- Corresponding Author:
- S. R. Deshpande
Department of Medicinal and Pharmaceutical Chemistry, H. S. K. College of Pharmacy, BVVS Campus, Bagalkote-587 101, India
E-mail: srinidesh71@gmail.com
| Date of Submission | 26 October 2013 |
| Date of Revision | 17 October 2014 |
| Date of Acceptance | 06 January 2015 |
| Indian J Pharm Sci 2015;77(1):24-33 |
Abstract
A series of novel 3, 4-methylenedioxybenzene scaffold incorporated 1,3,5-trisubstituted-2-pyrazoline derivatives was synthesised as potent antitubercular agents via chalcone intermediates by reaction with hydrazines. The structures of the compounds were confirmed by IR, 1HNMR, 13CNMR and mass spectral data. The novel pyrazolines were screened for in vitro antitubercular activity by almar blue dye method against M. tuberculosis H37Rv. All the compounds exhibited excellent activity that could be due to the presence of 3,4-methylenedioxybenzene frame work in the molecules. Some of the compounds also showed in vitro cytotoxicity on EAC cell lines in tryphan blue exclusion assay suggesting their safety.
Keywords
Piperonal, chalcone, antitubercular, almar blue assay, tryphan blue exclusion.
Tuberculosis (TB), a devastating chronic necrotizing infectious disease with a wide variety of manifestations caused by Mycobacterium tuberculosis, is killing approximately two million people every year with more than eight million people developing active TB each year globally[1]. It is endemic in most developing countries and resurgent in developed countries with high rates of HIV infection. With the emergence of multi-drug resistant (MDR) TB, it became the global scourge by reaching epidemic proportions[2] associated with poor treatment outcomes indicated by low cure rates of around 60% and high recurrence rates close to 30% after standard short-course TB treatment[3,4]. It was forecasted that 1.3 million MDR-TB cases needed to be treated between 2010 and 2015 in 27 countries with high MDR-TB burden[5]. In a report from World Health Organisation (WHO) for 2011, India is quoted as the highest TB burden country with an estimated incidence of 2.2 million cases[6]. Poor primary health-care infrastructure in rural areas, unregulated private health care leading to widespread irrational use of first-line and second-line antiTB drugs, spreading HIV infection, and poverty are some of the factors making difficult to control TB in India[7]. In the year 2005, 0.04% of the TB cases in India were diagnosed as MDR-TB, which rose to 0.15% (~4 times) in the year 2007[8]. This alarming scenario calls for an immediate need to develop newer, safer and potent antituberculosis drugs for effective therapy.
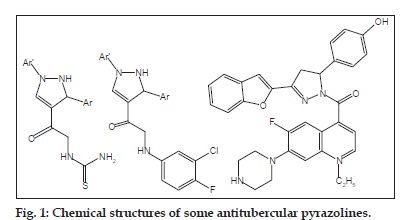
The exploration of new heterocyclics that can accommodate potency to multiple biological targets remains a continuous intriguing scientific endeavor. Pyrazolines have been known to play an important role in medicinal chemistry. Diversely substituted pyrazoline derivatives embedded with variety of functional group are important biological agents[9] and have been found to exhibit diverse biological and pharmacological activities such as antibacterial[10,11], antiamoebic[12], antidepressant[13,14], antioxidant[15,16], antiinflammatory[17], analgesic[18], hypotensive[19], anticoagulant[20], fungicidal and insecticidal[21] actions. Some pyrazoline derivatives possessing thiourea, 3-chloro-4-fluoro aniline[22] and quinoline moieties[23] (fig. 1) were also reported for good antitubercular activity.
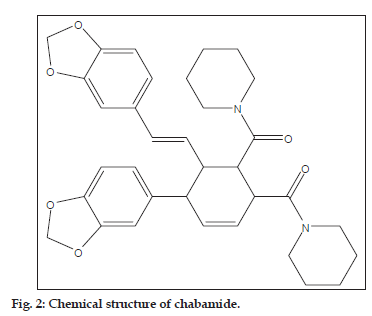
The alkaloid piperine possesses 3,4-methylenedioxybenzene scaffold in its structure and is known to be bestowed with diverse biological activities. Recently a novel piperine dimer chabamide (fig. 2), isolated from stems of Piper chaba Hunter displayed good antituberculosis activity[24]. Some semi synthetic analogs of piperine have also been reported to exhibit significant antitubercular activity[25]. These facts aroused an interest in developing novel molecular framework containing 1,3,5-trisubstituted-2-pyrazolines incorporated with 3,4-methylenedioxybenzene scaffold 2a-g and 3a-g with a hope to have noteworthy antituberculosis activity.
Materials and Methods
Piperonal and almar blue were purchased from Sigma-Aldrich (St. Louis, MO, USA) and substituted acetophenones from Sisco Research Labs Ltd (Mumbai, India). Tryphan blue, Middle brook 7H9 broth and Hank’s balanced salt solution (HBBS) were procured from Hi-Media laboratories (Mumbai, India). Rest of the chemicals used were of analytical grade. The chemicals were used as received without purification. The standard drugs isoniazid and pyrazinamide were generously gifted by K. P. Pharmaceuticals (Bidar, India), while 5-fluorouracil by Taj Pharmaceuticals (Mumbai, India). Mycobacterium tuberculosis H37Rv ATCC 25618 obtained from Microbiology Department of Maratha Mandal’s N. G. H. Institute of Dental Sciences and Research, Belgaum and EAC cell lines maintained at H. S. K. College of Pharmacy, Bagalkote were used in this study. Progress of the reaction and purity of the compounds was checked by thin layer chromatography using silica gel G coated on glass plates. The spots were resolved by iodine vapours. All yields refer to crude products before purification.
Melting points were determined on an open capillary Veego VMP-DS apparatus (Mumbai, India) and are uncorrected. UV spectra were recorded on a Systronics V530 spectrophotometer (Ahmadabad, India). IR spectra were recorded in KBr pellets on a Thermo Nicolet 200 FT-IR (Madison, USA). 1H NMR spectra were obtained in DMSO-d6 on Bruker AC200F (Rheinstatten, Germany) at 400 MHz. Chemical shifts were measured on δ scale in parts per million downfield to tetramethylsilane. Peak multiplicities are indicated as s (singlet), d (doublet), dd (doublet of doublet), t (triplet) and m (multiplet) and coupling constant (J) values are given in Hz. 13C NMR spectra were taken at 50 MHz. The mass spectra were recorded on AB Sciex API 2000 LC/MS (Framingham, USA) by ESI technique.
Synthesis of chalcones (1a-g):
Substituted acetophenones (0.06 mol) and piperonal (0.06 mol) were dissolved in ethanol (20 ml). The reaction mixture was cooled in an ice bath to 10-15° and 10 ml aqueous solution of 50% sodium hydroxide was added drop wise with continuous stirring for 4 h and then left overnight. The reaction mixture was poured into crushed ice and neutralised with dilute hydrochloric acid if necessary. The solid obtained was filtered and washed thoroughly with ice-cold water, dried and recrystallised with appropriate solvent.
