- *Corresponding Author:
- S. Harakeh
King Fahd Medical Research Center and Yousef Abdul Latif Jameel Scientific Chair of Prophetic Medicine Application, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia
E-mail: sharakeh@gmail.com
| Date of Received | 05 September 2023 |
| Date of Revision | 05 June 2024 |
| Date of Acceptance | 08 November 2024 |
| Indian J Pharm Sci 2024;86(6):1922-1934 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Most of the probiotic bacterial candidates currently available in the market consist of various strains belonging to different classes. Nevertheless, the gastrointestinal microbiome is a collection of undefined microbial agents that may contribute some medical benefits to human beings. Hence, currently, researchers from different corners are centered on investigating and identifying gastrointestinal-derived probiotic strains for the advancement of next-generation probiotics. The term next-generation probiotics refers to genetically modified microorganisms created by deleting, adding or modifying specific genes to produce a probiotic strain that modulates the metabolism, gastrointestinal health and delivered directly to the mucosa. Next-generation probiotics generated from different probiotic strains are intended to provide one or more health benefits to a host and for the efficient control and/or treatment of multiple diseases. Even though some next-generation probiotics are auspicious of the control and treatment of several chronic sicknesses, studies on human beings are still sporadic and confirmations from regulatory authorities are thus rare. Furthermore, some problems need to be resolved by releasing their broad application to the public. Probiotic strains such as Faecalibacterium prausnitzii, Prevotella copri, Bacteroides uniformis, Bacteroides thetaiotaomicron, Bacteroides acidifaciens, Clostridium butyricum, Christensenella minuta, Akkermansia muciniphila and Parabacteroides goldsteinii, have been postulated as next-generation probiotic candidates, as a result of their therapeutic or preventive effects on diseases such as colitis, obesity, diabetes and liver diseases. This review highlights the probiotic potential of next-generation probiotics and discusses the potential existing and emerging next-generation probiotics.
Keywords
Lactic acid bacteria, butyrate, next-generation probiotics, gastrointestinal tract
It has been estimated that the human microbiota contains 100 trillion bacteria and forms a symbiotic relationship with the host by controlling both onset and progression of illness as well as the promotion of health[1,2]. Bacteria that colonize the intestinal tract are involved in biological processes that control metabolic phenotype, epithelial lining development and innate immunity. It has been reported that intestinal dysbiosis (altered composition of intestinal microbiota) has the capability to produce a multitude of disorders such as obesity, diabetes mellitus, neurodegenerative diseases, asthma, and inflammatory bowel disease[3]. The identification of beneficial bacterial candidates that promote health and those that can be used to treat intestinal dysbiosis has been achieved through an in-depth understanding of the intestinal microbiome[4].
Medical experts and the public in general use the term "probiotics" to describe a means of improving human and/or animal health. The term "probiotic" is often used in reference to both foods and medications[5]. According to World Health Organization/Food and Agriculture Organization probiotics are defined as "living microorganisms that, when introduced into the body in adequate quantities, provide health benefits"[6].
Most of the bacterial strains licensed and sold as probiotics in the market today belong to the Lactic Acid Bacteria (LAB) group, which is mainly designated by the genus Lactobacillus[7]. They are metabolically recognized by the production of lactic acid from carbohydrates, producing an acidic milieu that inhibits the emergence of some disease-causing bacterial species[8]. They may also generate secondary metabolites such as bacteriocins, exopolysaccharides and enzymes, all of which are beneficial to human health[9].
Despite the benefits mentioned above, current probiotic development trends seek to reduce the usage of probiotic groups like Lactobacillus and enhance the use of other genera and species of bacteria that are more suited to the intestinal environment[10]. These bacteria are termed Next-Generation probiotics (NGPs). The growing popularity of NGPs in recent years can be linked to the various benefits they provide over conventional probiotics[11,12]. In-depth research into this new generation of probiotics will allow for the development of more advanced tools to aid in the treatment of emerging and re-emerging illnesses[13]. The aim of this review is to highlight the different types of NGPs and their benefits to human health.
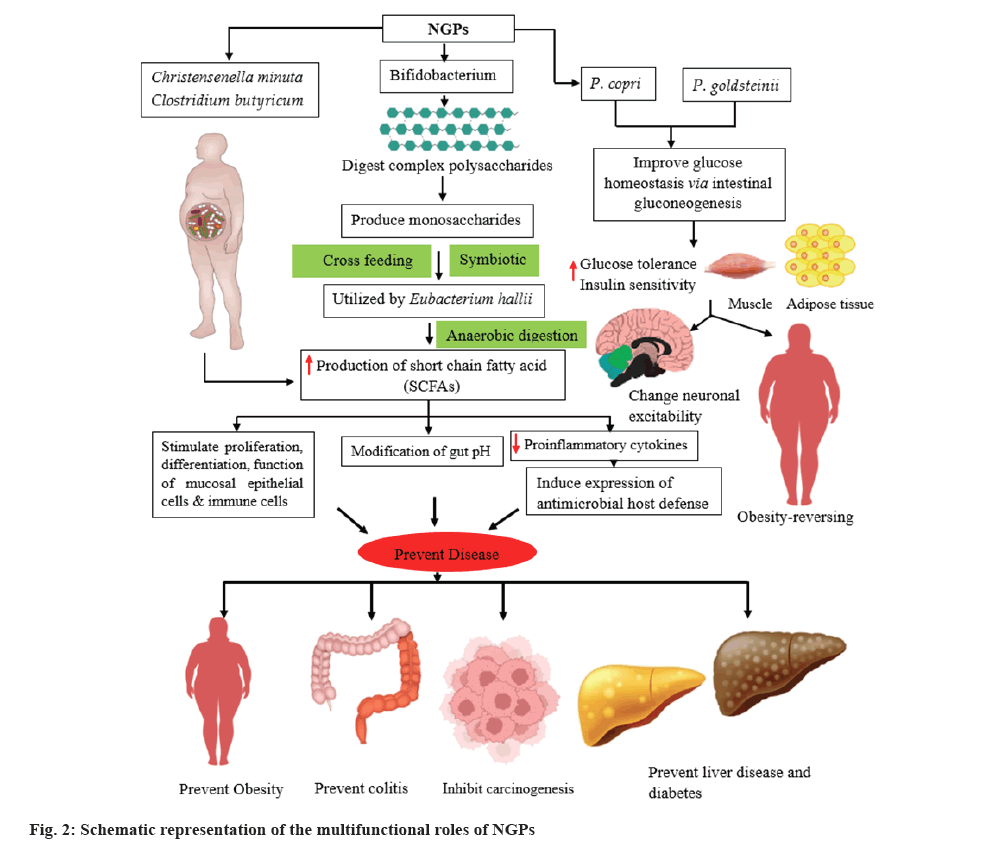
Probiotics display numerous modes of action, including the regulation of intestinal health, and its pH over the secretion of Short-Chain Fatty Acids (SCFAs), inhibition of the growth and occupation of gastrointestinal (GIT) pathogens, and help to prevent related illnesses[14]. It has been found that butyrate, produced from SCFAs, reduces the proliferation of pro-inflammatory cytokines and supports the expression of antimicrobial substances in the intestinal system[15]. Moreover, it facilitates the differentiation, proliferation and physiology of the epithelial cells of the immune and mucosal system. Butyrate facilitates the propagation and the intestinal integrity in Crohn's disease patient[16]. Bacterial candidates such as Bacteroides uniformis, Bacteroides thetaiotaomicron, Bacteroides acidifaciens, Akkermansia muciniphila, Faecalibacterium prausnitzii, Clostridium butyricum (C. butyricum), Prevotella copri (P. copri), Christensenella minuta (C. minuta) and Parabacteroides goldsteinii (P. goldsteinii), have been hypothesized as NGP candidates, due to their soothing and defensive effects on diseases such as colitis, diabetes, obesity and liver diseases[17,18]. This review highlights the probiotic potential of NGPs and discusses the potential existing and emerging NGPs.
