have a particle size of about 100 nm along with high entrapment efficiency. It was found that the preparation was safe and had significant antioxidant and wound healing activities. The Phytosomal vesicles proved to novel drug delivery system by enhancing the biological activity of Au nanoparticles and Calendula extract.
Mazumder et al.[101] developed phytosomes of sinigrin to enhance its bioavailability and therapeutic efficacy and studied the wound healing and cytotoxic activity. The phytosomes formed were found to have an average particle size of 153±39 nm, a zeta potential of 10.09±0.98 mV and an entrapment efficiency of 69.55 %. The phytosomal complex exhibited complete wound healing after 42 h as compared to only sinigrin which showed only about 71 % wound closure. As far as cytotoxic studies are concerned a nominal toxicity towards HaCaT cells and a potent activity towards A-375 was found. Moringa oleifera leaves are found to possess wound healing activity due to the presence of polyphenols. These polyphenols have large molecular size and low lipid solubility so therefore their permeation across cell membrane is low. To enhance the bioavailability of these polyphenols, aqueous extract of leaves of Moringa oleifera was complexed with phospholipids to form phytosomes[102]. Cell cytotoxicity study and cell migration assay for prepared phytosomes were determined using in vitro normal human dermal fibroblast cells. The phytosomes were found to be nontoxic and can be successfully used as a wound healing dressing.
Films/Membranes:
For the purpose of an effective wound healing it is desirable to cover the wound with a suitable covering or dressing to prevent contamination from microorganism[103]. Various materials like animal fat, plant fibers and honey etc. have been used earlier for covering the wounds. Several studies have reported use of polymers for preparing films to be used as wound dressing. Chin et al.[104] developed Moringa oleifera standardised aqueous leaf extract-loaded hydrocolloid film dressing for studying wound healing in diabetic rat model along with in vivo dermal safety. The polymers used for the preparation of films were pectin and sodium alginate. Based on the wound healing assay and cell proliferation studies films were prepared in polymer concentration of 0.1, 0.5 and 1 % by using solvent casting method. The 0.5 % film remarkably augmented the wound closure by 77.67 %±7.28 % at the 7th d as compared to the control group. Whereas in abrasion wounds, 0.5 % films expedited the wound closure notably at 81 %±4.5 % as compared to the control group. The study proves the use of Moringa leaves extract loaded films for accelerated wound healing in diabetic rat model.
Huanbutta et al.[105] utilized the pharmacological activities of propolis extract for preparing a natural gum- based film for enhancing its effectiveness for wound healing. Tamarind seed gum was used as a film forming agent. Several ratios of the solvents water, ethanol and concentration of gum were tried and finally a ratio of 55 %:45 % of water: ethanol, 7.5 % of tamarind seed gum and 1 % Polyethylene Glycol 400 (PEG400) as plasticizer was obtained as an optimized formulation. This optimized formulation exhibited a fast drying time and excellent rheological properties to be used as a spray formulation for film forming. The propolis extract loaded film system was found to inhibit Staphylococcus aureus and Staphylococcus epidermidis. Tong et al.[106] developed antimicrobial cellulose nanocrystals film wound dressings loaded with curcumin. The cellulose nanocrystals films were encapsulated with curcumin. These films were found to be flexible and soft. A remarkable inhibitory efficacy against, Yersinia sp, E. coli, Pseudomonas aeruginosa and Proteus mirabilis was seen in the antimicrobial studies of the films. The in vivo wound healing activity was studied using diabetic rat models which demonstrated a decrease in the wound area on 7th d after the topical treatment of films loaded with curcumin. Curcumin speeded up the wound healing by enhancing the level of TGF-β1 which is essential for cell growth and proliferation. Curcumin also safeguard the skin cells from damage by oxidation.
Nanofibers:
Nanofibers are fibers in which drug is inserted for topical application in wound healing. These nanofibers act as barrier for invasion of microorganisms due to high specific surface area, extensive permeability of about 60 % to 90 %. The nanofibers also show a nano-porosity as a network which inhibits microbes. The electrospinning technique provides sustained drug release. Thus, these nanofibers provide an excellent barrier and a controlled environment for wound healing[107]. Yao et al.[108] developed Lithospermi radix (LR) extract containing bilayer nanofiber scaffold for stimulating wound healing in a rat model. LR is a traditional herbal drug used for wound healing. They used different mammalian gelatin and fish collagen for electrospinning on to chitosan scaffolds to prepare bilayer nanofiber scaffold. The chitosan scaffold had a porous structure with a very high swelling ratio of about 1909 wt % and thus enabled the material to absorb the exudates properly. The gelatin nanofibers showed a greater cell attachment in vitro using the fibroblasts. From the cytotoxicity analysis it was found that the gelatin, fish collagen and chitosan released from the membranes were non-toxic to L929 fibroblasts. These LR loaded bilayer nanofiber scaffold exhibited the highest wound healing rate.