3-(benzo[d][1,3]-dioxol-5-yl)-1-phenylprop-2-en-1- one (1a)
Yellow needles; Rf: 0.42, petroleum ether (60-80):benzene (7:3); % yield: 72.07; m.p.: 104°; UV (λmax, nm, DMSO): 355.0; IR, cm-1: 3012.37 (ArCH), 2922.24 (aliphatic CH), 1658.40 (C=O), 1589.16 (Ar C=C), 1020.02 (sym C-O-C), 1248.67 (asym C-O-C); 1H NMR: δ 8.01-7.98 (m, 2H, ArH), 7.74-7.71 (d, J=15.56, 1H, chlcone αH), 7.58-7.46 (m, 4H, ArH), 7.38-7.34 (d, J=15.56, 1H, chlcone βH), 7.16-7.10 (m, 2H, ArH), 6.84-6.82 (d, J=8.00, 1H, ArH), 6.01 (s, 2H, CH2 dioxymethylene).
3-(benzo[d][1,3]-dioxol-5-yl)-1-(4-bromophenyl) prop-2-en-1-one (1b)
Yellow needles; Rf: 0.42, petroleum ether (60-80): benzene (7:3); % yield: 79.09; m.p.: 143°. UV (λmax, nm, DMSO): 361.0; IR, cm-1: 3015.28 (ArCH), 2881.34 (aliphatic CH), 1655.59 (C=O), 1590.28 (Ar C=C), 1035.78 (sym C-O-C), 1250.49 (asym C-O-C), 796.48 (C-Br); 1H NMR: δ 7.87-7.85 (d, J=8.48, 2H, ArH), 7.75-7.71 (d, J=15.52, 1H, chlcone αH), 7.64-7.61 (d, J=8.48, 2H, ArH), 7.31-7.28 (d, J=15.52, 1H, chlcone βH), 7.15-7.10 (m, 2H, ArH), 6.85-6.83 (d, J=7.96, 1H, ArH), 6.02 (s, 2H, CH2 dioxymethylene).
3-(benzo[d][1,3]-dioxol-4-yl)-1-(4-chlorophenyl)prop- 2-en-1-one (1c)
Creamy needles; Rf: 0.45, petroleum ether (60-80):benzene (8:2); % yield: 76.25; m.p.: 120o; UV (λmax, nm, DMSO): 360.0; IR, cm-1: 3004.67 (ArCH), 2885.47 (aliphatic CH), 1656.96 (C=O), 1592.31 (Ar C=C), 1094.11 (sym C-O-C), 1251.22 (asym C-O-C), 799.67 (C-Cl); 1H NMR: δ 7.96-7.93 (m, 2H, ArH), 7.75-7.72 (d, J=15.6, 1H, chlcone αH), 7.48-7.45 (m, 2H, ArH), 7.33-7.29 (d, J=15.48, 1H, chlcone βH), 7.16-7.11 (m, 2H, ArH), 6.85-6.83 (d, J=8.00, 1H, ArH), 6.03 (s, 2H, CH2 dioxymethylene).
3-(benzo[d][1,3]-dioxol-5-yl)-1-(3-nitrophenyl)prop- 2-en-1-one (1d)
Brown quartz; Rf: 0.32, petroleum ether (60-80):benzene (8:2); % yield: 88.26; m.p.: 165°; UV (λmax, nm, DMSO): 364.0; IR, cm-1: 3079.03 (ArCH), 2916.79 (aliphatic CH), 1661.61 (C=O), 1587.33 (ArC=C), 1524.13 (Ar-NO2), 1086.49 (sym C-O-C), 1208.39 (asym C-O-C); 1H NMR: δ 8.82-8.81 (m, 1H, ArH), 8.44-8.32 (m, 2H, ArH), 7.83-7.79 (d, J=15.48, 1H, chlcone αH), 7.72-7.68 (t, J=1.88, 7.92, 1H, ArH), 7.37-7.34 (d, J=15.44, 1H, chlcone βH), 7.19-7.15 (m, 3H, ArH), 6.87-6.85 (d, J=8.00, 1H, ArH), 6.05 (s, 2H, CH2 dioxymethylene).
3-(benzo[d][1,3]-dioxol-5-yl-1-(3-hydroxyphenyl) prop-2-en-1-one (1e)
Yellow needles; Rf: 0.74, acetone:o-xylene (3:7); % yield: 88.30; m.p.: 163°; UV (λmax, nm, DMSO): 362.0; IR, cm-1: 3386.40 (OH), 3066.51 (ArCH), 2916.83 (aliphatic CH), 1657.48 (C=O), 1573.82 (Ar C=C), 1032.34 (sym C-O-C), 1219.22 (asym C-O-C); 1H NMR: δ 9.35 (s, 1H, OH), 7.68-7.64 (d, J=15.56, 1H, chlcone βH), 7.47-7.45 (m, 2H, ArH), 7.41-7.37 (d, J=15.52, 1H, chlcone αH), 7.33-7.29 (t, J=15.76, 7.52, 1H, ArH), 7.22 (s, 1H, ArH), 7.14- 7.12 (d J=8.04, 1H, ArH), 7.06-7.04 (m, 1H, ArH), 6.86-6.84 (d, J=8.04, 1H, ArH), 6.04 (s, 2H, CH2 dioxymethylene).
3-benzo[d][1,3]-dioxol-5-yl)-1-p-tolylprop-2-en-1-one (1f)
Yellowish green needles; Rf: 0.50, acetone:o-xylene (3:7); % yield: 78.94; m.p.: 133°; UV (λmax, nm, DMSO): 354.0; IR, cm-1: 3032.58 (ArCH), 2915.76 (aliphatic CH), 1649.70 (C=O), 1580.66 (Ar C=C), 1030.93 (sym C-O-C), 1241.79 (asym C-O-C); 1H NMR: δ 7.92-7.89 (d, J=8.20, 2H, ArH), 7.73-7.69 (d, J=15.56, 1H, chlcone αH), 7.37-7.33 (d, J=15.56, 1H, chlcone βH), 7.28-7.26 (d, J=8.04, 2H, ArH), 7.14- 7.08 (m, 2H, ArH), 6.82-6.80 (d, J=8.00, 1H, ArH), 5.99 (s, 2H, CH2 dioxymethylene), 2.41 (s, 3H, CH3).
