- *Corresponding Author:
- Priti J. Mehta
Department of Pharmaceutical Analysis, Institute of Pharmacy, Nirma University, Sarkhej-Gandhinagar Highway, Ahmedabad-382 481, India
E-mail: drpritimehta@nirmauni.ac.in
| Date of Submission | 01 March 2012 |
| Date of Revision | 21 February 2013 |
| Date of Acceptance | 05 March 2013 |
| Indian J Pharm Sci 2013;75(2):211-216 |
Abstract
A rapid, nondestructive Raman spectroscopic method was developed for quantitative estimation of paracetamol and nimesulide in their combined dosage form. A Raman univariate calibration model was developed by measuring the peak intensities of paracetamol and nimesulide at 853 cm−1 and 1336 cm−1 , respectively. The developed method was successfully applied for in situ, concurrent estimation of paracetamol and nimesulide in their combined dosage and method was also validated according to International Conference on Harmonisation guidelines. Thus, the developed Raman spectroscopic method can be applied for simultaneous estimation of paracetamol and nimesulide in their combined dosage form as a process analytical technology tool by pharmaceutical industries for routine quality control.
Keywords
In situ analysis, nimesulide, nondestructive, paracetamol, Raman spectroscopy
Development in drug discovery and drug development approaches resulted in addition of plenty of newer drugs in the pharmaceutical market. With the advent of green chemistry, newer eco‑friendly analytical methodologies gained significant importance. Considering environmental concerns as well as operator safety, there is a huge demand for development of rapid, reliable and nondestructive eco‑friendly techniques for qualitative as well as quantitative analysis of pharmaceutical formulations. Nondestructive techniques are in demand nowadays owing to following main advantages; these are simple, economic and time saving as it does not involve any kind of sample preparation. This does not involve any organic as well as inorganic solvents in them so these are eco‑friendly as well. Laser Raman spectroscopy has been proved to be an excellent nondestructive, green analytical chemistry tool, not only for qualitative purposes, but also for quantitative analysis [1‑5].
Raman is noninvasive and solvent free technique. It is possible to analyse sample through plastic bags, glass vials and in aqueous solution with the help of Raman technique. It require very small amount of sample and analysis time ranges from seconds to only few minutes. In spite of the fact that Raman spectroscopy has so many advantages it has failed to live up to its original expectation when the technique was discovered. The most important reason behind this was instrumental inadequacies, high cost associated with Raman analyser systems, which gives a high resolution and high signal‑to‑noise ratio and the inability to reject or eliminate fluorescence. However, due to sophisticated instrumentation, the use of a near infrared laser with Fourier transform‑Raman, the introduction of fibre optics and availability of chemometrics to assist in the analysis of data, number of applications has escalated in various pharmaceutical fields in recent time and soon it will become integral part of quality control arsenal as number of applications of Raman are getting explored gradually [5].
Raman is a particularly useful tool in the analysis of products with a high active pharmaceutical ingredient (API) content more than 10% [1‑7], but it has also been portrayed to quantify API content 2‑5%. [8,9]. API which contains aromatic groups in them gives stronger Raman signals as compare with to aliphatic. In favourable cases it is even possible to perform a reliable quantitative analysis of API content below 0.1% (w/w) level with this method [10]. Moreover; Raman technique enables also quantification of multicomponent pharmaceutical preparations [11,12].
Paracetamol (PCM) is chemically N‑(4‑hydroxyphenyl) acetamide. It is a centrally and peripherally acting nonopioid analgesic and antipyretic, widely used over the counter analgesic. Nimesulide (NIM) is an antiinflammatory drug, chemically known as N‑(4‑nitro‑2‑phenoxyphenyl) methane sulphonamide. It is approved for use in treatment of musculoskeletal disorder, dysemenorrhoea, thrombophlebitis and dental pain, inflammation. Their combination is widely used in India for musculoskeletal disorders owing to their synergistic effect, longer duration of action. Various UV/Vis spectrophotometric [13,14], high‑performance liquid chromatography (HPLC) [15‑17] and high‑performance thin liquid chromatography [18] techniques have been reported for their simultaneous estimation.