Almasian et al.[109] developed Polyurethane (PU) based nanofibers containing Malva sylvestris extract. The effect of these nanofibers was evaluated on diabetic wound model. To improve the absorption ability of wound exudates various concentrations of Carboxymethyl Cellulose (CMC) were tried. It was found that smooth morphology with a moderate release of the phytoconstituent in 85 h was due to 20 % w/w CMC in the polymer mixture and formed nanofibers with a mean diameter of 386.5 nm. This extract loaded PU based nanofiber dressing showed significant antibacterial effect against Staphylococcus aureus and Escherichia coli. The wound dressing containing 15 % w/w herbal extract demonstrated the wound healing rate of 95.05 %±0.24 % by the 14th d. It was found from the histological analysis that macrophage infiltration, neovascularization activity and fibroblastic proliferation were enhanced on the 7th d. Also, the degree of collagenization and epithelium regeneration was increased on the 14th d.
Maintain its activity by protecting itself from proteolysis in the wound area incorporated standardized extract of four reputed wound healing plants namely Acalypha indica, Aristolochia bracteolate, Lawsonia inermis and Thespesia populnea in Electrospun nanofibrous Guar gum based nanofibrous scaffold matrix for wound healing[110]. The prepared nanofibrous scaffold or dressing was found to be non-toxic during dermal toxicity studies on female wistar rats. Thus, by this study a herbal drug loaded nanofibrous mat as a dressing and also a skin like scaffold with Gingival of Mesenchymal Stem Cells (GMSC’s, a novel concept by combining the biological and technological advantage of a herbal drug and stem cell therapy can be successfully used for regeneration of skin.
Recent Patents
Several herbal formulations have been patented for wound healing. Patents in the past few years are listed in the Table 4[111-129].
| S No | Patent no. | Title | Indication | Reference |
|---|---|---|---|---|
| 1 | WO2013091056A1 | Pharmaceutical compositions comprising Arrabidaea chica extract in controlled release systems, production process and use thereof | Tissue wound healing | [111] |
| 2 | US 20140199367A1 | Topical transdermal method for delivering nutrients through the skin for expedited wound healing and skin rejuvenation | Riboswitch-like activation and curation of wounds | [112] |
| 3 | WO2014147638A1 | A multifunctional natural wound healing matrixalso limited. Therefore, these extracts can be formulated | Wound healing | [113] |
| 4 | US8741353B1 | Ointment for healing burns and wounds. | Burns and wounds | [114] |
| 5 | EP 2586450B1 | Cream for burns | For effective recovery of burn without leaving any scar | [115] |
| 6 | US9101508B2 | Electro spun nanofibrous wound dressing and a method of synthesizing the same | Dressing for wounds | [116] |
| 7 | WO2017060535 | A topical herbal healing formulation | Treatments of wounds | [117] |
| 8 | WO2017122224A1 | A nano-biocomposite formulation for wound healing and a process for the preparation thereof | Wound healing | [118] |
| 9 | EP 2704729B1 | Antimicrobial silver hydrogel composition for the treatment of burns and wounds | Antimicrobial silver and acemannan composition for the treatment of wounds or lesions or burns. | [119] |
| 10 | US 20180185428A1 | Herbal combinations for wound healing in fibroblasts | Enhancing fibroblast cell migrations | [120] |
| 11 | US 20180318375A1 | Herbal preparations for accelerating wounds and skin inflammation healing and its application | Healing of wound and skin inflammation | [121] |
| 12 | US 20190183954A1 | Herbal preparation for accelerating wounds and skin inflammations especially for treatment of herpes and acne and its application | Treating herpes and acne | [122] |
| 13 | IN201721046295 | A herbal antiseptic and wound healing preparation | Healing of wounds and burns | [123] |
| 14 | EP2895209B1 | Improved wound healing compositions comprising microspheres | Wound healing | [124] |
| 15 | US10206886B2 | Lipid nanoparticles for wound healing | For promoting wound healing | [125] |
| 16 | US20200330543A1 | A topical herbal healing formulation | Topical herbal formulation for wound | [126] |
| 17 | US20180015133A1 | Topical formulation for promoting wound healing | Promotion of wound healing | [127] |
| 18 | RU2736214C1 | Ointment for wound healing | For wound healing, burn and antibacterial purpose | [128] |
| 19 | US20200376170A1 | Biomimetic pro-regenerative scaffolds and methods of use thereof | Scaffolds for wound healing | [129] |
Table 4: Recent Patents on Herbal Formulations for Wound Healing
Conclusion
Various novel herbal wound healing formulations like microspheres, nanoparticles, liposomes, nano- emulsions, nanoparticles, nanofibers, hydrogels, dressing etc. were reviewed. The main discussion was mainly concerned about their in vitro drug release and in vivo wound healing studies. It was found that these novel formulations not only cover the wounds but also stimulate the tissue regeneration and remodelling. They also speed up the regeneration of skin thus minimizes the size of scar and its formation. These herbal drugs work by various mechanisms and help in the healing of wounds in a more efficient way. The disadvantage of antibiotic resistance and serious adverse effects is also minimized while using herbal approaches for wound care. Treatment with conventional dosage is time consuming and also the release of drug is not in a sustained manner. Novel herbal formulations utilize the traditional phytoconstituents and convert them into a new advanced delivery system thus improving patient convenience and compliances. These novel formulation approaches when compared to the traditional pure herbs were found to be more effective in facilitating the wound healing and the repairing process. In future more and more traditional herbs can be explored and utilized in novel drug delivery systems for better management of chronic wounds.