What are Probiotics
Probiotics are live microbial agents, primarily bacteria and yeasts that generate health benefits when up taken in sufficient concentration. Mostly present in fermented products like yogurt, kefir, sauerkraut and supplements[19]. Probiotics promote a conducive gut microbiome niche, facilitate digestive function, strengthen immune response and may prevent or treat certain GIT issues, such as diarrhea and Irritable Bowel Syndrome (IBS)[20].
Physiological homeostasis, enhancement of intestinal integrity, and antimicrobial peptide production are all benefits of probiotics and their metabolites[21,22]. Probiotics are efficiently maintaining the levels of microflora of the intestinal tract[23]. Traditional Probiotics (TPs) have a long history of safe utilization with a broad range of health-promoting effects, such as preventing neonatal Necrotizing Enterocolitis (NEC)[24], alleviating the frequency intestinal colic in infants[25], uplifting the quality of life in IBS patients[26], preventing children’s diarrhea[27] and reversing antibiotic induced intestinal inflammation[28]. In the last few years, probiotics have become very popular and are becoming a multibillion-dollar business around the world, which is expected to exceed $65.9 billion by 2024. Probiotics categorized by constituent type (yeast and bacteria), by preparation form (dry or liquid probiotic), by application (dietary supplements, food & beverages, and animal feed), by end user (Animal or human probiotics): Global industry perspective, comprehensive analysis and forecast, 2019–2026.
Most of the TPs which are available on the market are derived from the conventional Streptococcus, Lactobacillus, Bacillus, Bifidobacterium and yeast[29]. For example, Streptococcus thermophilus secreted β-galactosidase in yogurt can degrade lactose into galactose and glucose, which is crucial process for those with lactose intolerance. Nevertheless, probiotics are not considered as antibiotics in several countries and are not regulated very well[30]. Consequently, a cocktail of probiotic products is mostly promoted to buyers without consistent proof of safety and efficiency[5]. NEC represents a severe intestinal disorder predominantly affecting preterm infants and is exemplified by abdominal distension, eating intolerance, high mortality, dysentery and unknown etiology[31]. Previous meta-analyses study revealed that the supplement of probiotic minimized the occurrence of NEC in newborns (<1500 g birth weight) ranging from 6 %-2 %, nonetheless Lactobacillus and Bifidobacterium are administered individually[32]. However, a phase III clinical trial indicated that no significant reduction was detected linked to the use of Bifidobacterium brevis BBG-001[33]. Thus, there are scarce of studies on the probiotic potential of Lactobacillus rhamnosus (L. rhamnosus) ATCC 53103, L. rhamnosus R0011, L. rhamnosus ATCC 53103, Saccharomyces cerevisiae, Saccharomyces boulardii and Lactobacillus helveticus R0052 as probiotics[34]. In United States of America, according to the Gastroenterological Association, the use of probiotics for children with acute gastroenteritis is not suggested or recommended[34]. Hence, even if probiotics are crucial in many different aspects, the research findings on their effectiveness are inconstant. Additionally, all human beings have a typical gut microflora; therefore, the probiotic profile can vary from individual to individual. For those reasons, thorough investigations to explore the actual profile and capacity of probiotics are necessary in future. The impacts of TPs on the human gut microbiota are insufficient and the therapeutic potential of gut flora intervention is mainly treated at the level of strains.
Closely 80 % of the gut microflora are uncharacterized[35], and several types of immune and metabolic illnesses are linked with the total mass of flora in the body[36]. Though, very limited investigations have been carried out on probiotics because these agents are highly sensitive to oxygen which makes it difficult to generate their pure isolates.
With the introduction of culturomics, pure bacterial isolates such as Faecalibacterium prausnitzii, Akkermansia muciniphila, Eubacterium hallii (E. hallii), Bacteroides fragilis (B. fragilis) and Roseburia sp. have efficaciously been attained in vitro. These have been termed as NGPs[37]. Nature Microbiology officially introduced the idea of NGPs in 2017 for the first time[37]. They realized that NGPs differ from TPs and conceptually refer to “active biological entities” which fulfil the suggestion presented by United States Food and Drug Administration guidelines. Comprise live microbial agent; they are utilized for treatment, prevention, control of a disease in humans and they are not vaccine. These days, NGPs candidates are being extensively investigated and reported. NGPs suggested overcoming the deficiencies of the existing TPs and can contribute an imperative role in the prevention or treatment of human diseases all over the globe[38].
Overview of the Mode of Action of Probiotics
Probiotics exert their beneficial effects through diverse mechanisms. The mode of action of probiotics involve colonization and maintaining balanced gut microbial populations in both adults and children; suppression of intestinal bacteria and bacteriocin synthesis; regulation of enzymatic pathways linked to the metabolization of several number of toxic substances and carcinogens; and synthesis of volatile fatty acids, branched chain fatty acids and SCFAs, which contribute a substantial benefit in the preservation of energy homeostasis and management of the activity of the peripheral tissues. Besides, probiotics enhance adhesion in the enterocytes and mucin generation and regulate the action of gut-linked lymphoid tissue and the immune system[39].
The immunomodulatory activity of probiotics is mainly because of the interaction of probiotic bacteria with DCs, epithelial cells, and with monocytes/macrophages and lymphocytes[40]. Likewise, probiotic metabolites can interact with the brain-gut axis and play a role in behavior[39]. This multifaceted mechanism underscores the potential of probiotics in preserving gut health.
NGPs:
The term NGPs consider the Genetically Modified Micro-organisms (GMMs) which have been developed either by deletion, addition, or upregulation of specific genes to generate an efficient probiotic candidates in terms of metabolism, GIT survivability, technological stress tolerance and mucosal directed delivery of prophylactic and/or therapeutic agents to confirm one or multidimensional benefits on the host (also called “bio-drug”) for the treatment or prevention of various illnesses[41].
While NGPs have not yet been functioning as health regulators, they have the potential to serve as alternative modulators for conventional probiotics. NGPs are probiotic which comprised strains such as Bifidobacterium, Lactobacilli etc., nevertheless large-scale genomic manipulation have identified potential probiotic candidates with considerable health benefits, mostly from the genera Verrucomicrobia, Bacteroides, Akkermansia, Firmicutes, Eubacterium and Faecalibacterium[42].
B. fragilis is one of the promising NGPs candidates which has been modulating the immune system associated with the T-cell immune response[43]. Likewise, Bacteroides acidifaciens have been recognized to promote the production of Immunoglobulin (IgA) in murine models and as a result uplifting the IgA+ B cells and B cells production which is crucial in maintaining homeostasis of the intestine and avoiding the pathogen attachment in the GIT[44]. Investigations have indicated that the NGPs candidate, Akkermanisa, has the potential to regulate obesity, diabetes and inflammation in humans[45]. In endurance athletes, Akkermansia muciniphilia plays an important role in maintaining gut barrier function[46], and glucose homeostasis and its direct relation to the capability of the athletics[47], and most importantly it has the capacity to induce the immune system[48]. Recent research findings indicated that the bacteria Akkermansia muciniphilia synthesize Vitamin B12, but the product's efficiency in humans has yet to be determined[49].
Another potential NGP is Faecalibacterium prausnitzii which has played immunodulatory activities via induction of T-cell and interleukin-10 responses in murine and human dendric cells[50]. Another worthy NGP E. hallii L2-7 has been reported to enhance insulin sensitivity (IS) and raise the metabolism of energy in diabetic and obese murine models[51], E. hallii DSM 17630 and E. hallii DMS 3353 have been suggested to generate Vitamin B12 and sustain intestinal homeostasis via the consumption of glucose and several fermentation intermediates like lactate and acetate in vitro investigation[52].