Process analytical technology (PAT) proposed by US Food Drug Administration provides information on, innovative pharmaceutical development, designing and controlling manufacturing and to assess critical quality and performance attributes, which will ensure quality of pharmaceutical product. Raman has the potential to give valuable insight into process understanding, optimization and monitoring when used in development and scale‑up operations. It is used to quantify changes in situ during processes such as wet granulation and batch crystallization. Quantitative and qualitative trends can be assessed and adjustments can be made quickly during the routine processing based on Raman analysis [5].
All above discussed techniques, except Raman, are time consuming, destructive and require tedious and lengthy sample preparation, which is not easily constituted in PAT analysis. Hence, our aim is to develop a rapid, nondestructive and in situ analytical technique for the simultaneous estimation of PCM and NIM in their combined dosage form, which can be easily adopted by pharmaceutical industries for routine in‑line or at‑line analysis.
Materials and Methods
PCM API was obtained from S. D. Fine Chemicals Ltd., Mumbai, India and NIM was kindly gifted by Torrent Research Center, Bhat, Gujarat, India. Excipients such as lactose, hydroxypropyl methylcellulose (HPMC), microcrystalline cellulose (MCC), sucrose, magnesium stearate and talc were of analytical grade (Central Drug House Pvt. Ltd., New Delhi, India). HPLC grade methanol was used (S. D. Fine Chemicals Ltd., Mumbai, India).
Tablets of different brands were procured from the local pharmacy like Dolamide Tablets (Ranbaxy Ltd., India); Artifen Tablets (Aglowmed Pharmaceuticals ltd., India); Nimek Para Tablets (J.B. Chemicals Ltd., India) having label claim 500 mg (71.4%) of PCM and 100 mg (14.3%w/w) of NIM and Biogesic Plus Tablets (Biochem, India); Surgimide Plus Tablets (Focus Healthcare, India) having label claim 325 mg (46.4%w/w) of PCM and 100 mg (14.3%w/w) of NIM.
Raman instrumentation
Fully integrated bench top dispersive R‑3000 Raman spectrophotometer, (Raman Systems Inc., USA), consisting of diode laser optic sampling probe fitted with a charge‑coupled device detector was used. The laser excitation wavelength used during the experiments was 785 nm and probe was equipped with special focusing caps for solids and liquids. All spectra were recorded at approximately 250 mW laser power at spectral resolution 4 cm-1 and the scattered light was collected at an angle of 180°.
The instrument was calibrated using verification probe consisting of Teflon coating. Data collection and transfer were automated using RSI scan software.
HPLC instrumentation and chromatographic conditions
A PU‑2080 plus intelligent pump, (Jasco, Japan) and Rheodyne injector with the volume injection set to 20 μL were used for HPLC analysis. Detection was through MD‑2015 plus multi wavelength detector operated at 276 nm. The chromatographic peaks were recorded by using the Borwin PDA software. Separation was achieved on a Luna, Phenomenex C‑18 column (150×4.6 mm) with particle size 5 μm. Mobile phase and other conditions were mimicked as reported [16]. The flow rate was 1 ml/min, whereas the mobile phase was degassed by filtering through a Millipore 0.45 μm pore membrane filter.
Raman spectral measurements
Samples were prepared by triturating substances in a mortar for 10 min to homogenize the powder mixture properly. The spectra of all samples were recorded by taking 100 mg powder in a low density polyethylene bag and spreading it to form a layer of uniform thickness. PCM was estimated at 853 cm-1 and NIM at 1336 cm-1. Each Raman spectrum corresponded to an accumulation of 4 scans with an exposure time of 5 s for each scan, accounting to a total integration time of 20 s. To take into account its heterogeneity, five measurements were made on each sample; the spectrum presented is the average of all recorded spectra. Tablets were analysed directly under the probe, horizontally without any sample preparation within its primary packaging system if blister pack is noncolored. If the blister pack is coloured then tablets are required to be removed from primary packaging material and then analysed.
Method validation
Method validation was performed according to International Conference on Harmonisation guidelines [19,20].