Conflict of interests:
The authors declare no conflict of interest.
References
- Goldman P. Herbal medicines today and the roots of modern pharmacology. Ann Intern Med 2001;135(8 Pt 1):594-600.
[Crossref] [Google Scholar] [PubMed]
- Norman GB. Herbal drugs and phytopharmaceuticals: A Handbook for Practice on a Scientific Basis. 2nd ed. New York: Medpharm Scientific Publishers; 2001.
- Charman WN, Chan HK, Finnin BC, Charman SA. Drug delivery: A key factor in realising the full therapeutic potential of drugs. Drug Dev Res 1999;46(3‐4):316-27.
- Singer AJ, Dagum AB. Current management of acute cutaneous wounds. N Engl J Med 2008;359(10):1037-46.
[Crossref] [Google Scholar] [PubMed]
- Adhirajan N, Shanmugasundaram N, Shanmuganathan S, Babu M. Functionally modified gelatin microspheres impregnated collagen scaffold as novel wound dressing to attenuate the proteases and bacterial growth. Eur J Pharm Sci 2009;36(2-3):235-45.
[Crossref] [Google Scholar] [PubMed]
- Zhang Z, Michniak-Kohn BB. Tissue engineered human skin equivalents. Pharmaceutics 2012;4(1):26-41.
[Crossref] [Google Scholar] [PubMed]
- Xu QY, Yang JS, Li Y, Wang YY. Effects of different scraping techniques on body surface blood perfusion volume and local skin temperature of healthy subjects. J Tradit Chin Med 2011;31(4):316-20.
[Crossref] [Google Scholar] [PubMed]
- Alam G, Singh MP, Singh A. Wound healing potential of some medicinal plants. Int J Pharm Sci Rev Res 2011;9(1):136-45.
- Järbrink K, Ni G, Sönnergren H, Schmidtchen A, Pang C, Bajpai R, et al. The humanistic and economic burden of chronic wounds: A protocol for a systematic review. Sys Rev 2017;6(1):1-7.
- Nusgart M. Alliance of Wound Care Stakeholders update: Demonstrating the impact and cost of chronic wounds. Ostomy Wound Manage 2017;63(10):1943-2720.
- Gupta N, Gupta SK, Shukla VK, Singh SP. An Indian community-based epidemiological study of wounds. J Wound Care 2004;13(8):323-5.
[Crossref] [Google Scholar] [PubMed]
- Negut I, Grumezescu V, Grumezescu AM. Treatment strategies for infected wounds. Molecules 2018;23(9):2392.
[Crossref] [Google Scholar] [PubMed]
- Lipsky BA. Treating diabetic foot osteomyelitis primarily with surgery or antibiotics: Have we answered the question? Diabetes Care 2014;37(3):593-5.
[Crossref] [Google Scholar] [PubMed]
- Mani R, Margolis DJ, Shukla V, Akita S, Lazarides M, Piaggesi A, et al. Optimizing technology use for chronic lower-extremity wound healing: A consensus document. Int J Low Extrem Wounds 2016;15(2):102-19.
[Crossref] [Google Scholar] [PubMed]
- Frykberg Robert G. Challenges in the treatment of chronic wounds. Adv Wound Care 2015;4(9):560-82.
[Crossref] [Google Scholar] [PubMed]
- Lipsky BA, Hoey C. Topical antimicrobial therapy for treating chronic wounds. Clin Infect Dis 2009;49(10):1541-9.
[Crossref] [Google Scholar] [PubMed]
- Thomas GW, Rael LT, Bar-Or R, Shimonkevitz R, Mains CW, Slone DS, et al. Mechanisms of delayed wound healing by commonly used antiseptics. J Trauma 2009;66(1):82-91.
[Crossref] [Google Scholar] [PubMed]
- Kaye ET. Topical antibacterial agents. Infect Dis Clin North Am 2000;14(2):321-39.
[Crossref] [Google Scholar] [PubMed]
- Punjataewakupt A, Napavichayanun S, Aramwit P. The downside of antimicrobial agents for wound healing. Eur J Clin Microbiol Infect Dis 2019;38(1):39-54.
[Crossref] [Google Scholar] [PubMed]
- Giannelli M, Chellini F, Margheri M, Tonelli P, Tani A. Effect of chlorhexidine digluconate on different cell types: A molecular and ultrastructural investigation. Toxicol In Vitro 2008;22(2):308-17.
[Crossref] [Google Scholar] [PubMed]
- Poon VK, Burd A. In vitro cytotoxity of silver: Implication for clinical wound care. Burns 2004;30(2):140-7.
[Crossref] [Google Scholar] [PubMed]
- Ahmed KB, Nagy AM, Brown RP, Zhang Q, Malghan SG, Goering PL. Silver nanoparticles: Significance of physicochemical properties and assay interference on the interpretation of in vitro cytotoxicity studies. Toxicol In Vitro 2017;38:179-92.