3-benzo[d][1,3]-dioxol-5-yl)-1-(4-hydroxyphenyl) prop-2-en-1-one (1g):
Yellowish green needles; Rf: 0.56, petroleum ether (60-80):benzene (8:2); % yield: 70.27; m.p.: 204°; UV (λmax, nm, DMSO): 355.0; IR, cm-1: 3452, 84 (OH), 3036.32 (ArCH), 2907.63 (aliphatic CH), 1645.86 (C=O), 1537.94 (ArC=C), 1033.88 (sym C-O-C), 1255.70 (asym C-O-C); 1H NMR: δ 9.86 (s, 1H, OH), 7.95-7.93 (d, J=8.64, 2H, ArH), 7.67-7.63 (d, J=15.48. 1H, chlcone αH), 7.47-7.43 (d, J=15.48, 1H, chlcone βH), 7.23 (s, 1H, ArH), 7.13-7.11 (d, J=7.96, 1H, ArH), 6.91-6.89 (m, 2H, ArH), 6.85-6.83 (d, J=7.96, 1H, ArH), 6.04 (s, 2H, CH2 dioxymethylene).
Synthesis of 1,3,5-trisubstituted 2-pyrazoline derivatives (2a-g) and (3a-g)
Chalcones (0.02 mol) and hydrazine hydrate (0.03 mol) (for 2a-g)/phenyl hydrazine (0.024 mol) (for 3a-g) were taken into 15-20 ml glacial acetic and the reaction mixture was refluxed for 8 h. The resulting mixture was allowed to stand overnight and excess solvent was removed by distillation. The reaction mixture was poured into ice-cold water and neutralised with dilute sodium bicarbonate solution. The solid obtained was filtered and washed thoroughly with ice-cold water and recrystallised with appropriate solvent.
1-(5-(benzo[d][1,3]-dioxol-5yl)-3-phenyl-4,5-dihydro- 1H-pyrazol-1-yl)ethanone (2a):
Creamy yellowish needles; Rf: 0.96, butanol:petroleum ether (60-80) (3:7); % yield: 88.51; m.p.: 152°; UV (λmax, nm, DMSO): 307.0; IR, cm-1: 3058.85 (Ar-CH), 1637.62 (C=O), 1511.28 (C=N), 1058.96 (sym C-O-C), 1202.96 (asym C-O-C); 1H NMR: δ 7.74-6.67 (m, 8H, Ar-H), 5.88 (S, 2H, CH2 dioxymethylene), 5.51-5.47 (dd, J=16.36, 7.24, 4.56, 1H, pyrazoline 5H), 3.73-3.65 (dd, J=29.44, 5.88, 11.76, 1H, pyrazoline 4H), 3.14-3.08 (dd, J=22.28, 13.08, 4.60, 1H, pyrazoline 4H), 2.40 (s, 3H, COCH3); 13C NMR: δ 22.82, 40.68, 59.36, 103.65, 110.47, 111.90, 119.64, 128.85, 129.32, 132.67, 133.87, 137.32, 147.42, 149.31, 153.12, 169.50; MS: M/Z, 308.7 (M+), 309.7 (M++1), 310.7 (M++2), 267.1, 187.1, 145.0.
1-(5-(benzo[d][1,3]-dioxol-5yl)-3-(4-bromophenyl)- 4,5-dihydro-1H-pyrazol-1-yl)ethanone (2b)
Dull yellowish powder; Rf: 0.96 butanol:petroleum ether (60-80) (2:8); % yield: 43.08; m.p.: 189°; UV (λmax, nm, DMSO): 309.0; IR, cm-1: 3065.95 (Ar-CH), 2924.18 (aliphatic CH), 1650.16 (C=O), 1595.18 (C=N), 1032.92 (sym C-O-C), 1242.20 (asym C-O-C), 658.71 (C-Br); 1H NMR: δ 7.66-7.64 (d, J=8.44, 2H, Ar-H), 7.57-7.55 (d, J=8.52, 2H, Ar-H), 6.75-6.65 (m, 3H, ArH), 5.92 (s, H, CH2 dioxymethylene), 5.49-5.45 (dd, J=16.32, 7.28, 4.52, 1H, pyrazoline 5H), 3.79- 3.72 (dd, J=29.72, 5.96, 11.88, 1H, pyrazoline 4H), 3.12-3.06 (dd, J=22.48, 13.28, 4.60, 1H, pyrazoline 4H), 2.34 (s, 3H, COCH3); 13C NMR: δ 23.93, 40.16, 60.36, 102.27, 110.26, 113.36, 119.51, 126.83, 127.92, 132.63, 136.10, 137.49, 147.98, 149.74, 152.85, 169.53; MS: M/Z, 387.8 (M+), 388.8 (M++1), 386.8 (M+-1), 344.8, 265, 224.9, 222.8, 144.0.
1-(5-(benzo[d][1,3]dioxol-5yl)-3-(4-chlorophenyl)-4,5- dihydro-1H-pyrazol-1-yl)ethanone (2c)
Yellow needles; Rf: 0.94 butanol:petroleum ether (60- 80) (2:8); % yield: 22.66; m.p.: 183°. UV (λmax, nm, DMSO): 308.0; IR, cm-1: 3064.91 (Ar-CH), 2896.99 (aliphatic CH), 1649.70 (C=O), 1419.49 (C=N), 1097.40 (sym C-O-C), 1236.24 (asym C-O-C), 725.25 (C-Cl); 1H NMR: δ 7.74-7.72 (d, J=8.32, 2H, Ar-H), 7.43-7.41 (d, J=8.32, 2H, ArH), 6.76-6.66 (m, 3H, ArH), 5.93 (s, 2H, CH2 dioxymethylene), 5.49-5.45 (dd, J=16.08, 7.36, 4.36, 1H, pyrazoline 5H), 3.80- 3.73 (dd, J=29.76, 5.96, 11.88, 1H, pyrazoline 4H), 3.12-3.07 (dd, J=22.4, 13.48, 4.44, 1H, pyrazoline 4H), 2.33 (s, 3H, COCH3); 13C NMR: δ 23.63, 41.28, 61.30, 101.55, 109.36, 113.94, 119.74, 128.67, 134.75, 137.43, 145.89, 149.22, 151.70, 170.13; MS: M/Z 342.9 (M+), 343.9 (M++1), 344.9 (M++2), 301, 220.9, 179.
1-(5-(benzo[d][1,3]-dioxol-5yl)-3-(3-nitrophenyl)-4,5- dihydro-1H-pyrazol-1-yl)ethanone (2d)
Brown powder; Rf: 0.85, butanol:petroleum ether (60-80) (8:2); % yield: 56.36; m.p.: 135°; UV (λmax, nm, DMSO): 308.0; IR, cm-1: 3076.56 (Ar-CH), 2924.18 (aliphatic CH), 1657.87 (C=O), 1530.57 (C=N), 1034.84 (sym C-O-C), 1239.31 (asym C-O-C); 1H NMR: δ 8.52-8.51 (m, 1H, Ar-H), 8.27-8.07 (m, 2H, ArH), 7.64-7.60 (t, J=16.04, 8.04, 1H, ArH), 6.75-6.66 (m, 3H, ArH), 5.90 (s, 2H,CH2 dioxymethylene), 5.58-5.54 (dd, J=16.64, 7.2, 4.72, 1H, pyrazoline 5H), 3.80-3.73 (dd, J=29.68, 5.88, 11.92, 1H, pyrazoline 4H), 3.20-3.14 (dd, J=22.52, 13.00, 4.76, 1H, pyrazoline 4H), 2.43 (s, 3H, COCH3); 13C NMR: δ 21.90, 41.11, 60.39, 101.86, 109.76, 113.77, 119.48, 126.32, 127.88, 132.74, 133.87, 135.60, 136.59, 147.79, 147.20, 151.19, 171.62; MS: M/Z, 353.9 (M+), 354.9 (M++1), 312.0, 295.0, 280.0, 232.0, 207.0, 190.0, 143.9.