Linearity
Mixed standard samples of PCM and NIM were prepared in different content ratios keeping the total weight of powder mixture 700 mg using talc. For linearity of PCM, constant 100 mg NIM 14.29% w/w) was taken and PCM was mixed in increasing concentration from 100 mg PCM (14.29% w/w), 200 mg PCM (28.57% w/w), 300 mg PCM (42.85% w/w), 400 mg PCM (57.14% w/w), 500 mg PCM (71.43% w/w) to 600 mg PCM (85.71% w/w). For linearity of NIM, constant 500 mg PCM (71.43% w/w) was taken and NIM was mixed in increasing concentration from 50 mg NIM (7.07% w/w), 75 mg NIM (10.71% w/w), 100 mg NIM (14.28% w/w), 125 mg NIM (17.86% w/w), 150 mg NIM (21.35% w/w), 175 mg NIM (25.00% w/w) to 200 mg NIM (28.57% w/w).
Precision
Intraday and interday precision were carried out by measuring Raman counts for PCM and NIM at three concentration levels as 200, 400, 600 mg (28.57, 57.14, 85.71% w/w) for PCM and corresponding 50, 100, 150 mg (7.43, 14.29, 21.43% w/w) for NIM in mixture where the final weight of the mixture was made up to 700 mg with talc. Repeatability was determined by taking six readings of mixture containing 500 mg PCM (71.4% w/w) and NIM 100 mg (14.3% w/w).
Accuracy
Recovery studies were carried out by addition of standard drug to tablet powder equivalent to 250 mg PCM and 50 mg NIM, at three different concentration levels 80, 100 and 120% in triplicate.
Robustness
Raman spectra were recorded by varying ±10% each of integration time, frame size and power strength to carry out robustness studies.
Results and Discussion
The intensity of a Raman line depends on a number of factors including the incident laser power, the frequency of the scattered radiation, the absorptivity of the materials involved in the scattering and the response of the detection system. Raman spectra of most commonly used excipients such as lactose; HPMC, MCC, sucrose, magnesium stearate and talc were recorded. All excipients except talc did not have any Raman scattering and fluorescence at excitation wavelength of laser (785 nm), but talc had two Raman peaks at 295 cm-1 and 1092 cm-1. Talc was selected as diluents so as to check interference of talc at the estimating Raman shifts of PCM and NIM, if any.
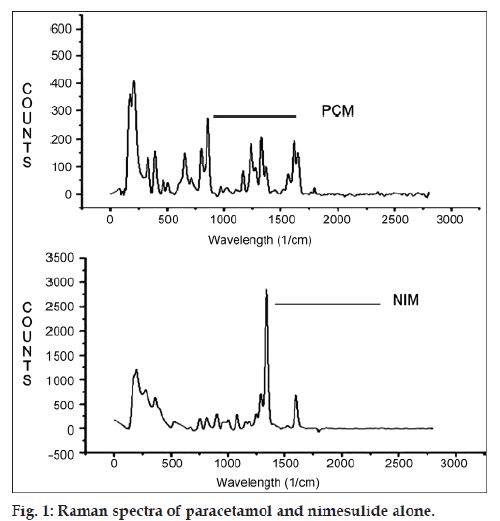
Raman spectra of PCM and NIM were recorded and shown in fig 1. It is apparent that the most intense vibration at 853 cm-1 for PCM and 1336 cm-1 for NIM should be used for the quantitative analysis. PCM can be estimated at 853 cm-1 without any interference from NIM and talc at the selected wavenumber. For quantification of NIM, 1336 cm-1 is selected as estimating wavenumber, but PCM shows Raman shift at 1336 cm-1 which interferes and gives error in estimation of NIM. It was observed that the ratio of Raman counts of PCM at 1336 cm-1 and at 853 cm-1 is 0.67. By deducting Raman counts of PCM from total counts at 1336 cm-1, Raman counts of only NIM can be obtained, which can be used for estimation of NIM in presence of PCM.
Quantitative Raman analysis of solid powder mixtures is usually complicated because the intensity depends on the reproducibility of some factors such as particle size, packing density of the sample, homogeneity of the mixture and variation of detector response. To assess these variations, all the samples were triturated for 10 min. Various spectra were taken from sub samples of the whole mixture and random positioning of the laser beam on the sample, which did not yield any significant differences in the Raman counts. The integration time and frame size were optimised to 20 and 5 s, respectively; to obtain a balance between signal to noise level and time of exposure of sample to laser beam. The laser power intensity was optimised at 250 mW; as at lower power, Raman counts get hampered and at higher laser power, the incidence of fluorescence is high.