[Crossref] [Google Scholar] [PubMed]
- Sato S, Miyake M, Hazama A, Omori K. Povidone-iodine-induced cell death in cultured human epithelial HeLa cells and rat oral mucosal tissue. Drug Chem Toxicol 2014;37(3):268-75.
[Crossref] [Google Scholar] [PubMed]
- Leung AM, Braverman LE. Consequences of excess iodine. Nat Rev Endocrinol 2014;10(3):136-42.
- Jacob SE, James WD. From road rash to top allergen in a flash: Bacitracin. Dermatol Surg 2004;30(4):521-4.
[Crossref] [Google Scholar] [PubMed]
- Bork K, Brauers J, Kresken M. Efficacy and safety of 2 % mupirocin ointment in the treatment of primary and secondary skin infections: An open multicentre trial. Br J Clin Pract 1989;43(8):284-8.
[Google Scholar] [PubMed]
- Menezes de Pádua CA, Schnuch A, Lessmann H, Geier J, Pfahlberg A, Uter W. Contact allergy to neomycin sulfate: Results of a multifactorial analysis. Pharmacoepidemiol Drug Saf 2005;14(10):725-33.
[Crossref] [Google Scholar] [PubMed]
- Ansari IA, Onyema E. Severe generalised hypersensitivity reaction to topical neomycin after cataract surgery: A case report. J Med Case Rep 2008;2(1):1-3.
[Crossref] [Google Scholar] [PubMed]
- Lee AR, Moon HK. Effect of topically applied silver sulfadiazine on fibroblast cell proliferation and biomechanical properties of the wound. Arch Pharma Res 2003;26(10):855-60.
[Crossref] [Google Scholar] [PubMed]
- Albert LT, Lewis NS, Warpeha RL. Late pulmonary complications with use of mafenide acetate. J Burn Care Rehab 1982;3(6):375-6.
- Firoz EF, Firoz BF, Williams JF, Henning JS. Allergic contact dermatitis to mafenide acetate: A case series and review of the literature. J Drug Dermatol 2007;6(8):825-8.
[Google Scholar] [PubMed]
- Santos-Buelga C, Mateus N, De Freitas V. Anthocyanins. Plant pigments and beyond. J Agric Food Chem 2014;62(29):6879-84.
[Crossref] [Google Scholar] [PubMed]
- Maver T, Maver U, Stana Kleinschek K, Smrke DM, Kreft S. A review of herbal medicines in wound healing. Int J Dermatol 2015;54(7):740-51.
[Crossref] [Google Scholar] [PubMed]
- Ghosh PK, Gaba A. Phyto-extracts in wound healing. J Pharm Pharm Sci 2013;16(5):760-820.
[Crossref] [Google Scholar] [PubMed]
- Akkol EK, Koca U, Pesin I, Yilmazer D. Evaluation of the wound healing potential of Achillea biebersteinii Afan.(Asteraceae) by in vivo excision and incision models. Evid Based Complement Alternat Med 2011;2011:474026.
[Crossref] [Google Scholar] [PubMed]
- Ghosh PK, Gupta VB, Rathore MS, Hussain I. Wound-healing potential of aqueous and ethanolic extracts of Apamarga leaves. Int J of Green Pharm. 2011;5(1):12-5.
- Subhashini S, Arunachalam KD. Investigations on the phytochemical activities and wound healing properties of Adhatoda vasica leave in Swiss albino mice. Afr J Plant Sci 2011;5(2):133-45.
- Majewska I, Gendaszewska-Darmach E. Proangiogenic activity of plant extracts in accelerating wound healing-A new face of old phytomedicines. Acta Biochim Pol 2011;58(4):449-60.
[Crossref] [Google Scholar] [PubMed]
- Gadgoli CH. Research in Phyto‐Constituents for Treatment of Wounds. In: Worldwide Wound Healing-Innovation in Natural and Conventional Methods; 2016.
- Raina R, Parwez S, Verma PK, Pankaj NK. Medicinal plants and their role in wound healing. Online Vet J 2008;3(1):21-4.
- Nayak S, Nalabothu P, Sandiford S, Bhogadi V, Adogwa A. Evaluation of wound healing activity of Allamanda cathartica. L. and Laurus nobilis. L. extracts on rats. BMC Complement Alternat Med 2006;6(1):1-6.
[Crossref] [Google Scholar] [PubMed]
- Nithya V, Baskar A. A preclinical study on wound healing activity of Lawsonia ulba Linn. Res J Phyto Chem 2011;5:123-9.
- Xu L, Choi TH, Kim S, Kim SH, Chang HW, Choe M, et al. Anthocyanins from black soybean seed coat enhance wound healing. Ann Plast Surg 2013;71(4):415-20.
[Crossref] [Google Scholar] [PubMed]
- Ponrasu T, Suguna L. Efficacy of Annona squamosa on wound healing in streptozotocin‐induced diabetic rats. Int Wound J 2012;9(6):613-23.