1-(5-(benzo[d][1,3]-dioxol-5yl)-3-(3-hydroxyphenyl)- 4,5-dihydro-1H-pyrazol-1-yl)ethanone (2e)
White powder; Rf: 0.90, butanol:petroleum ether (60-80) (8:2); % yield: 58.06; m.p.: 177°; UV (λmax, nm, DMSO): 308.0; IR, cm-1: 3236.66 (OH), 2923.22 (Ar-CH), 2853.78 (aliphatic CH), 1636.65 (C=O), 1494.02 (C=N), 1030.99 (sym C-O-C), 1208.44 (asym C-O-C); 1H NMR: δ 9.21 (s, 1H, OH), 7.23-7.14 (m, 3H, Ar-H), 6.9-6.8 (d, J=7.48, 1H, ArH), 6.73-6.65 (m, 3H, ArH), 5.91 (s, 2H, CH2 dioxymethylene), 5.47-5.43 (dd, J=15.36, 7.32, 4.12, 1H, pyrazoline 5H), 3.74-3.67 (dd, J=29.36, 6.04, 11.68, 1H, pyrazoline 4H), 3.09-3.03 (dd, J=21.88, 13.48, 4.28, 1H, pyrazoline 4H), 2.36 (s, 3H, COCH3); 13C NMR: δ 24.43, 39.71, 60.60, 101.39, 110.63, 112.47, 115.38, 119.86, 121.72, 129.32, 131.52, 135.22, 136.49, 147.53, 148.60, 150.86, 159.55, 167.98; MS: M/Z, 325.1 (M++1), 326.1 (M++2), 283.0, 266.2, 203.1, 161.0.
1-(5-(benzo[d][1,3]-dioxol-5yl)-3-p-tolyl-4,5-dihydro- 1H-pyrazol-1-yl)ethanone (2f)
White needles; Rf: 0.96, (butanol:petroleum ether (60-80) (2:8); % yield: 73.34; m.p.: 160°; UV (λmax, nm, in DMSO): 307.0; IR, cm-1: 3001.96 (ArCH), 2903.67 (aliphatic CH), 1646.40 (C=O), 1489.89 (C=N), 1028.35 (sym C-O-C), 1237.50 (asym C-O-C); 1H NMR: δ 7.62-7.60 (d, J=8.16, 2H, Ar-H), 7.22-7.20 (d, J=8.04, 2H, ArH), 6.71-6.69 (m, 3H, ArH), 5.88 (s, 2H, CH2 dioxymethylene), 5.48-5.44 (dd, J=16.24, 7.24, 4.52, 1H, pyrazoline 5H), 3.70-3.62 (dd, J=29.44, 5.88, 11.76, 1H, pyrazoline 4H), 3.11-3.06 (dd, J=22.16, 13.12, 3.80, 1H, pyrazoline 4H), 2.39 (s, 3H, COCH3); 13C NMR: δ 20.89, 23.42, 40.98, 59.26, 101.25, 109.87, 112.23, 119.94, 128.00, 129.12, 133.47, 134.27, 140.72, 147.82, 149.61, 151.72, 168.52; MS: M/Z, 322.7 (M+), 323.7 (M++1), 324.7 (M++2), 281.0.
1-(5-(benzo[d][1,3]-dioxol-5yl)-3-(4-hydroxyphenyl)- 4,5-dihydro-1H-pyrazol-1-yl)ethanone (2g)
Yellowish white needles; Rf: 0.90, butanol:petroleum ether (60-80) (3:7); % yield: 72.82; m.p.: 140°. UV (λmax, nm, DMSO): 307.0; IR, cm-1: 3450 (OH), 3008.12 (ArCH), 2925.18 (aliphatic CH), 1613.51 (C=O), 1447.62 (C-N), 1037.74 (sym C-O-C), 1276.92 (asym C-O-C); 1H NMR: δ 9.78 (s, 1H, OH), 7.59-7.56 (m, 2H, Ar-H), 6.83-6.64 (m, 5H, ArH), 5.94 (s, 2H, CH2 dioxymethylene), 5.43-5.39 (dd, J=16.00, 7.28, 4.40, 1H, pyrazoline 5H), 3.75- 3.67 (dd, J=29.44, 6.12, 4.44, 1H, pyrazoline 4H), 3.06-3.00 (dd, J=22.16, 13.32, 4.40, 1H, pyrazoline 4H), 2.92 (s, 3H, COCH3); 13C NMR: δ 24.63, 40.00, 60.13, 101.97, 110.98, 112.37, 117.77, 119.59, 129.62, 133.84, 147.83, 149.90, 151.71, 161.22, 169.71; MS: M/Z, 324.6 (M+), 325.6 (M++1), 282.5.
5-(Benzo[d][1,3]-dioxol-5-yl]-1,3-diphenyl-4,5- dihydro-1H-pyrazole (3a)
Yellow needles; Rf: 0.94, butanol:petroleum ether (60- 80) (3:7); % yield: 76.13; m.p.: 127°; UV (λmax, nm, DMSO): 362.0; IR, cm-1: 3061.13 (ArCH), 2918.40 (aliphatic CH), 1594.22 (C=N), 1037.74 (sym C-O-C), 1244.13 (asym C-O-C); 1H NMR: δ 6.72-6.74 (m, 13H, Ar-H), 5.91 (s, 2H, CH2 dioxymethylene), 5.20- 5.15 (dd, J=19.44, 5.12, 7.16, 1H, pyrazoline 5H), 3.82-3.75 (dd, J=29.4, 4.76, 12.32, 1H, pyrazoline 4H), 3.13-3.07 (dd, J=24.24, 9.92, 7.2, 1H, pyrazoline 4H); 13C NMR: δ 39.65, 54.82, 101.34, 110.75, 112.96, 117.10, 119.28, 121.00, 128.75, 129.55, 131.66, 137.28, 144.65, 147.31, 149.21, 152.34; MS: M/Z, 343 (M+), 344 (M++1), 344.9 (M++2), 340.9 (M+-1), 250.1, 239.9, 221.1, 195.1, 147.0.
