- *Corresponding Author:
- N. Siddiqui
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Jamia Hamdard (Hamdard University), New Delhi-110 062, India.
E-mail: nadeems_03@yahoo.co.in
| Date of Submission | 12 May 2005 |
| Date of Revision | 04 April 2006 |
| Date of Acceptance | 04 September 2006 |
| Indian J Pharm Sci, 2006, 68 (5): 549-555 |
Abstract
The human immunodeficiency virus has been shown to be the causative agent for acquired immunodeficiency syndrome (AIDS). The human immunodeficiency virus encodes for unique aspartyl protease that is essential for the production of enzymes and proteins in the final stages of maturation. Protease inhibitors act by preventing the formation of functional proteins from precursor proteins, which are vital for production of mature infectious viral particles. This review summarizes the data documenting the pharmacology and chemistry of non-peptide protease inhibitors that may be used as therapeutic agents against the human immunodeficiency virus infection. These agents are structural mimics of peptides with little or no peptidic character, thus overcoming various pharmacological problems of peptidic protease inhibitors. Structure-based drug designs of potent protease inhibitors, discovered through broad screening, have been developed into various clinical candidates for the treatment of AIDS. Non-peptide protease inhibitors could provide a useful model in the further search for novel compounds with even more pertinent pharmacological and pharmacokinetic profiles.
Human immunodeficiency virus (HIV), the causative agent of acquired immunodeficiency syndrome (AIDS) [1–4] now infects an estimated 60 million people worldwide, out of which 4 million are in India. Cure has not yet been found for this fatal disease. Rapid advances in molecular biology, along with 3-D elucidation of HIV protein, have led to new drug-targeting approaches for designing antiviral agents that specially bind key regulatory proteins that are essential for HIV replication. The HIV-1 protease is one such enzyme crucial for maturation and assembly of infectious viral particles. Thus by inhibiting HIV-1 protease activity [5-16], a potential cure for AIDS is at hand. Armed with 3-D crystal structure of HIV-1 protease, rational drug design approaches have been widely employed in the development of HIV protease inhibitors; while in clinical trials, existent HIV-1 protease inhibitors show promise in causing an initial dramatic decline in viral plasma RNA in HIV-infected individuals, most patients taking protease inhibitors alone. Failure of HIV-1 protease inhibitor therapy is attributed to the development of resistant viral protease.
AIDS, the acquired immunodeficiency syndrome, was first reported in the Morbidity and Mortality Weekly Report, Los Angles, as pneumocystis pneumonia in 1981. Since then, AIDS has become the most devastating disease that mankind has ever faced. India ranks second after South Africa in the number of people with HIV infection. Tuberculosis is the leading opportunistic infection in HIV positive persons. The AIDS epidemic is one of the greatest challenges being faced by the medical community. Infection with HIV is a dynamic process characterized by vigorous viral replication, CD4 lymphocyte depletion and profound immunodeficiency. The error-prone nature of HIV reverse transcriptase promotes rapid evolution of genetic diversity and a remarkable propensity to develop resistance to antiretroviral agents. Improved understanding of viral pathogenesis, as well as genetic basis of resistance, has fuelled the rapid and rational development of numerous effective drugs that target either HIV reverse transcriptase or HIV protease. Various multidrug regimens have been shown to inhibit viral replication effectively, reverse CD4 cell depletion and reduce morbidity and mortality dramatically. Despite much progress, many patients do not benefit from antiretroviral therapy due to emergence of viral resistance, adverse effects of chronic therapy or inability to adhere to complex regimens.
AntiHIV therapy
Antiretroviral treatment for HIV-positive persons was first introduced in 1986. It includes nucleoside reverse transcriptase inhibitors (NRTIs), e.g., zidovudine, stavudine, lamvudine, didanosine and abacavir; nonnucleoside reverse transcriptase inhibitors (NNRTIs), e.g., nevirapine, efavirenz and delavirdine; protease inhibitors (PIs), e.g., indinavir, ritonavir, lopinavir, nelfinavir, saquinavir, tipranavir; and others at various stages of clinical trial, e.g., KNI-272, mozenavir and atazanavir. An increasing number of HIV protease inhibitors are currently undergoing clinical evaluations [17–28]. The peptide-derived protease inhibitors have poor pharmacokinetic profile, which includes low oral bioavailability and rapid excretion. Hence there is a need of potent non-peptide protease inhibitors. Recent advances have resulted in HIV protease inhibitors with reduced peptidic character and more bioavailability without cross resistance to the currently approved antiviral agents. Although protease inhibitors have radically improved the life of AIDS patients by highly active antiretroviral therapy, the rapid emergence of several viral strains resistant to one or more of drugs for AIDS has now become the most important issue in the treatment of HIV infection.