Probiotics of the new generation appear to be beneficial in preliminary studies, however, more testing and proof is needed to confirm their effectiveness and safety in humans[53]. Lactobacillus sp. and other TPs are biologically safe and some are practically effective. According to evidence-based medicine, a statistically insignificant effect is likely to result from their use. Moreover, TPs are also not implemented to cure certain disorders[54]. Thus, in the current scientific landscape, there is a critical need to identify and use more potent and disease-specific NGPs.
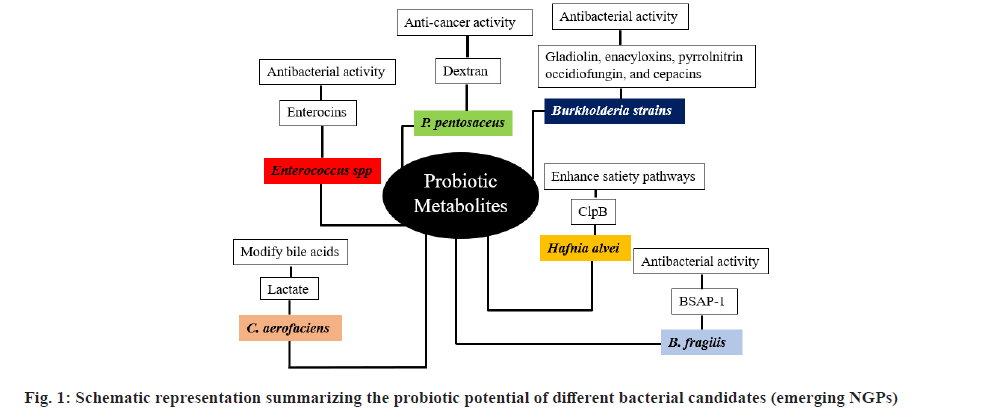
By using modern next-generation sequencing techniques, many previously unknown probiotic bacterial candidates have been identified from the intestinal microbiota and these NGPs have become ideal sources for novel therapeutic agents for several diseases in humans[53]. In comparison to TPs, NGPs have several benefits. To date, many metabolites such as indoles, secondary bile acids, folate, serotonin, Trimethylamine-N-Oxide (TMAO), Gamma-Aminobutyric Acid (GABA), SCFAs acetate, butyrate, propionate and others have been identified from NGPs, all of which can contribute a significant role in maintaining the physiological host phenotype (fig. 1)[55].
NGPs strains:
B. fragilis:B. fragilisis the species within the different species found under the class Bacteroides. Documented findings indicated that enterotoxin genelacking strain B. fragilis show relatively several benefits[56]. The capsular Polysaccharide(PSA) of this strain has been recognized as the structure which is typically involved in the interaction to the host cell. PSA boosts the anti-inflammatory memory of T-cell[57].
C. minuta: C. minuta is a gram-negative bacterium, was recently discovered as a member of the Christensenella genus and the Christensenellaceae family. C. minuta was first introduced from the stool of an apparently healthy individual. This bacterium is a natural inhabitant of the human GIT which is a conducive niche for it[58]. C. minuta is the member of the Firmicutes family and has been known to have probiotic potential against metabolic illnesses and obesity as well. According to reports, the population of Christensenellaceae was found to be higher in individuals whose body mass index is lower than those weighing higher[59]. It has also been proven that the usage of C. minuta may enhance microbiota related with obesity[60] and it’s also been discovered that taking C. minuta increases the formation of SCFAs.
P. copri: P. copri is non-spore forming anaerobic, gram-negative bacterium and belonging to Bacteroidetes phylum. P. copri is a common inhabitant of the intestinal microbiome of humans that has been both negatively and positively linked with the health of the host[61]. Like other anaerobes, it can perpetuate easily and efficiently in the GIT of humans. This probiotic candidate can enhance glucose tolerance and the level of glycogen in the liver[62]. P. copri has been documented as a potential curing agent for metabolic sicknesses including obesity and type-2 diabetes[63]. Its NGP potential is mainly associated with its ability to enhance glucose homeostasis through intestinal gluconeogenesis. Moreover, P. copri is involved in intensification of glucose tolerance and boost insulin resistance which happens before the progression of type 2 diabetes and cardiovascular sickness.
P. goldsteinii : P. goldsteinii is an anerobic, gram-negative bacterium in the family Porphyromonadaceae and belongs to the genus Parabacteroides. It is considered as an NGP for obesity[64]. Mice fed a high-fat diet had significantly lower P. goldsteinii concentrations in the microbiota, while mice treated with prebiotic PSA showed significantly higher levels. The reduction in the weight of mice has been tied to augmented intestinal permeability, inflammation, and metabolic endotoxemia, which culminates in insulin resistance and obesity reversing. This NGP candidate has also been reported to possess insulin stimulating and anti-inflammatory properties[42].
E. hallii: E. hallii is an anaerobic, gram-positive bacterium pertaining to the phylum Firmicutes and the family Lachnospiraceae. E. hallii has the capability to ferment carbohydrates and generate butyrate as a product[65]. E. hallii is advantageous in the intestinal food chain. The organism can influence metabolic balance and gut microbiota structure by generating various SCFAs from the host and/or dietary PSA[52]. It has been documented that daily oral dose of E. hallii enhances IS and upgrade metabolic energy in diabetic and obese mice. Interestingly, increasing the dosage of E. hallii did not affect the body weight or food intake of the treated mice, indicating that this bacterium may serve as a novel, safe and potential probiotic candidate for enhancing IS in the treatment of obesity and diabetes[51].
In the gut microbiota and breast milk, Bifidobacteria (naturally strain) can break down complex carbohydrates into monosaccharides. These monosaccharides can then be utilized by E. hallii to produce SCFAs[66]. This symbiotic relationship between Bifidobacteria and E. hallii suggests a significant and advantageous association for the host.
C. butyricum: C. butyricum is an obligatory anaerobe, gram-positive and spore-producing bacterium. The name 'butyricum' is derived from its ability to produce substantial amounts of butyric acid[67]. This specific strain has the sole future to metabolize non-carbohydrate substrates, resulting in the production of SFA, primarily butyric acid. In a study by Cao et al.[68], oral administration of C. butyricum after gastrectomy reduced early postoperative inflammation, boosted the immune system, maintained GIT microbiota, raised intestinal SCFAs, minimized the postoperative problems, and finally helped the quick recovery[68].
Documented reports indicated that that C. butyricum displays notable potential in plummeting the occurrence of GIT tumors in mice that are induced by a high-fat diet. Therefore, it could be utilized in the prevention and treatment of cancer. Besides, C. butyricum has been recorded to inhibit the propagation of GIT tumor and facilitate apoptosis[69]. Additionally, in the context of depression, combining therapy with C. butyricum strains and antidepressants has shown significant improvement in individuals suffering from depression[70].
Odoribacter laneus (O. laneus): O. laneus is a rod-shaped, anaerobic, gram-negative bacterium that was first detected from the fecal maters of a healthy donors in Japan[71]. In a recent investigation performed by Hueber-Ruano et al.[72], O. laneus was determined as an NGP because of its capability to enhance the sensitivity for insulin and minimize the inflammatory markers in a mouse model. The researchers introduced O. laneus to the obese mice via oral gavage and visualized a considerable decline in the levels of serum succinate. They postulated that raised succinate levels might be due to a compromised integrity of intestinal barrier, chiefly in cases of dysbiosis of the gut. The increase in serum succinate levels is correlated with situations such as obesity and type 2 diabetes mellitus (T2DM), which are known by chronic inflammation[73]. Additionally, the activation of Succinate Receptor 1 (SUCNR1) in macrophages is linked to the development of obesity and T2DM, causing the adipose tissue inflammation and infiltration by inflammatory cells[73]. In general, O. laneus displays promising therapeutic power in the treatment of T2DM and obesity (fig. 2).