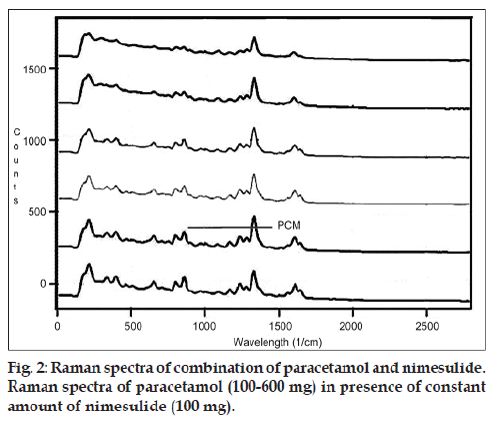
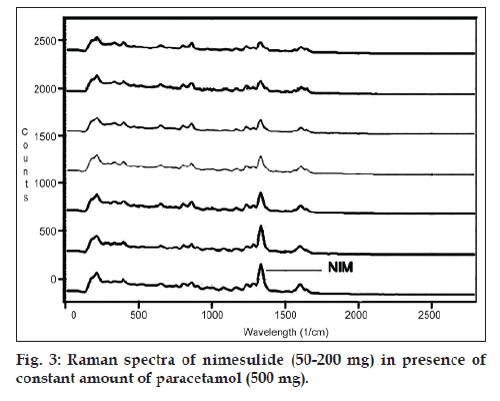
The developed Raman spectroscopic method was based on a simple linear regression model. The linearity of PCM and NIM simultaneously were evaluated by analysis of different concentrations of both drugs. In this study, six concentrations were chosen, ranging between 100 and 600 mg (14.28‑85.71% w/w) for PCM and 50‑200 mg (7.07‑28.56% w/w) for NIM (figs. 2 and 3). The intensity of the Raman peaks corresponding to the drug molecules shows a concentration‑dependent change in the spectra. Six Raman spectra were recorded from each mixed sample and a plot of intensity versus concentration was plotted, yielding linear equations depicted as, IPCM=0.767CPCM+59.62...(1) and INIM=8.05CNIM-125.3... (2). The correlation coefficient were 0.998 and 0.992 for PCM and NIM, respectively.
Accuracy of reported method was estimated in terms of % recovery of both PCM and NIM from marketed tablet formulation and given in Tables 1 and 2. The % relative standard deviation values for intraday, interday precision and repeatability were found to be less than 2, which indicate that developed method is precise for simultaneous estimation of PCM and NIM.
| Level (%) | Standard Conc. (mg) | Amount added (mg) | Total amount (mg) | Amount recovered (mg) | Mean % recoverya ± SD |
|---|---|---|---|---|---|
| 80 | 250 | 200 | 450 | 444.65 | 98.79 ± 0.46 |
| 250 | 200 | 450 | 442.45 | ||
| 250 | 200 | 450 | 446.56 | ||
| 100 | 250 | 250 | 500 | 496.64 | 98.77 ± 0.64 |
| 250 | 250 | 500 | 490.4 | ||
| 250 | 250 | 500 | 494.49 | ||
| 120 | 250 | 300 | 550 | 548.24 | 98.94 ± 0.65 |
| 250 | 300 | 550 | 542.72 | ||
| 250 | 300 | 550 | 541.54 |
Table 1: Accuracy Studies Of Pcm
| Level (%) | Standard conc. (mg) | Amount added (mg) | Total amount (mg) | Amount recovered (mg) | Mean % recoverya ± SD |
|---|---|---|---|---|---|
| 80 | 50 | 40 | 90 | 88.94 | 98.45 ± 2.194 |
| 50 | 40 | 90 | 90.39 | ||
| 50 | 40 | 90 | 86.48 | ||
| 100 | 50 | 50 | 100 | 99.43 | 99.03 ± 0.350 |
| 50 | 50 | 100 | 98.8 | ||
| 50 | 50 | 100 | 98.85 | ||
| 120 | 50 | 60 | 110 | 109.48 | 99.60 ± 0.095 |
| 50 | 60 | 110 | 109.67 | ||
| 50 | 60 | 110 | 109.52 |
Table 2: Accuracy Studies Of Nim
Robustness of the method was reported by changing frame size ±1 s and Integration time ±5 s and all the results are within ±2% of the obtained assay values of tablets. All the validation parameters are summarized in Table 3.