Structure and function of HIV protease
Through X-ray crystallography techniques, the 3-D structure of HIV-1 protease has been extensively studied and characterized. In its mature form, the viral protease exists as a dimer, whose subunits each consists of 99 amino acids. The folded subunits together interact to form a core hydrophobic, cylindrical catalytic cavity (24 A long × 6-8 A in diameter) and two flexible flaps (one per unit) that can close around the substrate. Centred in the hydrophobic active sites are two symmetrically disposed substratecatalytic aspartyl residues (Asp 25 and Asp 25') that are involved in the hydrolysis of the peptide bond [29]. Studies have shown that the hydrophobic cavern can hold six amino acids of the substrate in an extended conformation for cleavage. Because structural and enzymatic proteins of HIV virus are translated as part of two polyprotein precursors (Gag and Gag/Pol), cleavage of these precursors to generate gag matrix (p17), capsid (p24), nucleocapsid (p15) protein, Pol reverse transcriptase, integrase enzymes and other viral proteins is vital for the production of mature infectious viral particles [30]. Since this cleavage process is critical to viral propagation, inhibition of viral protease proves to be an attractive drug target. Protease inhibitors act by binding reversibly to the active site of HIV proteases. This prevents the proteases from cleaving the viral precursor polypeptide and blocks subsequent viral maturation. Most HIV protease inhibitors have poor absorption, poor oral bioavailability, short serum half life value, high susceptibility to hydrolysis by degradative enzymes and undergo oxidative metabolism by CYP3A4 and rapid excretion [31]. Toxicities include nausea, vomiting, diarrhoea, paraesthesias, glucose intolerance, diabetes, hypercholesterolemia and hypertriglyceridemia. Because of these limitations of peptidomimetic inhibitors, efforts have been made to minimize pharmacological problems by developing inhibitors that are structural mimics of peptides but with little or no peptidic character. Despite rapid advances in developing potent protease inhibitors, their effectiveness has been hindered by the emergence of drug-resistant viral variants. Emergence of multidrug-resistant virus among patients treated with current antiHIV agents has created an urgent need for new antiretroviral agents, especially non-peptide protease inhibitors.
Dihydropyran analogues as potent nonpeptidic inhibitors of HIV protease
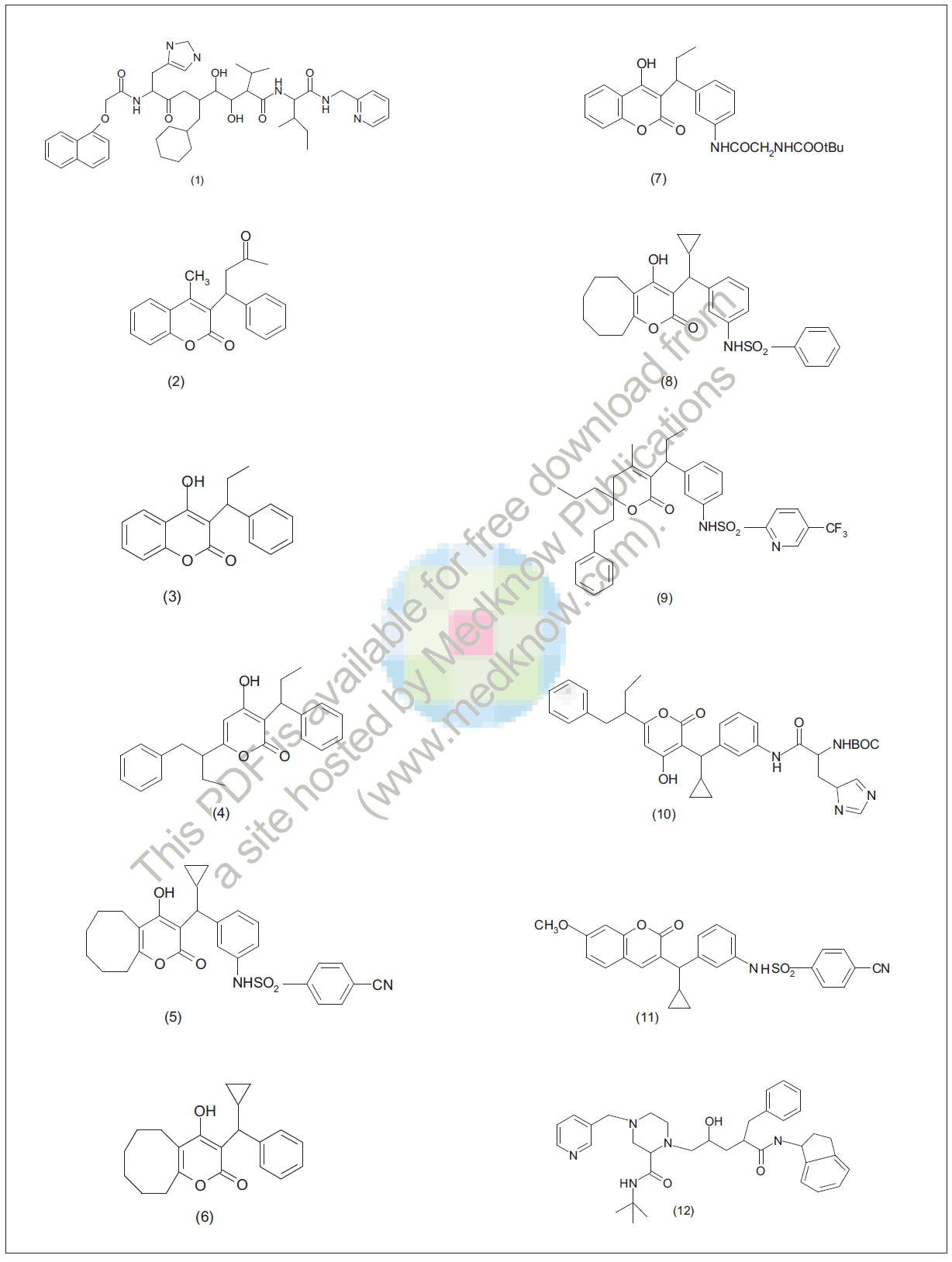
The X-ray structure showed that 4 hydroxy group of coumarin ring forms hydrogen bonds with two key catalytic aspartic residues (Asp 25 and Asp 25´), and two oxygen of lactone ring form hydrogen bonds with isoleucine residues, thus replacing the water molecule of peptide-type inhibitors bound to HIV protease. During lead optimization, the phenyl and ethyl groups on compound (3) each occupied a peptide-binding pocket. The rigid coumarin ring on the other hand prevented access to good binding to additional sites; thus, substituted dihydropyran analogues were investigated.