Emerging NGPs:
Emerging NGPs are probiotic strains that go beyond TPs in their capabilities and therapeutic potential. These NGPs include specific bacterial species and engineered microbes that are designed to target and modulate gut microbiota, enhance immune responses and improve metabolic health. Unlike conventional probiotics, emerging NGPs typically include strains mostly encompassing lesser-known and newly discovered bacterial species as discussed below and summarized in fig. 1.
Eubacterium:
Eubacterium sp. are anaerobic, rod-shaped gram-positive bacteria that are commonly found in the oral cavity and GIT. These bacteria are considered beneficial microbes and are part of the core human gut microbiome[74]. Within the genus Eubacterium, there are 26 officially recognized species, but the key genotypes are limited to Eubacterium callanderi, Eubacterium barkeri, Eubacterium limosum and Eubacterium aggregans[75]. Eubacterium spp. has been associated with human health, particularly in relation to Colorectal Cancer (CRC) development. Notably, Eubacterium rectale, E. hallii and Eubacterium ventriosum are found in reduced abundance in CRC patients[76], and metagenomic investigations have consistently shown that Eubacterium ventriosum is more prevalent in healthy control groups, indicating its potential as a biomarker for lower CRC risk[77]. Additionally, Eubacterium sp. are known producers of SCFAs, including butyrate, acetate and formic acid, with butyrate playing a role in inhibiting carcinogenesis and inducing apoptosis in cancer cells[78].
Collinsella aerofaciens (C. aerofaciens):
C. aerofaciens is a gram-positive, anaerobic bacterium commonly found in the human gut microbiota. It plays a crucial role in the digestive process by helping to break down complex carbohydrates and produce SCFAs, which are vital for health. C. aerofaciens is also involved in the modulation of the immune system and maintaining the integrity of the gut barrier[79]. Within the human colon, C. aerofaciens plays a significant role in the metabolism of lactose. Numerous studies have indicated that Collinsella and Bifidobacterium have the ability to modify host bile acids, thereby influencing the pathogenicity and virulence of enteric pathogens[80]. More recently, it has been suggested that variations in the abundance of Collinsella may also impact the levels of plasma cholesterol in the host[81].
Burkholderia sp.:
Bacteria belonging to the Burkholderia genus are renowned due to their adaptive metabolic pathways and varied ecological niches[82]. The taxonomical revisions within this genus have resulted in the use of the term "Burkholderia" to encompass the Burkholderia sensu lato, acknowledging its heterogeneity[83]. These bacteria are commonly found in terrestrial and aquatic ecosystems and can exist as free-living organisms or establish close associations with protozoans, fungi, animals, plants, and humans[84]. Such connection can have either advantageous or detrimental effects on the eukaryotic host. For instance, candidates of the Burkholderia cepacia complex, which comprises no fewer than 22 taxonomically related species, act as opportunistic pathogens, particularly in individuals with compromised immune systems like those with cystic fibrosis[85].
Various strains of Burkholderia exhibit killing properties by generating specialized metabolites such as pyrrolnitrin, occidiofungin and cepacins[86]. Despite prior studies chiefly aimed at eco-friendly management of phytopathogenic fungi, there is a growing interest in utilizing Burkholderia bacteria for the discovery of novel antibiotics. For example, Burkholderia ambifaria produces enacyloxins that exhibit activity against gram-negative pathogens, including Acinetobacter baumannii[87]. Additional illustrations involve gladiolin synthesized by Burkholderia gladioli and thailandamides produced by Burkholderia thailandensis, both of which demonstrate encouraging efficacy against Mycobacterium tuberculosis and staphylococcus aureus, respectively[88]. These recent findings, coupled with the identification of numerous 'cryptic' clusters of genes governing biosynthetic pathways within Burkholderia genomes, highlight the potential of these organisms as a valuable resource for drug discovery[89].
Enterococcus sp.:
Enterococci, which are LAB, encompass a wide range of microorganisms that can be both pathogenic and commensal. They are commonly found in various environments, including the gut as symbionts. Enterococcus sp. strains have developed a high tolerance to salts and acids, making them well-suited for adaptation to different food systems. They play a role in the fermentation process of traditional cheese and dry sausages, where they are thought to provide a peculiar sensory characteristic of these dairy products[90]. Additionally, numerous strains of Enterococcus have been recognized for their antimicrobial substance, including bacteriocins. Bacteriocins could be applied for preserving a range of food commodities and are now known as a potential probiotic agent[91]. Furthermore, these biomolecules are being determined as promising alternative therapeutic agents in combating the development of antimicrobial resistance[92].
Enterococcus strains, belonging to the Enterococcus genus, produce a diverse array of bacteriocins often referred to as enterocins. These bacteriocins have been extensively studied, particularly for their activity against gram-positive foodborne bacteria such as L. monocytogenes[93].
Two Enterococcus strains mainly Enterococcus faecium and Enterococcus faecalis, are the chief producers of enterocins in comparison to the contributions obtained from Enterococcus hirae, Enterococcus avium, Enterococcus mundtii and Enterococcus durans. According to reports, the bacteriocins generated from Enterococcus faecalis and relatedisolates have clinical relevance and are considered as emerging NGP candidates[94].
Ruminococcus sp.:
Ruminococcus genus is characterized as obligatory anaerobic, gram-positive, non-motile cocci that do not form endospores. Their growth is dependent on fermentable carbohydrates. Several species of Ruminococcus are present in significant quantities in the human gut microbiota and are essential for proper digestion. Recent advances in next-generation sequencing have revealed their wide distribution among various animal hosts. Among these species, Dysosmobacter welbionis, a member of the Ruminococcaceae family, has been identified as a novel bacterium that produces butyrate[95].
This newly discovered bacterium has been detected in 62.7 %-69.8 % of healthy individuals. Notably, in obese individuals with syndrome of metabolic dysfunction, the abundance of Dysosmobacter genus is inversely correlated with body mass index, fasting glucose levels and glycated hemoglobin[96]. Live Dysosmobacter welbionis J115 supplemented mice, but not with pasteurized bacteria, partially mitigated the development of diet-induced obesity, fat mass gain, insulin resistance, white adipose tissue enlargement and inflammation. Furthermore, administration of live Dysosmobacter welbionis J115 protected mice from inflammation in brown adipose tissue, accompanied by increased mitochondrial count and enhanced non-shivering thermogenesis. These effects were observed with minimal impact on the composition of the mouse gut microbial community. These findings indicated that Dysosmobacter welbionis J115 has a direct and beneficial influence on host metabolism and holds promise as a potential NG beneficial bacterium for addressing obesity and associated with metabolic disorders. The presence of Ruminococcus bacteria in the gut microbiome is crucial for the digestion of resistant starches. The slow digestion of these specific carbohydrates by Ruminococcus has been related to a variety of wellness benefits, including the reversal of infectious diarrhea and a reduced risk of diabetes and colon cancer[97].
Pediococcus pentsaceus: According to Jiang et al.[98], Pediococcus pentosaceus (P. pentosaceus) is a non-motile, gram-positive, cocci-shaped, homofermentative LAB with facultative anaerobic metabolism and carbohydrate catabolism features. The ability of some P. pentosaceus strains to be used in fermentation, as a bioactive growth enhancer for animals and as a probiotic was already established in the 1990s[99]. Nevertheless, most of P. pentosaceus' characteristics were not thoroughly researched at the time. Many additional previously unknown traits of P. pentosaceus have also been researched after more than 20 y of research. As of today, there are still issues with the actual application of P. pentosaceus as a probiotic, such as a lack of understanding of processes, adverse effects, usage and dose. There is mounting information that P. pentosaceus and its bacteriocins are effective for maintaining intestinal health as well as the food industry[100].