5-(Benzo[d][1,3]-dioxol-5-yl]-3-(4-bromophenyl)-1- phenyl-4,5-dihydro-1H-pyrazole (3b)
Yellow needles; Rf: 0.92, butanol:petroleum ether (60-80) (3:7); % yield: 22.56; m.p.: 169°; UV (λmax, nm, DMSO): 364.0; IR, cm-1: 3012.64 (ArCH), 2922.25 (aliphatic CH), 1498.74 (C=N), 1036.77 (sym C-O-C), 1247.02 (asym C-O-C), 687.65 (C-Br); 1H NMR: δ 7.79-6.69 (m, 12H, Ar-H), 5.91 (s, 2H, CH2 dioxymethylene), 5.22-5.17 (dd, J=19.48, 5.16, 7.16, 1H, pyrazoline 5H), 3.79-3.72 (dd, J=29.44, 4.72, 12.32, 1H, pyrazoline 4H), 3.10-3.03 (dd, J=24.24, 9.92, 7.20, 1H, pyrazoline 4H); 13C NMR: δ 40.54, 53.97, 102.32, 110.63, 111.87, 117.35, 119.52, 121.26, 126,37, 128.85, 130.25, 131.94, 136.11, 137.34, 144.35, 147.28, 148.86, 152.57; MS: M/Z, 420.8 (M+), 421.8 (M++1), 419.8 (M+-1), 338.2, 330, 272.8, 239.9, 160.9.
5-(Benzo[d][1,3]-dioxol-5-yl]-3-(4-chlorophenyl)-1- phenyl-4,5-dihydro-1H-pyrazole (3c)
Yellow needles; Rf: 0.96, butanol:petroleum ether (60-80) (3:7); % yield: 43.82; m.p: 161°; UV (λmax, nm, DMSO): 364.0; IR, cm-1: 3025.34 (ArCH), 2920.32 (aliphatic CH), 1497.78 (C=N), 1036.77 (sym C-O-C), 1247.99 (asym C-O-C), 749.37 (C-Cl); 1H NMR: δ 7.86-6.70 (m, 12H, Ar-H), 5.92 (s, 2H, CH2 dioxymethylene), 5.22-5.17 (dd, J=19.52, 5.12, 7.20, 1H, pyrazoline 5H), 3.79-3.72 (dd, J=29.40, 4.72, 12.28, 1H, pyrazoline 4H), 3.10-3.04 (dd, J=24.32, 9.88, 7.20, 1H, pyrazoline 4H); 13C NMR: δ 40.23, 54.62, 101.72, 110.43, 113.33, 116.80, 119.35, 121.26, 128.69, 129.58, 130.58, 134.89, 137.63, 143.95, 147.27, 149.43, 151.89; MS: M/Z, 377 (M+), 378 (M++1), 379 (M++2), 338.2, 283.9, 239.9, 229, 177.
5-(Benzo[d][1,3]-dioxol-5-yl)-3-(3-nitrophenyl)-1- phenyl-4,5-dihydro-1H-pyrazole (3d)
Dull brown powder; Rf: 0.92, butanol:petroleum ether (60-80) (8:2); % yield: 50.18; m.p.: 132°; UV (λmax, nm, DMSO): 364.0; IR, cm-1: 3027.87 (ArCH), 2923.22 (aliphatic CH), 1595.18 (C=N), 1037.74 (sym C-O-C), 1244.13 (asym C-O-C); 1H NMR: δ 8.41-6.71 (m, 12H, Ar-H), 5.89 (s, 2H, CH2 dioxymethylene), 5.26-5.22 (dd, J=19.48, 5.48, 7.00, 1H, pyrazoline 5H), 3.81-3.73 (dd, J=29.64, 4.60, 12.52, 1H, pyrazoline 4H), 3.12-3.06 (dd, J=24.16, 10.12, 7.00, 1H, pyrazoline 4H); 13C NMR: δ 41.20, 54.77, 102.24, 110.65, 112.69, 117.34, 119.12, 121.26, 126.34, 127.20, 130.42, 132.75, 134.87, 137.12, 143.98, 147.55, 149.31, 152.68; MS: M/Z, 387 (M+), 388 (M++1), 339.2, 338.2, 321.1, 239.9.
5-(Benzo[d] [1, 3] dioxol-5-yl)-3-(3-hydroxyphenyl)- 1-phenyl-4, 5-dihydro-1H-pyrazole (3e)
Brown powder; Rf: 0.91, butanol:petroleum ether (60-80) (8:2); % yield: 48.25; m.p.: 147°; UV (λmax, nm, DMSO): 361.0; IR, cm-1: 3420.87 (OH), 3017.34 (ArCH), 2926.78 (aliphatic CH), 1578.16 (C=N), 1038.28 (sym C-O-C), 1249.16 (asym C-O-C); 1H NMR: δ 9.18 (s, 1H, OH), 7.62-6.67 (m, 12H, ArH), 5.92 (S, 2H, CH2 dioxymethylene), 5.46-5.42 (dd, J=19.50, 5.10, 7.18, 1H, pyrazoline 5H), 3.75-3.68 (dd, J=29.38, 4.70, 12.26, 1H, pyrazoline 4H), 3.10- 3.03 (dd, J=24.30, 9.86, 7.18, 1H, pyrazoline 4H); 13C NMR: δ 40.22, 53.92, 100.86, 110.45, 112.30, 115.38, 117.46, 119.35, 121.36, 130.73, 131.27, 136.85, 144.35, 147.72, 148.76, 152.82, 159.42; MS: M/Z, 358.8 (M+), 359.7 (M++1), 360.7 (M++2), 314.7, 313.6.
5-(Benzo[d][1, 3]-dioxol-5-yl]-1-phenyl-3-p-tolyl-4,5- dihydro-1H-pyrazole (3f)
Light yellowish spongy crystals; Rf: 0.65, butanol:petroleum ether (60-80) (2:8); % yield: 26.31; m.p.: 156°; UV (λmax, nm, DMSO): 360.0; IR, cm-1: 3037.57 (ArCH), 2910.93 (aliphatic CH), 1594.74 (C=N), 1034.21 (sym C-O-C), 1247.02 (asym C-O-C); 1H NMR: δ 7.61-6.70 (m, 12H, Ar-H), 5.90 (s, 2H, CH2 dioxymethylene), 5.17-5.12 (dd, J=19.4, 4.96, 7.20, 1H, pyrazoline 5H), 3.80-3.73 (dd, J=29.28, 4.76, 12.00, 1H, pyrazoline 4H), 3.11-3.05 (dd, J=24.24, 9.84, 7.20, 1H, pyrazoline 4H), 2.36 (s 3H, CH3); 13C NMR: δ 22.62, 40.53, 54.68, 101.50, 110.58, 112.35, 117.68, 119.93, 121.72, 127.46, 130.28, 130.62, 135.31, 137.47, 140.86, 144.69, 147.52, 148.13, 152.63; MS: M/Z, 356.7 (M+), 357.8 (M++1), 358.8 (M++2), 312.7, 311.6.