Initial approaches for developing HIV-1 protease inhibitors were based on characterizing substrate- protease. The first generation of HIV protease inhibitors are peptidomimetic compounds containing transition state inserts in place of dipeptide cleavage sites of natural substrates for viral enzyme. Optimization of peptide leads produced potent protease inhibitor PNU- 75875 (1) having extremely poor pharmacokinetic properties. Anticoagulant warfarin (2) selected as a lead had only weak enzyme inhibitory activity. another related compound phenprocoumon (3) having improved potency, both having excellent pharmacokinetic properties. Kinetic analysis of phenprocoumon indicated that it was a competitive inhibitor of HIV protease. Substituted pyrone analogues were investigated, leading to first generation clinical candidate PNU-96988 (4); it was at least 25 times more potent than the original lead template phenprocoumon. The modelling hypothesis that increasing the flexibility of coumarin nucleus in compound (2) would provide additional binding, led to cyclooctylpyrone
analogues PNU-103017 (6). Based upon drug design, additional binding site was realized by attaching an amide group at the meta position of C-3 phenyl group (7). Further modelling based on the structure of compound (7) bound to HIV protease suggested that replacement of the amide carbonyl with an SO2 group (8) could result in an additional hydrogen bonding; subsequent optimization of the sulphonamide moiety led to clinical candidate PNU140690 (5) bound in the active site of HIV protease. It revealed that there was a small pocket in the enzyme that permitted the favourable insertion of para-cyano group, a small electron-withdrawing moiety. It was discovered that cellular activity of PNU-103017 was greatly reduced when human serum protein was added to cell culture, and high protein binding limited its effectiveness; therefore, search continued for protease inhibitors. Attachment of the sulfonamide side chain at meta position on the dihydropyran-type analogue resulted in compounds with exceedingly interesting properties. PNU-140690 (tipranavir), a trifluoromethyl pyridyl sulfonamide (9) resulted after optimization of sulphonamide [32]. Efficient asymmetric synthesis of the compound has been developed [33]. The increasing number of reported crystal structures of inhibitor / HIV protease complexes has provided numerous successful examples of structure- based design of potent HIV protease inhibitors [34, 35] Thaisrivongs et al. [36] reported that non-peptide HIV. protease inhibitor phenprocouman (3), previously identified as lead template, led to the identification of first generation drug candidate compound (4). Further molecular modelling studies based on crystal structures of HIV protease inhibitor complexes suggested the incorporation of carboxamide functionality at the meta position of the benzyl side chain at C-3-carboxamide containing 4- hydroxy-2-pyrone compound (7) showed improved inhibitory activity, and replacement of carboxamide group with sulphonamide functionality resulted in non-peptide compounds with improved activity. The most active diasteromer of compound (11) showed potent protease inhibitory activity with a ki value of 0.5 μM. Cyclooctylpyranone derivatives with mcarboxamide substituents were identified as potent nonpeptide HIV protease inhibitors, but these compounds lacked significant antiviral activity in cell culture.