P. pentosaceus, a member of LAB, has been gaining increasing attention, resulting in a notable surge in experimental research. When employed as an additive, P. pentosaceus enhances the flavor and nutritional value of food, as well as improving the preservation of animal-sourced products. Scientific evidence suggests that P. pentosaceus produces substances known as bacteriocins or bacteriocin-like compounds, which demonstrate effective antibacterial properties within the microbial environment. Moreover, numerous strains of P. pentosaceus have been recognized for their potential as probiotics due to their abilities to reduce inflammation, combat cancer, provide antioxidant effects, aid in detoxification and lower lipid levels[101,102].
In recent years, P. pentosaceus, a specific type of LAB, has gained significant importance in LAB applications. This bacterium has been found in various sources, including fermented food, raw animal and plant products, aquatic products, as well as feces, with some strains showing association with the human GIT[99]. There is a widening body of experimental evidence suggesting that P. pentosaceus could be utilized as a potential bio preservative for foods, animals, or plants, as well as a potential probiotic candidate.
P. pentosaceus has displayed positive activity on intestinal substance absorption and excretion, along with the ability to enhance detoxification processes in the liver by reducing levels of blood ammonia, heavy metal ions, and endotoxins[103]. Additionally, P. pentosaceus has exhibited anti-inflammatory properties by regulating Lipopolysaccharides (LPS) and cytokines in the host[104]. Recent in vitro study indicated that the P. pentosaceus strain OBK05 displayed strong antidiabetic activity by inhibition of α-glucosidase and α-amylase activity[105].
Butyricoccus pullicaecorum (B. pullicaecorum):
B. pullicaecorum, anaerobic, gram-positive, non-motile coccoid cells typically arranged in pairs, displayed potential for therapeutic interventions in various bladder cancers and Colorectal Cancers (CRC). This is primarily due to its ability to secrete butyrate. An oral dose of B. pullicaecorum or its metabolites has demonstrated improved clinical outcomes in CRC by activating secrete butyrate transporters and/or receptors, indicating its potential as an anti-CRC probiotic. Moreover, B. pullicaecorum displayed a probiotic characteristic with positive effects on chronic inflammations. In a rat colitis model, oral dose of B. pullicaecorum exhibited a marked protective effect, reducing lesion sizes and inflammation. Additionally, the supernatant of B. pullicaecorum cultures withstand cytokine-initiated deterioration of epithelial integrity in an in vitro experimental model[106].
The production of butyrate, known for its beneficial properties in maintaining GIT health, has led to the exploration of butyrate-producing bacteria as the NGPs. B. pullicaecorum, belonging to clostridial cluster IV, emerges as a promising probiotic candidate for individuals with inflammatory bowel disease. However, before generating a stable formulation for oral dose, understanding its bile and intrinsic acid tolerance is crucial[107].
Clinical trials have shown that B. pullicaecorum, a safe butyrate-producing microbe, may potentially reduce cancer progression, offering opportunities for therapeutic interventions in different types of bladder cancers via the secretion of butyrate[108].
Hafnia alvei (H. alvei):
H. alvei is a rod, gram-negative bacteria which belongs to the genus Hafnia under the order Enterobacterales. Lucas et al.[109], displayed that the gavage of the H. alvei HA4597TM strain in High fat diet-mice caused a reduction in food intake and reduced fat mass as well as weight gain[109]. Besides, the treatment group that acquired H. alvei HA4597™ exhibited reductions in triglycerides, serum glucose and the level of anino-transferase[109].
Legrand et al.[110], reported that oral administration of H. alvei in High fat diet-mice for 18 d and 46 d, respectively, reduced the mass of fat in treated mice and lessened food intake in 16 d of gavage mice. In general, the gavage of H. alvei HA4597TM had weight-dropping effects[110]. In a different study, Dechelotte et al.[111], documented the effect of orally administered H. alvei HA4597® for 236 overweight patients coupled with slightly hypocaloric food for a period of 12 w. They encountered a significant body weight loss (around 3 %) in the original body weight. A weight loss of 3 %-5 % of body weight is suggested by international baseline for overweight individuals. Moreover, Dechelotte et al.[111] reported a reduction in the circumference of hip and a significant decline in the circumference of waist and the level of serum glucose. The documented decline in body weight may be accredited to metabolic activity of the ClpB protein generated by H. alvei which improve satiety pathways through melanocortin receptor activation (essential for energy metabolism)[110]. This pathway is associated with the conduction of anorexigenic signals and a rise in the expenditure of energy linked with peripheral lipolytic effects[111]. As well, obese individuals have been reported to display a low concentration of bacterial strains encoding ClpB protein. A high number of bacteria encoding for ClpB protein in the intestinal microbiota may be attributed to a decline in the human body weight[112]. Thus, H. alvei could be a potential NGP bacterium for obesity and other illnesses in humans.
Conclusion
In conclusion, next-generation and emerging probiotics hold significant present as viable therapeutic effect for metabolic and GIT disorders, offering innovative solutions through their ability to maintain gut flora, immune responses and metabolic pathways. This review addressed different bacterial species which have probiotic potential, highlighting their diverse mechanisms of action and therapeutic benefits. With continued interdisciplinary collaboration and rigorous scientific investigation, NGPs have the potential to revolutionize the management and treatment of metabolic and GIT disorders, improving patient outcomes and overall health.
Acknowledgments:
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University (KAU) Jeddah, Saudi Arabia, under grant no. (GPIP:930-305-2024). The authors, therefore, gratefully acknowledge with thanks DSR for the technical and financial support.
Conflict of interest:
The authors declared no conflict of interests.
References
- Ramadevi S, Meenakshi S. Communication with gut microbiota: An emerging strategy to predict and prevent cancer. Role of Microbes in Sustainable Development 2023;471-86.
- Ursell LK, Metcalf JL, Parfrey LW, Knight R. Defining the human microbiome. Nutr Rev 2012;70(Suppl_1):S38-44.
[Crossref] [Google Scholar] [PubMed]
- Mostafavi Abdolmaleky H, Zhou JR. Gut microbiota dysbiosis, oxidative stress, inflammation, and epigenetic alterations in metabolic diseases. Antioxidants 2024;13(8):985-90.
[Crossref] [Google Scholar] [PubMed]
- Adak A, Khan MR. An insight into gut microbiota and its functionalities. Cell Mol Life Sci 2019;76(3):473-93.
[Crossref] [Google Scholar] [PubMed]
- Liang D, Wu F, Zhou D, Tan B, Chen T. Commercial probiotic products in public health: Current status and potential limitations. Crit Rev Food Sci Nutr 2024;64(19):6455-76.
[Crossref] [Google Scholar] [PubMed]
- Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document: The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 2014;11(8):506-14.
[Crossref] [Google Scholar] [PubMed]
- Ansari F, Bahadori A, Samakkhah SA, Pirouzian HR, Pourjafar H. Probiotic lactic acid bacteria: Taxonomy, properties and benefits. In Handbook of Food Bioactive Ingredients: Properties and Applications 2023;1473-503.
- Yeboah PJ, Wijemanna ND, Eddin AS, Williams LL, Ibrahim SA. Lactic acid bacteria: Review on the potential delivery system as an effective probiotic. In: Current Issues and Advances in the Dairy Industry 2023.
- Zhang J, Xiao Y, Wang H, Zhang H, Chen W, Lu W. Lactic acid bacteria-derived exopolysaccharide: Formation, immunomodulatory ability, health effects, and structure-function relationship. Microbiol Res 2023;274:1-12.