| Parameters | PCM | NIM |
|---|---|---|
| Linearity (% w/w)* | 14.28-85.71 | 7.07-28.56 |
| R2 | 0.998 | 0.991 |
| Linear regression equation | y=0.767 x+59.62 | y=8.050 x−125.3 |
| LOD (% w/w) | 2.77 | 0.37 |
| LOQ (% w/w) | 8.38 | 1.11 |
| Repeatability (% RSD)* | 1.19-2.34 | 1.28-2.07 |
| Intraday precision (% RSD)# | 0.2-2.03 | 0.89-1.60 |
| Interday precision (% RSD)# | 1.45-2.23 | 0.46-1.06 |
| % recovery | 98.77-98.94 | 98.45-99.60 |
Table 3: Summary of validation parameters For developed raman spectroscopic Technique
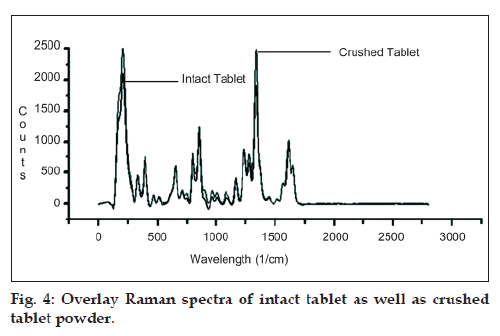
Proposed method was developed and validated using APIs of PCM and NIM; hence to apply the method for intact tablets, it was essential to check the effect of packing density and thickness of tablet on Raman shift of both the drugs. For the same, Raman spectra of both intact tablet as well as crushed tablet powder were recorded and are shown in fig 4. From the figure, it was indicative that there is no effect of packing density or thickness of material on estimating Raman shift of PCM and NIM when analysed in their intact tablet dosage form. The application of developed method was evaluated to determine the amounts of PCM and NIM in their marketed combined tablet dosage form. Two of the combined dosage forms of PCM and NIM were available in colourless blister packing and the tablets can be analysed in situ, non‑contact form, without removal of tablets from primary packaging system thus; making it possible to quantify the PCM and NIM in its combined dosage form within its packing material. Three dosage forms were available in coloured blister packing and thus it was required to remove tablets from its primary packing for analysis as the coloured blister pack causes quenching of Raman scattering and hence resulting in decreased peak intensities. PCM and NIM are available in various weight ratios in their combined dosage forms i.e., 500 and 100 mg and the other weight ratio being 325 and 100 mg, respectively. Hence for a wider applicability, the developed method was applied on various brands of marketed formulations having different weight ratios of PCM and NIM. Assay results of different marketed formulations are summarized in Table 4.
| Marketed formulation | Label claim | % assaya ± SD | ||
|---|---|---|---|---|
| PCM | NIM | PCM | NIM | |
| A | 500 mg | 100 mg | 99.90 ± 3.058 | 98.76 ± 0.626 |
| B | 98.19 ± 1.791 | 99.52 ± 0.773 | ||
| C | 99.11 ± 1.265 | 99.30 ± 0.724 | ||
| D | 325 mg | 100 mg | 102.73 ± 1.973 | 97.65 ± 0.993 |
| E | 100.22 ± 1.805 | 99.00 ± 1.137 | ||
Table 4: Results of various commercially Available combined tablet dosage form of Pcm-nimbydeveloped raman method
The assay results of marketed formulation obtained with developed Raman spectroscopic technique was compared with the reported reverse-phase HPLC method [16] using paired Student t‑test at a confidence level of 0.05. The t‑cal value was less than t‑tab value at 99.95% significance level. This result indicates that both the methods are in excellent agreement and there is no significant difference between assay results obtained by developed Raman and reported RP‑HPLC method.
A simple, rapid, nondestructive and solvent free Raman spectroscopic technique was developed and validated for simultaneous estimation of PCM and NIM in their combined dosage form. Tablets can be directly analysed through its colourless primary packing material using the developed Raman method; thus, it is a promising technique for its applicability as a PAT tool for in‑line and at‑line analysis in routine quality control check in pharmaceutical industries. In addition, Raman is also gaining popularity in the arsenal of green analytical chemistry.