SAR of protease inhibitors
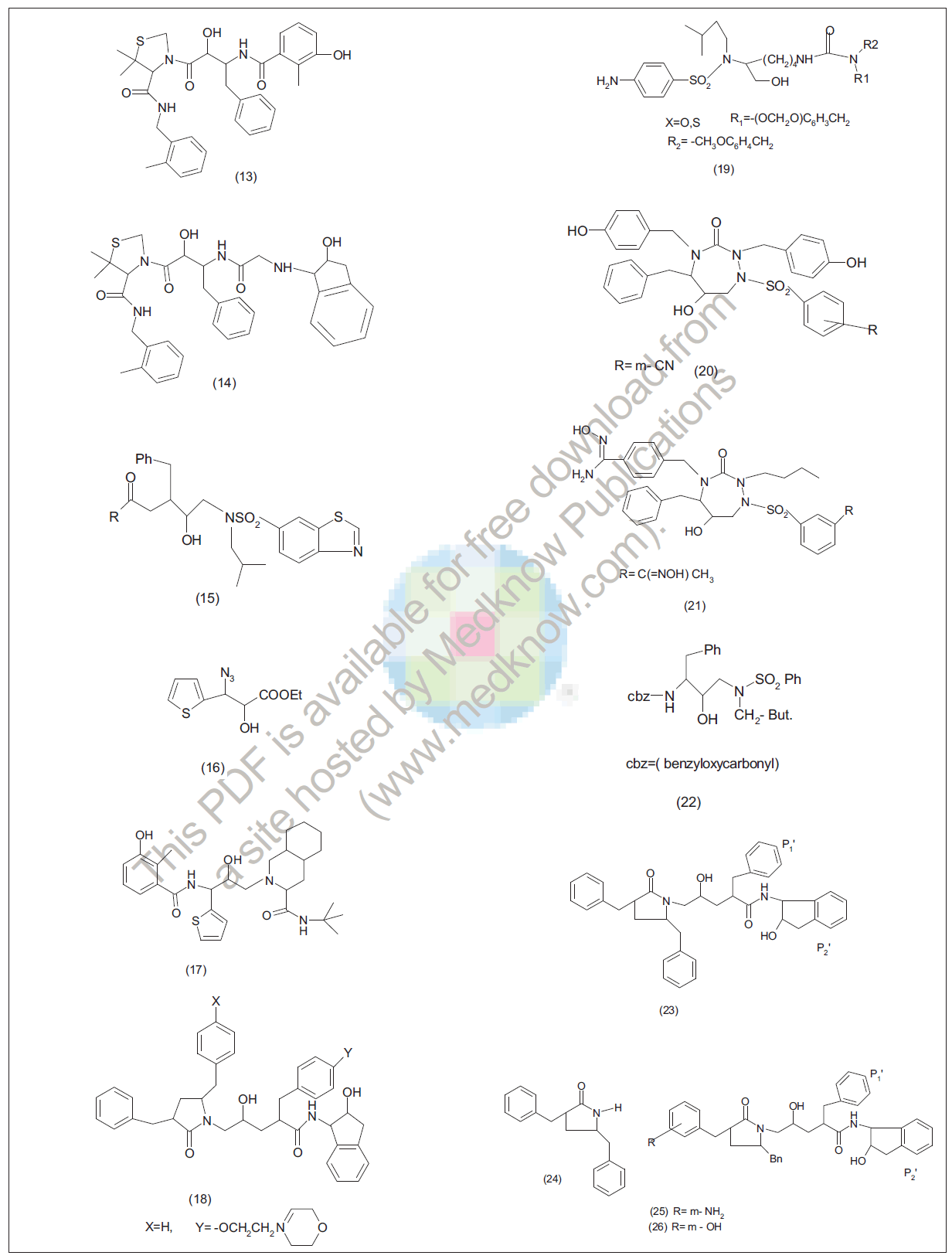
Lu et al. [37] prepared hybrid inhibitors (14) by combining the P3P2P1 ligand of a hydroxy amide based inhibitor JE2147 (13) with P1P3 portion of indinavir (12). They reported the SAR study of lead. Replacement of one of the methyl group with an electronegative atom on phenyl ring resulted in highly potent protease inhibitors. Dichloro-substituted analogues appeared to be one of the most potent compounds. However, they found that dichloro-substituted analogues were less potent than methyl- or chloro-substituted analogues due to smaller size, while methyl-substituted pyridyl methyl amides were found less active than 3-pyridyl analogues. Fivemembered heterocycles (oxazole and thiazole) were also studied. They found thiazole derivatives comparable to the most potent substituted phenyl analogues. Nagaranjan et al. [38] reported that inhibitors incorporate a variety of isosters including the hydroxy ethylurea at the protease cleavage site. They found that replacement of t-butylurea moiety with benzothiazolesulfonamide resulted in potent and improved inhibitors (15), where R=3- pyridylmethyloxy,-3-thiazolylmethyloxy,-2-(R)-methyl-3 methylsulfonyl ether, 3-hydroxy-2-methylphenyl;-2,6 dimethylphenoxymethyl;-3-tetrahydrofuranyloxy against proteases. Bonni et al. [39] reported the stereo-selective and efficient preparation of thiophene containing intermediate (16) from ethyl-3-thienyl propionate as core for new possible HIV protease inhibitors .They used chiral intermediate for the preparation of nelfinavir analogue (17). They designed and synthesized the first thiophene containing HIV protease inhibitor analogue. Sherrill et al. [40] reported the generation of novel series of inhibitors, where 2,5- disubstituted pyrrolidine (18) based HIV protease inhibitors with improved antiviral potency through modification at P1. These analogues demonstrated antiviral efficacy comparable to currently marketed agents (amprenavir). P1-substituted phenols demonstrated similar or increased enzyme inhibition relative to the unsubstituted analogue, and each of P1 analogues with the exception of benzyl analogue demonstrated increased antiviral activity. The derivatives of compound (18) demonstrated somewhat improved antiviral efficacy relative to the marketed protease inhibitor amprenavir. Stranix et al. [41] reported the synthesis of new series of lysine sulfonamide analogues bearing N ε-benzylic ureas (19) using both solution and solid phase approach through novel synthetic route Nα(alkyl)-Nα-(sulfonamide)lysinol using α-aminocaprolactam. Synthesized compounds were novel protease