[Crossref] [Google Scholar] [PubMed]
- Liu X, Mao B, Gu J, Wu J, Cui S, Wang G, et al. Blautia-a new functional genus with potential probiotic properties? Gut Microbes 2021;13(1):1-21.
[Crossref] [Google Scholar] [PubMed]
- Shyam KU, Krishnan R, Jeena K, Vijaysunderdeva G, Prasad KP. Next-generation probiotics-Future therapeutics for sustainable aquaculture. Aquaculture 2021;25(4):23-6.
- López-Moreno A, Acuña I, Torres-Sánchez A, Ruiz-Moreno Á, Cerk K, Rivas A, et al. Next generation probiotics for neutralizing obesogenic effects: Taxa culturing searching strategies. Nutrients 2021;13(5):16-7.
[Crossref] [Google Scholar] [PubMed]
- Sun F, Zhang Q, Zhao J, Zhang H, Zhai Q, Chen W. A potential species of next-generation probiotics? The dark and light sides of Bacteroides fragilis in health. Food Res Int 2019;126:1-10.
[Crossref] [Google Scholar] [PubMed]
- Thoda C, Touraki M. Immunomodulatory properties of probiotics and their derived bioactive compounds. Appl Sci 2023;13:1-14.
- Akhtar M, Chen Y, Ma Z, Zhang X, Shi D, Khan JA, et al. Gut microbiota-derived short chain fatty acids are potential mediators in gut inflammation. Anim Nutr 2022;8:350-60.
[Crossref] [Google Scholar] [PubMed]
- Geirnaert A, Calatayud M, Grootaert C, Laukens D, Devriese S, Smagghe G, et al. Butyrate-producing bacteria supplemented in vitro to Crohn’s disease patient microbiota increased butyrate production and enhanced intestinal epithelial barrier integrity. Sci Rep 2017;7(1):1-11.
[Crossref] [Google Scholar] [PubMed]
- Pesce M, Seguella L, Del Re A, Lu J, Palenca I, Corpetti C, et al. Next-generation probiotics for inflammatory bowel disease. Int J Mol Sci 2022;23(10):1-5.
[Crossref] [Google Scholar] [PubMed]
- Tan H, Zhai Q, Chen W. Investigations of Bacteroides spp. towards next-generation probiotics. Food Res Int 2019;116:637-44.
[Crossref] [Google Scholar] [PubMed]
- Maftei NM, Raileanu CR, Balta AA, Ambrose L, Boev M, Marin DB, et al. The potential impact of probiotics on human health: An update on their health-promoting properties. Microorganisms 2024;12(2):1-13.
[Crossref] [Google Scholar] [PubMed]
- El-Enshasy HA, Yang ST. Probiotics, the natural microbiota in living organisms. CRC Press 2021.
- Traina G. On probiotic integration in the management of inflammation and the maintenance of the intestinal epithelial barrier’s integrity. Journal of Biological Research-Bollettino della Società Italiana di Biologia Sperimentale 2024;97(2):1-5.
- Yu S, Qian Y, Gao Q, Yan Y, Huang Y, Wu Z, et al. Discovery, characterization, and application of a novel antimicrobial peptide produced by Lactiplantibacillus plantarum FB-2. Food Biosci 2024;58:1-10.
- Dasriya VL, Samtiya M, Ranveer S, Dhillon HS, Devi N, Sharma V, et al. Modulation of gut-microbiota through probiotics and dietary interventions to improve host health. J Sci Food Agric 2024;104(11):6359-75.
[Crossref] [Google Scholar] [PubMed]
- Underwood MA. Probiotics and the prevention of necrotizing enterocolitis. J Pediatr Surg 2019;54(3):405-12.
[Crossref] [Google Scholar] [PubMed]
- Ong TG, Gordon M, Banks SS, Thomas MR, Akobeng AK. Probiotics to prevent infantile colic. Cochrane Database Syst Rev 2019;13(3):1-9.
[Crossref] [Google Scholar] [PubMed]
- Ustaoğlu T, Tek NA, Yıldırım AE. Evaluation of the effects of the FODMAP diet and probiotics on Irritable Bowel Syndrome (IBS) symptoms, quality of life and depression in women with IBS. J Hum Nutr Diet 2024;37(1):5-17.
[Crossref] [Google Scholar] [PubMed]
- Martins EM, Nascimento da Silva LC, Carmo MS. Probiotics, prebiotics, and synbiotics in childhood diarrhea. Braz J Med Biol Res 2024;57:e13205.
[Crossref] [Google Scholar] [PubMed]
- Łukasik J, Dierikx T, Johnston BC, de Meij T, Szajewska H. Systematic review: Effect of probiotics on antibiotic-induced microbiome disruption. Benef Microbes 2024;15(5):431-47.
[Crossref] [Google Scholar] [PubMed]
- Douillard FP, De Vos WM. Functional genomics of lactic acid bacteria: From food to health. Microb Cell Fact 2014;13(Suppl 1):S1-8.
[Crossref] [Google Scholar] [PubMed]
- De Simone C. The unregulated probiotic market. Clin Gastroenterol Hepatol 2019;17(5):809-17.
[Crossref] [Google Scholar] [PubMed]
- Liebe HL, Ford HR, Camerini V, Hunter CJ. Necrotizing enterocolitis. InPediatric Surgery: Diagnosis and Management 2023;907-18.
- Robinson, J. Cochrane in context: Probiotics for prevention of necrotizing enterocolitis in preterm infants. Evid Based Child Health 2014;9(3):672-4.
[Crossref] [Google Scholar] [PubMed]
- Costeloe K, Hardy P, Juszczak E, Wilks M, Millar MR. Bifidobacterium breve BBG-001 in very preterm infants: A randomised controlled phase 3 trial. Lancet 2016;387(10019):649-60.
[Crossref] [Google Scholar] [PubMed]
- Su GL, Ko CW, Bercik P, Falck-Ytter Y, Sultan S, Weizman AV, et al. AGA clinical practice guidelines on the role of probiotics in the management of gastrointestinal disorders. Gastroenterology 2020;159(2):697-705.
[Crossref] [Google Scholar] [PubMed]
- Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, et al. Diversity of the human intestinal microbial flora. Science 2005;308(5728):1635-8.
[Crossref] [Google Scholar] [PubMed]
- Portincasa P, Khalil M, Graziani A, Frühbeck G, Baffy G, Garruti G, et al. Gut microbes in metabolic disturbances. Promising role for therapeutic manipulations?. Eur J Intern Med 2024;119:13-30.
[Crossref] [Google Scholar] [PubMed]
- O’Toole PW, Marchesi JR, Hill C. Next-generation probiotics: The spectrum from probiotics to live biotherapeutics. Nat Microbiol 2017;2(5):1-6.
[Crossref] [Google Scholar] [PubMed]
- Zhang H, Duan Y, Cai F, Cao D, Wang L, Qiao Z, et al. Next-generation probiotics: Microflora intervention to human diseases. BioMed Res Int 2022;2022(1):5633403.
[Crossref] [Google Scholar] [PubMed]
- Plaza-Diaz J, Ruiz-Ojeda FJ, Gil-Campos M, Gil A. Mechanisms of action of probiotics. Adv Nutr 2019;10:S49-66.
[Crossref] [Google Scholar] [PubMed]
- Yousefi B, Eslami M, Ghasemian A, Kokhaei P, Salek Farrokhi A, Darabi N. Probiotics importance and their immunomodulatory properties. J Cell Physiol 2019;234(6):8008-18.
[Crossref] [Google Scholar] [PubMed]
- Murali SK, Mansell TJ. Next generation probiotics: Engineering live biotherapeutics. Biotechnol Adv 2024;72:1-10.