[Crossref] [Google Scholar] [PubMed]
- Jaswanth A, Loganathan V, Manimaran S. Wound healing activity of Aegle marmelos. Indian J Pharm Sci 2001;63(1):41-4.
- Mukherjee PK, Mukherjee K, Pal M, Saha BP. Wound healing potential of Nelumbo nucifera (Nymphaceae) rhizome extract. Phytomedicine 2000;7(2):66-74.
- Daburkar M, Lohar V, Rathore AS, Bhutada P, Tangadpaliwar S. An in vivo and in vitro investigation of the effect of Aloe vera gel ethanolic extract using animal model with diabetic foot ulcer. J Pharm Bioallied Sci 2014;6(3):205.
[Crossref] [Google Scholar] [PubMed]
- Swamy HK, Krishna V, Shankarmurthy K, Rahiman BA, Mankani KL, Mahadevan KM, et al. Wound healing activity of embelin isolated from the ethanol extract of leaves of Embelia ribes Burm. J Ethnopharmacol 2007;109(3):529-34.
[Crossref] [Google Scholar] [PubMed]
- Nayak BS, Isitor GN, Maxwell A, Bhogadi V, Ramdath DD. Wound-healing activity of Morinda citrifolia fruit juice on diabetes-induced rats. J Wound Care 2007;16(2):83-6.
[Crossref] [Google Scholar] [PubMed]
- Lodhi S, Jain AP, Rai G, Yadav AK. Preliminary investigation for wound healing and anti-inflammatory effects of Bambusa vulgaris leaves in rats. J Ayurveda Integr Med 2016;7(1):14-22.
[Crossref] [Google Scholar] [PubMed]
- Gothai S, Arulselvan P, Tan WS, Fakurazi S. Wound healing properties of ethyl acetate fraction of Moringa oleifera in normal human dermal fibroblasts. J Intercult Ethnopharmacol 2016;5(1):1-6.
[Crossref] [Google Scholar] [PubMed]
- Singh SD, Krishna V, Mankani KL, Manjunatha BK, Vidya SM, Manohara YN. Wound healing activity of the leaf extracts and deoxyelephantopin isolated from Elephantopus scaber Linn. Indian J Pharmacol 2005;37(4):238.
[Crossref] [Google Scholar] [PubMed]
- Subramoniam A, Evans DA, Rajasekharan S, Nair GS. Effect of Hemigraphis colorata (Blume) HG Hallier leaf on wound healing and inflammation in mice. Indian J Pharmacol 2001;33(4):283-5.
- Umadevi S, Mohanta GP, Kalaichelvan VK, Manavalan R. Studies on wound healing effect of Flaveria trinervia leaf in mice. Indian J Pharm Sci 2006;68(1):106-8.
- Kaur J, Kaur J, Jaiswal S, Gupta G. Recent advances in topical drug delivery system. Pharm Res 2016;6(7):6353-69.
- Builders PF, Kabele-Toge B, Builders M, Chindo BA, Anwunobi PA, Isimi YC. Wound healing potential of formulated extract from Hibiscus sabdariffa calyx. Indian J Pharm Sci 2013;75(1):45-8.
[Crossref] [Google Scholar] [PubMed]
- Aslam MS, Ahmad MS, Mamat AS, Ahmad MZ, Salam F. Antioxidant and wound healing activity of polyherbal fractions of Clinacanthus nutans and Elephantopus scaber. Evid Based Complement Alternat Med 2016;2016:1-14.
- Talekar YP, Apte KG, Paygude SV, Tondare PR, Parab PB. Studies on wound healing potential of polyherbal formulation using in vitro and in vivo assays. J Ayurveda Integr Med 2017;8(2):73-81.
[Crossref] [Google Scholar] [PubMed]
- Mahboubi M, Taghizadeh M, Khamechian T, Tamtaji OR, Mokhtari R, Talaei SA. The wound healing effects of herbal cream containing Oliveria decumbens and Pelargonium graveolens essential oils in diabetic foot ulcer model. World J Plast Surg 2018;7(1):45-50.
[Google Scholar] [PubMed]
- Yaduvanshi B, Mathur R, Mathur SR, Velpandian T. Evaluation of wound healing potential of topical formulation of leaf juice of Tridax procumbens L. in mice. Indian J Pharm Sci 2011;73(3):303-6.
[Crossref] [Google Scholar] [PubMed]
- Fahimi S, Mortazavi SA, Abdollahi M, Hajimehdipoor H. Formulation of a traditionally used polyherbal product for burn healing and HPTLC fingerprinting of its phenolic contents. Iran J Pharm Res 2016;15(1):95-105.
- Elzayat EM, Auda SH, Alanazi FK, Al-Agamy MH. Evaluation of wound healing activity of henna, pomegranate and myrrh herbal ointment blend. Saudi Pharm J 2018;26(5):733-8.
- Namunana S, Lutoti S, Nyamaizi G, Agaba G, Apun I, Ssebunnya C, et al. Formulation, development and validation of a wound healing herbal ointment from extracts of Bidens pilosa and Aloe barbadensis. J Pharm Pharmacol Res 2018;2(2):32-8.