inhibitors with high potency against wild type HIV virus. Huang et al. [42] reported the synthesis of novel azacyclic urea (20,21) HIV protease inhibitors bearing a benzene sulfonamide group at P1 using parallel synthesis method. They did structural studies of early analogues bound in enzyme active sites to design more potent inhibitors. They also reported the effects of substituting P1 benzenesulfonyl group on antiviral activity and protein binding. Skulnick et al. [43] reported that substitution of a sulfonamide group at the meta position produces compounds with excellent HIV protease binding affinity and antiviral activity. They prepared and evaluated a number of these derivatives; a few of the most of the compounds were further evaluated. pcyanophenylsulphonamide derivatives emerged as promising inhibitors and entered phase I clinical trials. Boyer et al. [44] synthesized a series of compounds which possess various sulfonyl moieties substituted at 4-position of C-3-phenyl ring, substituent of dihydropyran-2-one ring system. The sulfonyl substituents were added in an attempt to fill the additional S(3)’ pocket to produce potent inhibitors of target enzyme. Selected inhibitors were tested against wild type HIV and HIV-1 strains that displayed resistance to currently marketed protease inhibitors. Vazquez et al. [45] prepared amino acid hydroxy ethylaminosulfonamide compounds as retroviral protease inhibitors (22). Shrill et al. [46] reported the preparation of 1- acylamino-3-(N-arylsulfonyl-N-alkoxyamino)-2 hydroxypropanes and related compounds as inhibitors of HIV aspartyl protease. Kazamierski et al. [47] developed efficient synthesis of HIV-1 protease inhibitor (23) and its analogues, which incorporate pyrrolidone scaffold (24) as P1-P2 moiety. They evaluated these analogues in HIV-1 protease enzyme assay. They found potent and more water-soluble meta-amino and meta hydroxy inhibitors (25, 26). They suggested that the polar meta but not para benzyl substituents in P2 could sidestep the hydrophobic S2 enzyme active pocket by rotating the P2 moiety around its Cβ-Cγ bond; such orientation allows to engage the unsubstituted hydrophibic edge of benzyl moiety in P2 in the requisite P2/S2 hydrophobic interaction and projects polar meta substituent into bound water. They found that meta position can be chemically derivatized without potency loss of thus resulting inhibitors. Finally they identified pyrrolidone-based inhibitors (25,26) which uniquely accommodate both high enzyme potency and which provide platform for fine tuning of drug-like properties in this class of PIs by additional chemical manipulations on the meta position.