[Crossref] [Google Scholar] [PubMed]
- Lin TL, Shu CC, Lai WF, Tzeng CM, Lai HC, Lu CC. Investiture of next generation probiotics on amelioration of diseases-strains do matter. Med Microecol 2019;1:1-10.
- Eribo OA, du Plessis N, Chegou NN. The intestinal commensal, Bacteroides fragilis, modulates host responses to viral infection and therapy: Lessons for exploration during Mycobacterium tuberculosis infection. Infect Immun 2022;90(1):e00321.
[Crossref] [Google Scholar] [PubMed]
- Yanagibashi T, Hosono A, Oyama A, Tsuda M, Suzuki A, Hachimura S, et al. IgA production in the large intestine is modulated by a different mechanism than in the small intestine: Bacteroides acidifaciens promotes IgA production in the large intestine by inducing germinal center formation and increasing the number of IgA+ B cells. Immunobiology. 2013;218(4):645-51.
[Crossref] [Google Scholar] [PubMed]
- Cani PD, de Vos WM. Next-generation beneficial microbes: The case of Akkermansia muciniphila. Front Microbiol 2017;8:1765-7.
[Crossref] [Google Scholar] [PubMed]
- Mo C, Lou X, Xue J, Shi Z, Zhao Y, Wang F, et al. The influence of Akkermansia muciniphila on intestinal barrier function. Gut Pathog 2024;16(1):41-8.
[Crossref] [Google Scholar] [PubMed]
- Zhang L, Liu Y, Sun Y, Zhang X. Combined physical exercise and diet: Regulation of gut microbiota to prevent and treat of metabolic disease: A review. Nutrients 2022;14(22):4774-79.
[Crossref] [Google Scholar] [PubMed]
- Zhao Q, Yu J, Hao Y, Zhou H, Hu Y, Zhang C, et al. Akkermansia muciniphila plays critical roles in host health. Crit Rev Microbiol 2023;49(1):82-100.
[Crossref] [Google Scholar] [PubMed]
- Kirmiz N, Galindo K, Cross KL, Luna E, Rhoades N, Podar M, et al. Comparative genomics guides elucidation of vitamin B12 biosynthesis in novel human-associated Akkermansia strains. Appl Environ Microbiol 2020;86(3):e02117-19.
[Crossref] [Google Scholar] [PubMed]
- Martín R, Miquel S, Chain F, Natividad JM, Jury J, Lu J, et al. Faecalibacterium prausnitzii prevents physiological damages in a chronic low-grade inflammation murine model. BMC Microbiol 2015;15:1-12.
[Crossref] [Google Scholar] [PubMed]
- Udayappan S, Manneras-Holm L, Chaplin-Scott A, Belzer C, Herrema H, Dallinga-Thie GM, et al. Oral treatment with Eubacterium hallii improves insulin sensitivity in db/db mice. NPJ Biofilms Microbiomes 2016;2(1):1-10.
[Crossref] [Google Scholar] [PubMed]
- Engels C, Ruscheweyh HJ, Beerenwinkel N, Lacroix C, Schwab C. The common gut microbe Eubacterium hallii also contributes to intestinal propionate formation. Front Microbiol 2016;7:713-20.
[Crossref] [Google Scholar] [PubMed]
- Abouelela, M.E.; Helmy, Y.A. Next-generation probiotics as novel therapeutics for improving human health: current trends and future perspectives. Microorganisms 2024, 12, 430. [Crossref] [Google Scholar] [PubMed]
- Prajapati K, Bisani K, Prajapati H, Prajapati S, Agrawal D, Singh S, et al. Advances in probiotics research: Mechanisms of action, health benefits, and limitations in applications. Syst Microbiol Biomanu 2024;4(2):386-406.
- Barbosa JC, Machado D, Almeida D, Andrade JC, Brandelli A, Gomes AM, et al. Next-generation probiotics. In Probiotics 2022;483-502.
- Wang Y, Deng H, Li Z, Tan Y, Han Y, Wang X, et al. Safety evaluation of a novel strain of Bacteroides fragilis. Front Microbiol 2017;8:435.
[Crossref] [Google Scholar] [PubMed]
- Fakruddin M, Shishir MA, Yousuf Z, Khan MS. Next-generation probiotics-the future of biotherapeutics. Microb. Bioact 2022;5:156-63.
- Pan T, Zheng S, Zheng W, Shi C, Ning K, Zhang Q, et al. Christensenella regulated by Huang-Qi-Ling-Hua-San is a key factor by which to improve type 2 diabetes. Front Microbiol 2022;13:1-10.
[Crossref] [Google Scholar] [PubMed]
- Tavella T, Rampelli S, Guidarelli G, Bazzocchi A, Gasperini C, Pujos-Guillot E, et al. Elevated gut microbiome abundance of Christensenellaceae, Porphyromonadaceae and Rikenellaceae is associated with reduced visceral adipose tissue and healthier metabolic profile in Italian elderly. Gut Microbes 2021;13(1):1-8.
[Crossref] [Google Scholar] [PubMed]
- Ang WS, Law JW, Letchumanan V, Hong KW, Wong SH, Ab Mutalib NS, et al. A keystone gut bacterium Christensenella minuta: A potential biotherapeutic agent for obesity and associated metabolic diseases. Foods 2023;12(13):2485-90.
[Crossref] [Google Scholar] [PubMed]
- Chang HW, Lee EM, Wang Y, Zhou C, Pruss KM, Henrissat S, et al. Prevotella copri and microbiota members mediate the beneficial effects of a therapeutic food for malnutrition. Nat Microbiol 2024;9(4):922-37.
[Crossref] [Google Scholar] [PubMed]
- Zang X, Xiao M, Yu L, Chen Y, Duan H, Zhang C, et al. Prevotella copri-a potential next‐generation probiotic. Food Front 2024.
- Tsai CY, Liu PY, Huang MC, Chang CI, Chen HY, Chou YH, et al. Abundance of Prevotella copri in gut microbiota is inversely related to a healthy diet in patients with type 2 diabetes. J Food Drug Anal 2023;31(4):599-608.
[Crossref] [Google Scholar] [PubMed]
- Vallianou NG, Kounatidis D, Tsilingiris D, Panagopoulos F, Christodoulatos GS, Evangelopoulos A, et al. The role of next-generation probiotics in obesity and obesity-associated disorders: Current knowledge and future perspectives. Int J Mol Sci 2023;24(7):6755-58.
[Crossref] [Google Scholar] [PubMed]
- Seegers JF, Bui TP, de Vos WM. Remarkable metabolic versatility of the commensal bacteria Eubacterium hallii and Intestinimonas butyriciproducens: Potential next-generation therapeutic microbes. Probiotic Bacteria and Postbiotic Metabolites: Role in Animal and Human Health 2021:139-51.
- Bunesova V, Lacroix C, Schwab C. Mucin cross-feeding of infant bifidobacteria and Eubacterium hallii. Microb Ecol 2018;75(1):228-38.
[Crossref] [Google Scholar] [PubMed]
- Sada RM, Matsuo H, Motooka D, Kutsuna S, Hamaguchi S, Yamamoto G, et al. Clostridium butyricum bacteremia associated with probiotic use, Japan. Emerg Infect Dis 2024;30(4):665-8.
[Crossref] [Google Scholar] [PubMed]
- Cao W, Zheng C, Xu X, Jin R, Huang F, Shi M, et al. Clostridium butyricum potentially improves inflammation and immunity through alteration of the microbiota and metabolism of gastric cancer patients after gastrectomy. Front Immunol 2022;13:1-10.