5-(Benzo[d][1,3]-dioxol-5-yl)-3-(4-hydroxyphenyl)- 1-phenyl-4,5-dihydro-1H-pyrazole (3g)
Green fluorescent powder; Rf: 0.92, butanol: Petroeum ether (60-80) (8:2); % yield: 44.35; m.p.: 140o; UV (λmax, nm, DMSO): 358.0; IR, cm-1: 3590.61 (OH), 3043.19 (ArCH), 2920.32 (aliphatic CH), 1594.22 (C=N), 1037.74 (sym C-O-C), 1246.06 (asym C-O-C); 1H NMR: δ 7.59-6.65 (m, 12H, Ar-H), 5.91 (s, 2H, CH2 dioxymethylene), 5.11 (s, 1H, pyrazoline 5H), 3.76-3.70 (dd, J=21.12, 7.08, 7.00, 1H, pyrazoline 4H), 3.06 (s, 1H, OH), 1.26-1.23 (dd, J=14.08, 2.08, 7.04, 1H, pyrazoline 4H); 13C NMR: δ 40.85, 54.36, 101.78, 110.35, 113.16, 116.80, 119.67, 121.34, 129.74, 137.47, 144.42, 147.55, 149.10, 152.32, 161.64; MS: M/Z, 358.8 (M+), 359.9 (M++1), 360.9 (M++2), 314.8, 313.7.
Antitubercular activity
The antitubercular activity of compounds was assessed against M. tuberculosis H37Rv by microplate alamar blue assay[26]. First, 200 μl of sterile deionised water was added to all outer perimeter wells of sterile 96 wells plate to minimize the evaporation of medium in the test wells during incubation. The wells received 100 μl of the Middle brook 7H9 broth and serial dilution of compounds dissolved in DMSO were made directly on plate from 100 to 0.2 μg/ml. The above said wells were inoculated with 100 μl of 2000 cfu/ml of organisms in Middle brook 7H9 broth. Plates were covered and sealed with parafilm and incubated at 37º for five days. After this, 25 μl of freshly prepared 1:1 mixture of almar blue reagent and 10% tween 80 was added to the plate and incubated for 24 h. The same method was followed for control (DMSO) and standard drugs (pyrazinamide and isoniazid). A blue colour in the well was interpreted as no bacterial growth, and pink colour was scored as growth. The MIC was defined as lowest drug concentration which prevented the colour change from blue to pink.
Cytotoxic activity
The synthesized compounds were tested for in vitro cytotoxicity by tryphan blue exclusion assay method[27] against EAC cells. On the 15th day, the EAC cells were aspirated aseptically from the peritoneal cavity of the mice and washed with HBBS and centrifuged for 15 min at 1500 rpm in a cold centrifuge. The pellet was resuspended with HBBS and the process was repeated three times. Finally the cells were suspended in a known quantity of HBBS and the cell count was adjusted to 1×106 cells/ ml. This suspension measuring 0.1 ml was taken in eppendoRf tubes and 0.1 ml of different concentration of sample and 5-fluorouracil in DMSO or plane DMSO was added and incubated at 37º for 3 h. The viable cells were counted on a hemocytometer using tryphan blue dye exclusion method. The percent cytotoxicity was determined using the formula, % cytotoxicity =100×(Tdead–Cdead)/Ttotal, where, Tdead is the number of dead cells in the treated group, Cdead is that in the control group, and Ttotal is the total number of cells in the treated group.
Results and Discussion
Despite numerous reported methods for the preparation of 2-pyrazolines, the Fischer and Knoevenagel method of reacting α,β-unsaturated aldehydes and ketones with phenyl hydrazine in acetic acid is still the most popular one[28]. Chalcones are 1,3-diaryl-2-propen 1-ones and serve as important intermediates for the construction of corresponding 2-pyrazolines. Since piperonal is an aldehyde with 3,4-methylenedioxy benzene moiety, it was aptly used as a precursor for the development of 3,4-methylenedioxy benzene incorporated 2-pyrazolines. A series of chalcones (1a-g) was synthesized according to Claisen-Schmidt reaction[29] by condensing various substituted acetophenones with piperonal in presence of 50% sodium hydroxide solution at 10-15º in good to excellent yields. The cyclocondensation of chalcones 1a-g with hydrazine or phenylhydrazine in acetic acid yielded 1-acetyl-3,5-disubstituted-2-pyrazolines 2a-g or 1-phenyl-3,5-disubstituted 2-pyrazolines 3a-g, respectively in moderate to good yields[30]. However, 2,4-dinitrophenylhydrazine did not afford the corresponding 2-pyrazolines under similar conditions probably due to its poor solubility in the reaction medium. The synthetic approach of title compounds 2a-g and 3a-g is illustrated in Scheme 1. The structure of the synthesized compounds was assigned on the basis of UV, IR, 1H NMR, 13C NMR and Mass spectral analysis.
The physicochemical data of compounds 1a-g, 2a-g and 3a-g are presented in Table 1. Chalcones 1a-g in their UV spectra exhibited absorbance in the range of 354-364 nm due to n-π* transitions typical of α,β- unsaturated ketones. The IR spectra of compounds 1a-g showed characteristic α,β-unsaturated C=O stretching at 1603-1661 cm-1 and C=C stretching frequencies at 1524-1590 cm-1 . In 1H NMR spectra, the protons of α, β-unsaturated carbon atoms have displayed two doublets in the range of δ 7.34-7.89 amidst other protons.
The IR spectra of the derivatives 2a-g and 3a-g showed C=N stretching frequencies of pyrazoline ring at 1498-1649 cm-1 , aromatic C-H stretching at 2903- 3068 cm-1 , symmetric C-O-C stretch at 1028-1097 cm-1 , aliphatic C-H stretch at 3080-2920 cm-1 and absence of bands at 1603-1661 cm-1 (characteristic of α,β-unsaturated ketones). In addition, compounds 2a-h displayed C=O stretching of amide at 1613- 1657 cm-1 . In 1HNMR spectra, the compounds 2a-g and 3a-g exhibited two doublets of doublets in the range of δ 3.10-3.78 due to two methylene protons at C-4 of pyrazoline ring. The proton at 5th position of pyrazoline also showed a doublet of doublet at the range of δ 5.44-5.48 suggesting pyrazoline ring protons are bonded with carbon atoms on a spatially different environment. The aromatic protons appeared as multiplet at δ 6.65-8.81 and two methylene protons of methylenedioxy portion as a singlet at δ 5.88-5.94. The compounds 2a-g also exhibited singlet at δ 2.29- 2.43 accounting three protons of acetyl group. In 13C NMR spectra, the pyrazoline C-3 and C-4 signals appeared at δ ~152 and 40 whereas pyrazoline C-5 in case of 2a-g appeared at δ ~60 and in 3a-g at δ ~55. The methylenedioxy carbon appeared at δ ~102 in both 2a-g and 3a-g. In addition, compounds 2a-g displayed acetyl carbonyl carbon at δ ~170. Further both the compounds 2a-g and 3a-g showed M+, M++1 peaks in their mass spectra which were in close agreement with their molecular weights.