- Kolhe SS. Evaluation of polyherbal ointment for wound healing activity in Wistar rats. J Drug Deliv Ther 2018;8(6):26-31.
- Jahandideh M, Hajimehdipoor H, Mortazavi SA, Dehpour A, Hassanzadeh G. Evaluation of the wound healing activity of a traditional compound herbal product using rat excision wound model. Iran J Pharm Res 2017;16:153-63.
[Google Scholar] [PubMed]
- KP MH, Saraswathi R, Mohanta GP, Nayar C. Formulation and evaluation of herbal gel of Pothos scandens Linn. Asian Pac J Trop Biomed 2010;3(12):988-92.
- Yusufoglu HS. Topical anti-inflammatory and wound healing activities of herbal gel of Ziziphus nummularia L. (F. Rhamnaceae) leaf extract. Int J Pharmacol 2011;7(8):862-7.
- Khan AW, Kotta S, Ansari SH, Sharma RK, Kumar A, Ali J. Formulation development, optimization and evaluation of Aloe vera gel for wound healing. Pharmacogn Mag 2013;9:10.
[Crossref] [Google Scholar] [PubMed]
- Lakshmi PK, Thangellapalli N, Chennuri A, Prasanthi D, Veeresh B. Wound healing activity of topical lawsone gel on rat model. Int J Pharm Sci Res 2017;8:3162-9.
- Hamidi SA, Naeini AT, Oryan A, Tabandeh MR, Tanideh N, Nazifi S. Cutaneous wound healing after topical application of Pistacia atlantica gel formulation in rats. Turk J Pharm Sci 2017;14(1):65-74.
[Crossref] [Google Scholar] [PubMed]
- Toppo FA, Pawar RS. Development, optimization and evaluation of different herbal formulations for wound healing. Int J Pharm Pharm Sci 2015;7:447-52.
- Tasić-Kostov M, Arsić I, Pavlović D, Stojanović S, Najman S, Naumović S, et al. Towards a modern approach to traditional use: In vitro and in vivo evaluation of Alchemilla vulgaris L. gel wound healing potential. J Ethnopharmacol 2019;238:111789. [Crossref]
[Google Scholar] [PubMed]
- Krausz AE, Adler BL, Cabral V, Navati M, Doerner J, Charafeddine RA, et al. Curcumin-encapsulated nanoparticles as innovative antimicrobial and wound healing agent. Nanomedicine 2015;11(1):195-206.
- R Johnson N, Wang Y. Drug delivery systems for wound healing. Curr Pharm Biotechnol 2015;16(7):621-9.
[Crossref] [Google Scholar] [PubMed]
- Chen X, Peng LH, Shan YH, Li N, Wei W, Yu L, et al. Astragaloside IV-loaded nanoparticle-enriched hydrogel induces wound healing and anti-scar activity through topical delivery. Int J Pharm 2013;447(1):171-81.
[Crossref] [Google Scholar] [PubMed]
- Beyranvand F, Gharzi A, Abbaszadeh A, Khorramabadi RM, Gholami M, Gharravi AM. Encapsulation of Satureja khuzistanica extract in alginate hydrogel accelerate wound healing in adult male rats. Inflamm Regen 2019;39(1):1-2.
- Wang T, Liao Q, Wu Y, Wang X, Fu C, Geng F, et al. A composite hydrogel loading natural polysaccharides derived from Periplaneta americana herbal residue for diabetic wound healing. Int J Biol Macromol 2020;164:3846-57.
- Mittal AK, Bhardwaj R, Arora R, Singh A, Mukherjee M, Rajput SK. Acceleration of wound healing in diabetic rats through poly dimethylaminoethyl acrylate–hyaluronic acid polymeric hydrogel impregnated with a Didymocarpus pedicellatus plant extract. ACS Omega 2020;5(38):24239-46.
- Adhirajan N, Shanmugasundaram N, Babu M. Gelatin microspheres cross-linked with EDC as a drug delivery system for doxycyline: Development and characterization. J Microencapsul 2007;24(7):659-71.
[Crossref] [Google Scholar] [PubMed]
- Bascha J, Murthy BR, Likhitha PR, Ganesh Y, Bai BG, Rani RJ, et al. In vitro and in vivo assessment of lawsone microsphere loaded chitosan scaffolds. Int J Phytopharm 2016;6:74-84.
- Liu X, You L, Tarafder S, Zou L, Fang Z, Chen J, et al. Curcumin-releasing chitosan/aloe membrane for skin regeneration. Chem Eng J 2019;359:1111-9.
- Pan J, Chan SY, Lee WG, Kang L. Microfabricated particulate drug‐delivery systems. Biotechnol J 2011;6(12):1477-87.
[Crossref] [Google Scholar] [PubMed]
- Krausz A, Adler B, Cabral V, Navati M, Doerner J, Charafeddine R, et al. Curcumin-encapsulated nanoparticles as innovative antimicrobial and wound healing agent. Nanomedicine 2015;11(1):195-206.