Conclusion
So far, the modifications of non-peptide protease inhibitors have proven highly effective, and modifications that have been made so far do not exhaust the possible changes that can be made to improve the potency and efficacy of these non-peptide protease inhibitors. It would be interesting to see whether these derivatives can be utilized as potent antiHIV in future.
References
- Laurence, J., AIDS Res. Hum. Restrov., 1994, 10, 1585.
- Aggleton, P.O., Reilly, K., Slutkin, G. and Daris, P., Science, 1994, 265, 341.
- Kempf, D.J. and Sham, H.L., Curr. Pharm. Des., 1996, 3, 225.
- Vacca, J.P. and Condra, J.H., Drug Discov. Today., 1997, 2, 261.
- Kohl, N.R, Emini, E.A. and Schleif, W.A., Proc. Natl. Acad. Sci. U.S.A., 1998, 85, 4686.
- Nitsuya, H. and Broder, S., Nature, 1987, 325, 773.
- Wei, X. Ghosh, S.K., Taylor, M.E., Johnson, V.A., Emini, E.A., Deutsh, P., Lifson, J.D., Bonhoeffer, J.D., Nowak, M.A. and Hahn, B.H., Nature., 1995, 373, 117.
- Blundell, T.L., Lappato, R., Wilderspin, A.F., Hemmings, A.M., Hobart, P.M., Danly, D.E. and White, P.G., Trends Biol. Sci., 1990, 15, 425.
- Debouck, C. and Metcalf, B.W., Drug Dev. Res., 1990, 21, 1.
- Tomasselli, A.G. and Heinrikson, R.L., Biochem. Biophys Acta., 2000, 189, 1477
- Randolph, J.R. and De Goey, D., Curr. Topics Med. Chem., 2004, 4, 1079
- Eron, J.J., J. Clin. Infec. Dis., 2000, 30, 5160.
- Pillay, D., Bryaut, M., Getman, D. and Richman, D. D., Rev. Med Virol. 1995, 5, 23.
- West, M.L. and Fairlie, D.P., Trends Pharmacol. Sci., 1995, 16, 67.
- Darke, P.L. and Huff, J.R., Adv. Pharmacol., 1994, 25, 399
- Pallela, F.J., Jrchemiel, J.S., Moorman, A.C. and Holnberg, S.D., AIDS, 2002,16,1617.
- Martin, J.A., Antiviral Res., 1992, 17, 265.
- Kempf, D.J., Codacovi, L., Wang, X.C., Kohlbremer, W.E., Wideberg N.E., Saldivar, A., Vasavanonda, S., Marsh, K.C., Bryant, P., ShamH.L., Green, B.E., Betebenner, D.A., Erickson, J. and Norbeck, D.W J. Med. Chem., 1993, 36, 320.
- Kempf, D.J., Marsh, K.C., Denissen, J.F., Mc Donald, E., Vasavanonda, S., Flentage, C.A., Green, B.E., Fino, L., Park, C.H Kong, X.P., Widerberg, N.E., Saldivar, A., Ruiz, L., Kati, W.M., Sham H.L., Robins, T., Stewart, K.D., Hsu, A., Plattener, J.J., Leonard, J.M and Norbeck, D.W., Proc. Natl. Acad. Sci. U.S.A., 1995, 92:2484.