References
- Skoulika SG, Georgiou CA. Rapid quantitative determination of ciprofloxacin in pharmaceuticals using solid-state FT-Raman spectroscopy. Appl Spectrosc 2001;55:1259-65.
- Szostak R, Mazurek S. Quantitative determination of acetylsalicylic acid and acetaminophen in tablets by FT-Raman spectroscopy. Analyst 2002;127:144-8.
- Skoulika SG, Georgiou CA. Rapid, non-invasive quantitative determination of acyclovir in pharmaceutical solid dosage forms through their poly (vinyl chloride) blister package by solid-state Fourier transform Raman spectroscopy. Appl Spectrosc 2003;57:407-12.
- Izolani AO, de Moraes MH, Tellez CS. Fourier transform Raman spectroscopy of drugs: Quantitative analysis of 1-phenyl-2, 3-dimethyl-5-pyrazolone-4 methyl amino methane sodium sulfonate: (dipyrone). J Raman Spectrosc 2003;34:837-43.
- Patel BD, Mehta PJ. An Overview: Application of Raman spectroscopy in pharmaceutical field. Curr Pharm Anal 2010;6:131-41.
- Szostak R, Mazurek S. FT-Raman quantitative determination of ambroxol in tablets. J MolStruct 2004;704:229-33.
- de Beer TR, Baeyens WR, Vermeire A, Broes D, Remon JP, Vervaet C. Raman spectroscopic method for the determination of medroxyprogesterone acetate in a pharmaceutical suspension: Validation of quantifying abilities uncertainty assessment andcomparison with the highperformance liquid chromatography reference method. Anal Chim Acta 2007;589:192-9.
- Dyrby M, Engelsen SB, Norgaard L, Bruhn M, Lundsberg-Nielsen L. chemometric quantitation of the active substance (Containing C≡N) in a pharmaceutical Tablet Using near-infrared (NIR) transmittance and NIR FT-raman spectra. Appl Spectrosc 2002;56:579-85.
- Mazurek S, Szostak R. Quantitative determination of captopril and prednisolone in tablets by FT-Raman spectroscopy. J Pharm Biomed Anal 2006;40:1225-30.
- Hancewicz TM, Petty C. Quantitative analysis of vitamin a using fourier transform raman spectroscopy. Spectrochim Acta 1995;51:2193-8.
- Laplant F, De Paepe A. Pharmaceutical Applications of Raman Spectroscopy. Hoboken: J Wiley and Sons; 2008. p. 85-115.
- De Spiegeleer B, Baert B, Diericx N, Seghers D, Verpoort F, Van Vooren L, et al. Assessment of the solid-state composition of an active salicylanilide compound by FT-Raman spectroscopy. J Pharm Biomed Anal 2007;44:254-7.
- Kirtawade R, Salve P, Seervi C, Kulkarni A, Dhabale P. Simultaneous UV spectrophotometric method for estimation of paracetamol and nimesulide in tablet dosage form. Int J Chem Tech Res 2010;2:818-21.
- Sahu S, Danta CC. Simultaneous spectrophotometric estimation of nimesulide and paracetamol in liquid dosage form. J Phar Res 2010;3:1973-5.
- Indrajeet S. Spectrophotometric and HPLC methods for simultaneous estimation of nimesulide and paracetamol from tablets. Philipp J Sci 2002;131:59-64.
- Battu PR. Simultaneous RP-HPLC determination of nimesulide and paracetamol in tablets. Int J Pharm Tech Res 2009;1:514-6.
- Gharge D. Simultaneous estimation of nimesulide and paracetamol in solid dosage form by RP-HPLC method. Int J PharmTech Res 2010;2:1330-3.
- Sane RT, Gadgil M. Simultaneous determination of paracetamol, chlorzoxazone, and nimesulide by HPTLC. J Planar Chromat 2002;15:76-8.
- ICH Q2A. Text on Validation of Analytical Procedures, International Conference on Harmonisation, Tripartite Guidelines;1996.
- ICH Q2B. Analytical Validation Methodology, International Conference on Harmonisation, Tripartite Guideli nes;1996.