[Crossref] [Google Scholar] [PubMed]
- Chereddy KK, Coco R, Memvanga PB, Ucakar B, des Rieux A, Vandermeulen G, et al. Combined effect of PLGA and curcumin on wound healing activity. J Control Release 2013;171(2):208-15.
[Crossref] [Google Scholar] [PubMed]
- Leu JG, Chen SA, Chen HM, Wu WM, Hung CF, Yao YD, et al. The effects of gold nanoparticles in wound healing with antioxidant epigallocatechin gallate and α-lipoic acid. Nanomedicine 2012;8(5):767-75.
[Crossref] [Google Scholar] [PubMed]
- Servat-Medina L, Gonzalez-Gomez A, Reyes-Ortega F, Sousa IM, Queiroz ND, Zago PM, et al. Chitosan–tripolyphosphate nanoparticles as Arrabidaea chica standardized extract carrier: Synthesis, characterization, biocompatibility, and antiulcerogenic activity. Int J Nanomedicine 2015;10:3897-909.
[Crossref] [Google Scholar] [PubMed]
- Sharma M, Yadav S, Ganesh N, Srivastava MM, Srivastava S. Biofabrication and characterization of flavonoid-loaded Ag, Au, Au–Ag bimetallic nanoparticles using seed extract of the plant Madhuca longifolia for the enhancement in wound healing bio-efficacy. Prog Biomater 2019;8(1):51-63.
[Crossref] [Google Scholar] [PubMed]
- Cui MD, Pan ZH, Pan LQ. Danggui Buxue extract-loaded liposomes in thermosensitive gel enhance in vivo dermal wound healing via activation of the VEGF/PI3K/Akt and TGF-β/Smads signaling pathway. Evid Based Complement Alternat Med 2017;2017.
[Crossref] [Google Scholar] [PubMed]
- Jangde R, Singh D. Preparation and optimization of quercetin-loaded liposomes for wound healing, using response surface methodology. Artif Cells Nanomed Biotechnol 2016;44(2):635-41.
[Crossref] [Google Scholar] [PubMed]
- Castangia I, Nácher A, Caddeo C, Valenti D, Fadda AM, Díez-Sales O, et al. Fabrication of quercetin and curcumin bionanovesicles for the prevention and rapid regeneration of full-thickness skin defects on mice. Acta Biomater 2014;10(3):1292-300.
[Crossref] [Google Scholar] [PubMed]
- Rao J, McClements DJ. Lemon oil solubilization in mixed surfactant solutions: Rationalizing microemulsion and nanoemulsion formation. Food Hydrocoll 2012;26(1):268-76.
- McClements DJ. Nanoemulsions versus microemulsions: Terminology, differences and similarities. Soft Matter 2012;8(6):1719-29.
- Krstić M, Medarević Đ, Đuriš J, Ibrić S. Self-nanoemulsifying drug delivery systems (SNEDDS) and self-microemulsifying drug delivery systems (SMEDDS) as lipid nanocarriers for improving dissolution rate and bioavailability of poorly soluble drugs. Lipid Nanocarriers Drug Target 2018:473-508.
- Pouton CW. Lipid formulations for oral administration of drugs: Non-emulsifying, self-emulsifying and self-microemulsifying drug delivery systems. Eur J Pharm Sci 2000;11:S93-8.
[Crossref] [Google Scholar] [PubMed]
- Ghosh V, Saranya S, Mukherjee A, Chandrasekaran N. Antibacterial microemulsion prevents sepsis and triggers healing of wound in wistar rats. Colloids Surf B Biointerfaces 2013;105:152-7.
[Crossref] [Google Scholar] [PubMed]
- Shakeel F, Alam P, Anwer MK, Alanazi SA, Alsarra IA, Alqarni MH. Wound healing evaluation of self-nanoemulsifying drug delivery system containing Piper cubeba essential oil. 3 Biotech 2019;9(3):1-9.
[Crossref] [Google Scholar] [PubMed]
- Marquele-Oliveira F, da Silva Barud H, Torres EC, Machado RT, Caetano GF, Leite MN, et al. Development, characterization and pre-clinical trials of an innovative wound healing dressing based on propolis (EPP-AF®)-containing self-microemulsifying formulation incorporated in biocellulose membranes. Int J Biol Macromol 2019;136:570-8.
[Crossref] [Google Scholar] [PubMed]
- Agarwal A, Kharb V, Saharan VA. Process optimisation, characterisation and evaluation of resveratrol-phospholipid complexes using Box-Behnken statistical design. Int Curr Pharm J 2014;3(7):301-8.
- Das MK, Kalita B. Design and evaluation of phyto-phospholipid complexes (phytosomes) of rutin for transdermal application. J Appl Pharm Sci 2014;4(10):51-7.
- Demir BÜ, Barlas FB, Guler E, Gumus PZ, Can M, Yavuz M, et al. Gold nanoparticle loaded phytosomal systems: Synthesis, characterization and in vitro investigations. RSC Adv 2014;4(65):34687-95.
- Mazumder A, Dwivedi A, Du Preez JL, Du Plessis J. In vitro wound healing and cytotoxic effects of sinigrin-phytosome complex. Int J Pharm 2016;498(1):283-93.