The compounds 2a-g and 3a-g were screened for in vitro antitubercular activity by almar blue dye method against the best studied virulent laboratory strain Mycobacterium tuberculosis H37Rv ATCC 25618. This method uses a thermally stable reagent and shows good correlation with proportional and BACTEC radiometric methods. The activity data is shown in Table 2. Generally, all the tested compounds exhibited potency more than pyrazinamide the secondline antitubercular drug and less than isoniazid (INH), the first-line antitubercular drug. All the compounds except 2c, 3a and 3e exhibited the MIC at 0.8 μg/ml. The compounds 2c, 3a and 3e showed the MIC at 1.6 μg/ml whereas pyrazinamide and INH exhibited MIC at 3.125 and 0.5 μg/ml, respectively. It is well documented that, the highly lipophilic compounds are bound to show good antitubercular activity, as they have high tendency to penetrate the high lipid content cell wall of M. tuberculosis[31]. The excellent activity exhibited by title compounds could be due to their higher lipophilicity and thereby enhanced cell wall permeation suggesting a probable intracellular mode of action. In case of 1,3,5-trisubstituted 2-pyrazolines 3a-g, presence of polar group on 3-position of 3-phenyl ring reduced the activity as observed with compound 3e whereas, no such effect was observed when it is in 4-position. Substitution with non polar substituents either electron releasing or withdrawing favoured the activity of compounds 3a-g. On the contrary, in case of 1-acetyl 3,5-disubstittuted pyrazolines 2a-g, the presence of polar substituent on 3-phenyl ring sustained activity. The excellent antitubercular activity exhibited by compounds 2a-g and 3a-g could be attributed to the presence of 3,4-methylenedioxy benzene scaffold in them.
| Compound | R | Mol. formula | Mol. Wt. | Recrystallisation solvent |
|---|---|---|---|---|
| 1a | H | C16H12O3 | 252.26 | EtOH |
| 2a | C18H16N2O3 | 308.33 | EtOH | |
| 3a | C22H18N2O2 | 342.39 | EtOH | |
| 1b | 4-Br | C16H11BrO3 | 331.16 | EtOH |
| 2b | C18H15BrN2O3 | 387.23 | EtOH | |
| 3b | C22H17BrN2O2 | 421.29 | EtOH | |
| 1c | 4-Cl | C16H11ClO3 | 286.71 | EtOH |
| 2c | C18H15ClN2O3 | 342.78 | EtOH | |
| 3c | C22H17ClN2O2 | 376.84 | EtOH | |
| 1d | 3-NO2 | C16H11NO5 | 297.26 | 1:1 EtOH-AcMe |
| 2d | C18H15N3O5 | 353.33 | 7:3 EtOH-EtOAc | |
| 3d | C22H17N3O4 | 387.39 | 7:3 EtOH-EtOAc | |
| 1e | 3-OH | C16H12O4 | 268.26 | EtOH |
| 2e | C18H16N2O4 | 324.33 | EtOH | |
| 3e | C22H18N2O3 | 358.39 | EtOH | |
| 1f | 4-CH3 C17H14O3 | 266.29 | EtOH | |
| 2f | C19H18N2O3 | 322.36 | EtOH | |
| 3f | C23H20N2O2 | 356.42 | EtOH | |
| 1g | 4-OH | C16H12O4 | 268.26 | EtOH |
| 2g | C18H16N2O4 | 324.33 | EtOH | |
| 3g | C22H18N2O3 | 358.39 | Aq. EtOH | |
strong>Table 1: Physicochemical data of compounds 1a-g, 2a-g and 3a-g
| Compound | Antitubercular | Cytotoxic activity % | ||
|---|---|---|---|---|
| activity | Inhibition (µg/ml) | |||
| MIC (µg/ml) | 50 | 100 | 200 | |
| 2a | 0.8 | - | - | - |
| 2b | 0.8 | - | - | - |
| 2c | 1.6 | - | - | - |
| 2d | 0.8 | - | - | - |
| 2e | 0.8 | - | - | - |
| 2f | 0.8 | - | - | - |
| 2g | 0.8 | - | - | - |
| 3a | 1.6 | 27.15 | 29.82 | 28.42 |
| 3b | 0.8 | 11.02 | 19.82 | 18.82 |
| 3c | 0.8 | 15.82 | 14.82 | 02.82 |
| 3d | 0.8 | 05.82 | 06.22 | 05.12 |
| 3e | 1.6 | 12.12 | 17.72 | 14.82 |
| 3f | 0.8 | 01.47 | 24.48 | 12.12 |
| 3g | 0.8 | 11.15 | 25.48 | 13.82 |
| INH | 0.5 | - | - | - |
| Pyrazinamide | 3.12 | - | - | - |
| 5-Fluorouracil | - | 50.83 | 57.38 | 66.19 |
Table 2: Antitubercular and cytotoxic activity of compounds 2a-g and 3a-g
In vitro short term cytotoxicity of test compounds can be assessed by determining the percentage viability of Ehrlich Ascites Carcinoma (EAC) cells using tryphan blue exclusion technique. The cytotoxicity results of 1-phenyl-3,5-disubstituted 2-pyrazolines 3a-g suggested that the activity did not increase in a dose dependant manner. The compounds showed a cut off activity at 100 μg/ml and beyond this the activity was either decreased or almost remained same. However, the standard drug 5-fluorouracil exhibited cytotoxicity that was found to be linear with its dose. The unsubstituted compound 3a showed highest activity among the series making it more toxic, while substitution with electron releasing substituents reduced activity moderately in case of compounds 3g and 3f. In compounds with electron withdrawing substituents (3b and 3c), the activity drastically decreased making those compounds safer. The overall % inhibition by compounds 3a-g was found to be in the range of 1-30% (Table 2) suggesting they are less cytotoxic and safer. The cytotoxicity of compounds 2a-g could not be determined owing to their precipitation in the test medium.
In this study, it was conceived and synthesized novel 1-acetyl-3,5-disubstituted 2-pyrazolines (2a-g) and 1-phenyl-3,5-disubstituted 2-pyrazolines (3a-g) incorporating 3,4-methylenedioxy benzene scaffold as potent antitubercular agents through a simple and well-known method via chalcone intermediates. Their structures were assigned based on IR, 1HNMR, 13CNMR and Mass spectral data. The compounds 2a-g and 3a-g exhibited an excellent and encouraging preliminary in vitro antitubercular activity that is higher than pyrazinamide and comparable with isoniazid against M. tuberculosis H37Rv when tested by almar blue method. The promising activity of these compounds could be probably attributed to the presence of 3,4-methylenedioxy benzene scaffold. The cytotoxicity of compounds 3a-g was found to be less against EAC cells by tryphan blue exclusion assay making them safer compounds. Among the compounds 3a-g, those with electron withdrawing substituents 3c and 3d emerged better in terms of the ratio of efficacy to toxicity. Thus, our study on 1,3,5-trisubstituted- 2-pyrazolines incorporated with 3,4-methylenedioxy benzene scaffold demonstrated them as new active leads for antitubercular activity that could provide a powerful incentive for further research in this area.