- Vacca, J.P., Dorsey, B.D., Schleif, W.A., Levin, R.B., Mc Daniel, S.L., Darke, P.L., Zugay, J., Quintero, J.C., Blahy, O.M., Roth, E., Sardana, V.V., Schlabach, A.J., Graham, P.I., Condra, J.H., Gotlib, L., Holloway, M.K., Lin, J., Chen, I.W., Vastag, K., Ostovic, D., Anderson, P.S., Emini, E.A. and Huff, J.R., Proc. Natl. Acad. Sci. U.S.A., 1994, 91, 4096
- Lam, P.Y.S., Jadhav, P.K., Eyermann, C.J., Hodge, R.Y., Bacheler,L.T., Meek, J.L., Otto, M.J.R., Wong, Y.N., Chang, C.H., Weber, P.C. Jackson, D.A., Shrey, T.R. and Erickson Vitanan , S., Science, 1994, 263, 380.
- Wong, Y.N., Burcham, D.L., Saxton, P.L., Erickson Vitanan, S., Grubb, M.F., Quon, C.Y. and Huang, S.M., Biopharm. Drug Disp., 1994, 15, 535
- Getman, D.P., Decresenzo, G.A., Heintz, R.M., Reed, K., Talley, J.J., Bryant, M.L., Clase, M., Honseman, K.A., Mau, J.J., Mueller, R.A., Vazquenz, M.L., Shieh, H.S., Stalling, C. and Stegman, R., J. Med. Chem., 1993, 36, 288.
- Kim, E.E., Baker, C.T., Dwger, M.D., Murcko, M.A., Rack, S.G., Tung, R.D. and Navia, M.A., J. Amer. Chem. Soc., 1995, 117, 1180.
- Patrik, A.K., Mo. H., Markowitz, M., Appelt, K., Wu, B., Munick, L., Kalish, V., Kaldor, S., Reich, S., Ho, D. and Webber, S., Antimicrob. Agents Chemother., 1996, 40, 292.
- Mimoto, T., Imai, J., Kisanuki, S., Enomoto, H., Hottori, N., Akaju, K. and Kiso, Y., Chem. Pharm. Bull., 1992, 40, 2251.
- Thaisrivongs, S., Tomich, P.K., Watenpaugh, K.D., Chong, P.T., Howe, W.J., Yang, C.P., Strohbach, J.W., Turner, McGrath, J.P., Bohanon, J., Lynn, J.C., Mulichak, A., Spinelli, P.A., Hinshaw, R.R., Pagano, P.J.,Moon, J., Ruward, M.J., Wilinason, K.F., Rush, B.D., Zipp, G.L., Dale K.J., Schwende, F.J., Howard, G.M., Padbury, G.E., Toth, N., Zhao, Z.,Koeplinger, K.A., Kakuk, T.J., Cole, S.L., Zan, R.M., Piper, R.C. andJeffrey, P.J., J. Med. Chem.,1994, 41, 3200.
- Skulnick, H.I., Johnson, P.D., Howe, W.J., Tomich, P.K., Chong, K.T.Watenpaugh, K.D., Janakiraman, M.N., Dola, L.A., Mc Grath, J.P.Lynn, J.C., Horng, M.M., Hinshaw, R., Zipp, G.L., Ruwart, M.J.,Schwende, F.J., Zhong, W., Padbury, G.E., Dalga, R.J., Shion, L.,Possert, P.I., Rush, B.G., Kakuk, T.J., Cole, S.L., Zaya, R.M.,Thaisrivongs, S. and Aristoff, P.A., J. Med. Chem., 1994, 38, 4968.
- West, M.L., and David, P., Trends Pharmacol. Sci., 1995, 16, 67.
- Molla, A., Granneman, R.G., Sun, E. and Kempf, D.J., AntiviralRes., 1998, 1, 39.
- Reich, S.H., Melnick, M., Davis, J.F., Appett, K., Fuhry, M.A., Pino,M.P., trippe, A.J., Nguyen, D., Dawson, H., Wu. B., Musick, L., Kosa,M., Kahil, D., Webber, S., Gehlhaar, D. K., Andrada, D. and Shetty,B., Proc. Natl. Acad. Sci. U.S.A., 1995, 92, 329.
- Aristoff, P.A., Drugs of the Future., 1998, 23, 995.
- Judge, T.M., Phillips, G. and Morris, J.K., J. Amer. Chem. Soc.,
- Wlodawer, A. and Erickson, J.W., Annu. Rev. Biochem., 1993,1997, 119, 3627.62, 543.