[Crossref] [Google Scholar] [PubMed]
- Lim AW, Ng PY, Chieng N, Ng SF. Moringa oleifera leaf extract–loaded phytophospholipid complex for potential application as wound dressing. J Drug Deliv Sci Technol 2019;54:101329.
- Majno G. The healing hand: Man and wound in the ancient world. London, UK: Harvard University Press, Cambridge, Mass; 2014.
- Chin CY, Ng PY, Ng SF. Moringa oleifera standardised aqueous leaf extract-loaded hydrocolloid film dressing: In vivo dermal safety and wound healing evaluation in STZ/HFD diabetic rat model. Drug Deliv Transl Res 2019;9(2):453-68. [Crossref]
[Google Scholar] [PubMed]
- Huanbutta K, Sittikijyothin W, Sangnim T. Development of topical natural based film forming system loaded propolis from stingless bees for wound healing application. J Pharm Investig 2020;50(6):625-34.
- Tong WY, bin Abdullah AY, bin Wahid MI, Hossain M, Ring LC, Lazim Y, et al. Antimicrobial wound dressing film utilizing cellulose nanocrystal as drug delivery system for curcumin. Cellulose 2018;25(1):631-8.
- Ambekar RS, Kandasubramanian B. Advancements in nanofibers for wound dressing: A review. Eur Polym J 2019;117:304-36.
- Yao CH, Chen KY, Chen YS, Li SJ, Huang CH. Lithospermi radix extract-containing bilayer nanofiber scaffold for promoting wound healing in a rat model. Mater Sci Eng C Mater Biol Appl 2019;96:850-8.
[Crossref] [Google Scholar] [PubMed]
- Almasian A, Najafi F, Eftekhari M, Ardekani MR, Sharifzadeh M, Khanavi M. Polyurethane/carboxymethylcellulose nanofibers containing Malva sylvestris extract for healing diabetic wounds: Preparation, characterization, in vitro and in vivo studies. Mater Sci Eng C Mater Biol Appl 2020;114:111039.
- Kalachaveedu M, Jenifer P, Pandian R, Arumugam G. Fabrication and characterization of herbal drug enriched guar galactomannan based nanofibrous mats seeded with GMSC's for wound healing applications. Int J Biol Macromol 2020;148:737-49.
[Crossref] [Google Scholar] [PubMed]
- Foglio MA. Pharmaceutical compositions comprising Arrabidaea chica extract in controlled release systems, production process and use thereof. Google Patents WO2013091056A1; 2013.
- Orofino DP. Topical transdermal method for delivering nutrients through the skin for expeditied wound healing and skin rejuvenation. U.S. Patent 20140199367 A1; 2014.
- Walia P, Walia A, Talwar T. A multifunctional natural wound healing matrix. WO2014147638A1; 2014.
- Al-Mutawaa MG. Ointment for healing burns and wounds. United States patent US 8,741,353; 2014.
- Calzon RL, Calzon DL. Cream for burns. EP2586450B1; 2015.
- Mirzaei E, Majidi RF, Sarkar S, Rezayat SM. Electro spun nanofibrous wound dressing and a method of synthesizing the same. United States patent US 9,101,508; 2015.
- Carlene KA, Freedom K, James N. A topical herbal healing formulation. WO2017060535; 2017.
- Yadav SK, Singla R, Kumari A. Nanobiocomposite formulation for wound healing and a process for the preparation thereof. WO2017122224A1; 2017.
- Yates KM, Proctor CA, Atchley DH. Antimicrobial silver and acemannan composition for the treatment of wounds or lesions or burns. EP2704729B1; 2018.
- Shraibom N, Singh AT, Jaggi M, Steinberg E. Herbal combinations for wound healing in fibroblasts. United States patent application 20180185428 A1; 2018.
- Tomulewicz M. Herbal preparation for accelerating wounds and skin inflammations healing and its application. C 20180318375; 2019.
- Tomulewicz M. Herbal preparation for accelerating wounds and skin inflammations healing, especially for treatment of herpes and acne, and its application. United States patent US 20190183954 A1; 2021.
- Purushottamdas AS. A herbal antiseptic and wound healing preparation. IN201721046295; 2019.
- Ritter V, Kleyman M, Bartfeld DH, Asculai E. Improved wound healing compositions comprising microspheres. EP2895209B1; 2019.
- Lafuente EG. Lipid nanoparticles for wound healing. United States patent US 10206886 B2; 2019.
- Weller KA, Weller KF, McLoughlin NJ. A topical herbal healing formulation. United States patent application US 20200330543 A1; 2020.
- Chen JW, Lu KM. Topical formulation for promoting wound healing. United States patent US 20180015133 A1; 2020.
- Yurevna EA, Vladimirovich KA, Ivanovna FI. Ointment for wound healing. RU2736214C1; 2020.
- Ahn S, Chantre C, Gonzalez GM, Parker KK. Biomimetic pro-regenerative scaffolds and methods of use thereof. United States patent application US 16/762,384; 2020.