Acknowledgements
The authors thank SAIF, Chandigarh for providing NMR and O2H Research Centre, Ahmadabad for Mass spectra. The expertise in antitubercular activity rendered by Prof. K. G. Bhat, Department of Microbiology, Maratha Mandal’s N. G. H. Institute of Dental Sciences and Research, Belgaum is highly appreciated.
References
- World Health Organization. Treatment of TB: Guidelines for National Programmes. Geneva: WHO; 2003.
- duToit LC, Pillay V, Danckwerts MP. Tuberculosis chemotherapy: Current drug delivery approaches. Respir Res 2006;7:118-35.
- Espinal MA, Kim SJ, Suarez PG, Kam KM, Khomenko AG, Migliori GB, et al. Standard short-course chemotherapy for drug-resistant tuberculosis:Treatment outcomes in 6 countries. J Am Med Assn 2000;283:2537-45.
- Migliori GB, Espinal M, Danilova ID, Punga VV, Grzemska M, Raviglione MC. Frequency of recurrence among MDR-tB cases ‘successfully’ treated with standardised short-course chemotherapy. Int J Tuberc Lung Dis 2002;6:858-64.
- World Health Organization. Global tuberculosis control: A short update to the 2009 report. Geneva: 2009. Available from: http://www.who.int/ tb/publications/global_report/2009/update/en/index.html [Last accessed on 2012 Dec 21].
- World Health Organization. Global Tuberculosis Control 2012. Geneva: WHO; 2012. Available from: Http://www.who.int/tb/publications/ global_report/[Last accessed on 2012 Dec 23].
- Sandhu GK. Tuberculosis: Current Situation, Challenges and Overview of its Control Programs in India. J Glob Infect Dis 2011;3:143-50.
- World Health Organization. Global Tuberculosis Report. Geneva: WHO; 2009.
- Rahman MA, Siddiqui AA. Pyrazoline derivatives: A worthy insight into the recent advances and potential pharmacological activities. Int J Pharma Sci Drug Res 2010;2:165-75.
- Kolhe SV, Doshi AG, Raut AW. Synthesis of new pyrazolines and their derivatives. Indian J HeterocyclChem 2002;11:281-2.
- Holla SB, Veerendra B, Shivananda MK, Latha KP, Vaidya VP. Antimicrobial, analgesic and anthelmentic activity of some arylfurylpyrazolines. Indian J HeterocyclChem 2003;12:385-6.
- Mbarki S. Dguigui K, Hallaoui ME. Construction of 3D-QSAR models to predict antiamoebic activities of pyrazoline and dioxazoles derivatives. J Mater Environ Sci 2011;2:61-70.
- Palaskaa E, Aytemira M, Uzbay IT, Erola D. Synthesis and antidepressant activities of some 3,5-diphenyl-2-pyrazolines. Eur J Med Chem 2001;36:539-43.
- Prasad YR, Rao AL, Prasoona L, Murali K, Kumar PR. Synthesis and antidepressant activities of some 1,3,5-triphenyl-2-pyrazolines and 3-(2-hydroxynapthalen-1-yl)-1,5-diphenyl-2-pyrazolines. Bioorg Med ChemLett 2005;15:5030-4.
- Hazra K, Nargund LV, Rashmi P, Sharadchandra JNN, Nanda B. Synthesis and antioxidant activity of fluorobenzothiazolopyrazoline. Der Chem Sin 2011;2:149-57.
- Mondal P, Jana S, Mahanti B, Kanthal LK, Banerjee. Synthesis and evaluation of some novel pyrazoline derivatives of indol 2,3-dione as a potential antioxidant and antibacterial agents. Int J Pharm Sci Tech 2009;3:8-11.
- Shoman ME, Abdelaziz M, Aly OM, Farag HH, Morsy MA. Synthesis and investigation of antiinflammatory activity and gastric ulcerogenicity of novel nitric oxide donating-pyrazoline derivatives. Eur J Med Chem 2008;22:1-9.
- Amir M, Kumar H, Khan SA. Synthesis and pharmacological evaluation of pyrazoline derivatives as new antiinflammatory and analgesic agents. Bioorg Med ChemLett 2008;18:918-22.
- Zitounia GT, Chevallet P, Kilic FS, Erolc K. Synthesis of some thiazolyl-pyrazoline derivatives and preliminary investigation of their hypotensive activity. Eur J Med Chem 2000;35:635-41.
- Levai A, Jeko J. Synthesis of 5-aryl-1-carboxyphenyl-3-(3-coumarinyl)- 2-pyrazolines. Arkivoc 2009;6:63-70. Zhao PL, Wang F, Zhang MZ, Liu ZM, Huang W, Yang GF. Synthesis, fungicidal and insecticidal activities of ß-methoxyacrylate containing N-acetyl pyrazoline derivatives. J Agric Food Chem 2008;56:10767-73.
- Chetan BP, Sreenivas MT, Bhat AR. Synthesis and evaluation of certain pyrazolines and related compounds for their anti-tubercular, antibacterial and antifungal activities. Indian J HeterocyclChem 2004;13:225-8.
- Babu HV, Manna SK, Sneha, Srinivasan KK, Bhat V. Synthesis and biological evaluation of 1,3,5-trisubstituted pyrazolines bearing benzofuran. Indian J HeterocyclChem 2004;13:253-6.
- Rukachaisirikul T, Prabpai S, Champung P, Suksamrarn A. Chabamide, a novel piperine dimer from stems of Piper chaba. Planta Med 2002;68:853-5.
- Gupta OP, Nath A, Gupta SC, Srivastava TN. Preparation of semi synthetic analogue of Piper amides and their antitubercular activity. Bull Med Ethnobot Res 1980;1:99-106.
- Collins EA, Franzblow SG. Microplatealamar blue assay versus BACTEC 460 system for high throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob Agents Chemother 1997;41:1004-9.
- Pienta KJ, Lehr JE. Inhibition of prostate cancer cell growth by estamurine and etoposide: Evidence for interaction of the nuclear matrix. J Urol 1993;49:1622-5.
- Levai A, Jeko J. Synthesis of carboxylic acid derivatives of 2-pyrazolines. Arkivoc 2007;1:134-45.
- Furniss BS, Hannaford AT, Smith PWG, Tatchell AR, editors. Vogel’s text book of practical organic chemistry. New Delhi: Pearson Education Pvt Ltd; 1989.
- Hafez OM, Ahmed KM, Haggag EE. Synthesis of some potentially bioactive compounds from visnaginone. Molecules 2001;6:396-405.
- Lemke TL, Williams DA, Roche VF, Zito SW. editors. Foye’s Principles of Medicinal Chemistry. 6th ed. New Delhi: Wolters Kluwer (India) Pvt. Ltd.; 2008. p. 1127-9.