- Appelt, K., Perspect. Drug Discov. Des., 1993, 1, 23.
- Thaisrivongs, S., Janakiraman, M.N., Chang, K.T., Tomich, P.K., Dolak,L.A., Turner, S.R., Strobach, J.W., Lynn, J.C., Horng, M.M., Hinshaw,R.R. and Watenpaugh, K.D., J. Med. Chem., 1996, 39, 2400.
- Lu, Z., Ragharan, S., Bohn, J., Charest, M., Stahlhut, M.W.,Rutkowski, C.A., Simcoe, A.L., Olsen, D.B., Schleif, W.A., Carella, A.,Gabryelski, L., Jin, l., Lin, J.H., Emini, E., Chapman, K. and Tata, J.R.,Bioorg. Med. Chem. Lett., 2003, 13, 1821
- Nagaranjan, S.R., Crescenzo, G.A., Getman, D.P., Lu, H.F., Sikorski,J.A., Walker, J.L., Mac Donald, J.J., Houseman, K.A., Kocan, G.P.,Kishore, N., Mehta, P.P., Shippy, C.L. F. and Bolystone, L., Bioorg.Med. Chem. Lett., 2003, 11, 4769.
- Bonini, C., Chiummiento, L., Bonis, M.D., Funiscello, M. and Lupattelli,P., Tetrahedron Lett., 2004, 45, 2797.
- Sherrill, R.G., Andrews, C.W., Bock, W.J., Davis Ward, R.G., Furfine, E.S., Hazen, R.J., Rutkowske, R.D., Spaltenstein, A. and Wright, L.L., Bioorg. Med. Chem. Lett., 2005, 15, 81.
- Stranix, B.R., Sauve, G.M., Bouzide, A., Cote, A., Sevigny, G., Yelle,J. and Perron, Y., Bioorg. Med. Chem. Lett., 2004, 14, 3971.
- Huang, P.P., Randolph, J.T., Klein, L.L., Vasavanonda, S., Dekhtyar, T.,Stoll, V.S. and Kempf, D.J., Bioorg. Med. Chem. Lett., 2004, 14,4075
- Skulnick, H.I., Johnson, P.D., Aristoff, P.A., Morris, J.K., Lovasz,K.D., Howe, W.J., Watenpaugh, K.D., Janakiraman, M.N., Anderson,D.J., Reischer, R.J., Schwart, T.M., Bannit, L.S., Tomich, P.K., Lynn,J.C., Horng, M.M., Chong, K.T., Henshaw, R.R., Dolak, L.A., Seest, E.P., Schwende, F.J., Rush, B.D., Howard, G.M., Toth, L.N., Wilinson,K.R., Kakuk, T.J., Johnson, C.W., Cole, S.L., Zaya, R.M., Zipp, G.L.,Possert, P.L., Dalga, R.J., Zhong, W.Z., Williams, M.G. and Romines,K.R., J. Med. Chem., 1997, 40, 1149
- Boyer, F.E., Varaprasad, J.V., Domagala, J.M., Elsworth, E.L., Gajda,C., Hagen, S.E., Markoski, L.J., Tait, B.D., Lunney, E.A., Palovsky, A.,Ferguson, D., Graham, N., Holler, T., Hupe, D., Nouhan, C., Tummino,P.J., Urumov, A., Zeikus, E., Zeikus, G., Gracheck, S.J., Sanders, J.M.,Vander Roest, S., Brodfuehrer, J., Iyer, K., Sinz, M. and Gulnick,S.V., J. Med. Chem., 2000, 43, 843.
- Vazquez, M.L., Mueller, R.A., Talley, J.J., Getman, D.P., Decrecenzo,G.A., Freskos, J.N., Heintz, R.M. and Bertenshaw, D.E. (G. D. Searle& Co. USA) US Patent No. 6060476., 2000, Chem. Abstr.,2000, 132, 576, 322147e.
- Sherril, R.G., Hale, M.R., Spaltenstein, A., Furfine, E.S., Andrews,C.W. and Thomas, L.G., (Vertex Pharmaceuticals Incorporated USA)PCT Int Appl WO 9965870., Chem. Abstr., 2000, 132, 670,49801v.
- Kazmierski, W.M., Andrews, W., Furfine, E., Spaltenstein, A. andWright, L., Bioorg. Med. Chem. Lett., 2004, 14, 5689