[Crossref] [Google Scholar] [PubMed]
- Chen D, Jin D, Huang S, Wu J, Xu M, Liu T, et al. Clostridium butyricum, a butyrate-producing probiotic, inhibits intestinal tumor development through modulating Wnt signaling and gut microbiota. Cancer Lett 2020;469:456-67.
[Crossref] [Google Scholar] [PubMed]
- Miyaoka T, Kanayama M, Wake R, Hashioka S, Hayashida M, Nagahama M, et al. Clostridium butyricum MIYAIRI 588 as adjunctive therapy for treatment-resistant major depressive disorder: A prospective open-label trial. Clin Neuropharmacol 2018;41(5):151-5.
[Crossref] [Google Scholar] [PubMed]
- Nagai F, Morotomi M, Watanabe Y, Sakon H, Tanaka R. Alistipes indistinctus sp. nov. and Odoribacter laneus sp. nov., common members of the human intestinal microbiota isolated from faeces. Int J Syst Evol Microbiol 2010;60(6):1296-302.
[Crossref] [Google Scholar] [PubMed]
- Huber-Ruano I, Calvo E, Mayneris-Perxachs J, Rodríguez-Peña MM, Ceperuelo-Mallafré V, Cedó L, et al. Orally administered Odoribacter laneus improves glucose control and inflammatory profile in obese mice by depleting circulating succinate. Microbiome 2022;10(1):135-41.
[Crossref] [Google Scholar] [PubMed]
- Serena C, Ceperuelo-Mallafré V, Keiran N, Queipo-Ortuño MI, Bernal R, Gomez-Huelgas R, et al. Elevated circulating levels of succinate in human obesity are linked to specific gut microbiota. The ISME J 2018;12(7):1642-57.
[Crossref] [Google Scholar] [PubMed]
- Liu FL, Abdugheni R, Ran CG, Zhou N, Liu SJ. Eubacterium album sp. nov., a butyrate-producing bacterium isolated from faeces of a healthy human. Int J Syst Evol Microbiol 2024;74(5):1-6.
[Crossref] [Google Scholar] [PubMed]
- Mukherjee A, Lordan C, Ross RP, Cotter PD. Gut microbes from the phylogenetically diverse genus Eubacterium and their various contributions to gut health. Gut microbes 2020;12(1):1-18.
[Crossref] [Google Scholar] [PubMed]
- Ai D, Pan H, Li X, Wu M, Xia LC. Association network analysis identifies enzymatic components of gut microbiota that significantly differ between colorectal cancer patients and healthy controls. Peer J 2019;7:1-15.
[Crossref] [Google Scholar] [PubMed]
- Yu J, Feng Q, Wong SH, Zhang D, yi Liang Q, Qin Y, et al. Metagenomic analysis of faecal microbiome as a tool towards targeted non-invasive biomarkers for colorectal cancer. Gut 2017;66(1):70-8.
[Crossref] [Google Scholar] [PubMed]
- Karim MR, Iqbal S, Mohammad S, Morshed MN, Haque MA, Mathiyalagan R, et al. Butyrate’s (a short-chain fatty acid) microbial synthesis, absorption, and preventive roles against colorectal and lung cancer. Arch Microbiol 2024;206(4):137-44.
[Crossref] [Google Scholar] [PubMed]
- Qin P, Zou Y, Dai Y, Luo G, Zhang X, Xiao L. Characterization a novel butyric acid-producing bacterium Collinsella aerofaciens subsp. shenzhenensis subsp. nov. Microorganisms 2019;7(3):78-85.
[Crossref] [Google Scholar] [PubMed]
- Rajilić-Stojanović M, De Vos WM. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol Rev 2014;38(5):996-1047.
[Crossref] [Google Scholar] [PubMed]
- Lahti L, Salonen A, Kekkonen RA, Salojärvi J, Jalanka-Tuovinen J, Palva A, et al. Associations between the human intestinal microbiota, Lactobacillus rhamnosus GG and serum lipids indicated by integrated analysis of high-throughput profiling data. Peer J 2013;1:e32-6.
[Crossref] [Google Scholar] [PubMed]
- Kadhim MJ, Ahmed ME. A comprehensive review of Burkholderia sp. J Curr Med Res Opin 2023;6(8):1701-16.
- Estrada-de Los Santos P, Palmer M, Chávez-Ramírez B, Beukes C, Steenkamp ET, Briscoe L, et al. Whole genome analyses suggests that Burkholderia sensu lato contains two additional novel genera (Mycetohabitans gen. nov., and Trinickia gen. nov.): Implications for the evolution of diazotrophy and nodulation in the Burkholderiaceae. Genes 2018;9(8):389-95.
[Crossref] [Google Scholar] [PubMed]
- Suárez-Moreno ZR, Caballero-Mellado J, Coutinho BG, Mendonça-Previato L, James EK, Venturi V. Common features of environmental and potentially beneficial plant-associated Burkholderia. Microb Ecol 2012;63(2):249-66.
[Crossref] [Google Scholar] [PubMed]
- Leitão JH, Sousa SA, Ferreira AS, Ramos CG, Silva IN, Moreira LM. Pathogenicity, virulence factors, and strategies to fight against Burkholderia cepacia complex pathogens and related species. Appl Microbiol Biotechnol 2010;87(1):31-40.
[Crossref] [Google Scholar] [PubMed]
- Bach E, Passaglia LM, Jiao J, Gross H. Burkholderia in the genomic era: From taxonomy to the discovery of new antimicrobial secondary metabolites. Crit Rev Microbiol 2022;48(2):121-60.
[Crossref] [Google Scholar] [PubMed]
- Rodríguez-Cisneros M, Morales-Ruíz LM, Salazar-Gómez A, Rojas-Rojas FU, Estrada-de Los Santos P. Compilation of the antimicrobial compounds produced by Burkholderia Sensu Stricto. Molecules 2023;28(4):1646-52.
[Crossref] [Google Scholar] [PubMed]
- Song L, Jenner M, Masschelein J, Jones C, Bull MJ, Harris SR, et al. Discovery and biosynthesis of gladiolin: A Burkholderia gladioli antibiotic with promising activity against Mycobacterium tuberculosis. J Am Chem Soc 2017;139(23):7974-81.
[Crossref] [Google Scholar] [PubMed]
- Esmaeel Q, Pupin M, Jacques P, Leclère V. Nonribosomal peptides and polyketides of Burkholderia: new compounds potentially implicated in biocontrol and pharmaceuticals. Environ Sci Pollut Res 2018;25(30):29794-807.
[Crossref] [Google Scholar] [PubMed]
- Zugic Petrović TD, Ilić PD, Grujović MZ, Mladenović KG, Kocić-Tanackov SD, Čomić LR. Assessment of safety aspect and probiotic potential of autochthonous Enterococcus faecium strains isolated from spontaneous fermented sausage. Biotechnol Lett 2020;42(8):1513-25.
[Crossref] [Google Scholar] [PubMed]
- Daba GM, El-Dien AN, Saleh SA, Elkhateeb WA, Awad G, Nomiyama T, et al. Evaluation of Enterococcus strains newly isolated from Egyptian sources for bacteriocin production and probiotic potential. Biocatal Agri Biotechnol 2021;35:1-10.
- Almeida-Santos AC, Novais C, Peixe L, Freitas AR. Enterococcus spp. as a producer and target of bacteriocins: A double-edged sword in the antimicrobial resistance crisis context. Antibiotics 2021;10(10):1-12.
[Crossref] [Google Scholar] [PubMed]
- Farias FM, Teixeira LM, Vallim DC, Bastos MD, Miguel MA, Bonelli RR. Characterization of Enterococcus faecium E86 bacteriocins and their inhibition properties against Listeria monocytogenes and vancomycin-resistant Enterococcus. Braz J Microbiol 2021;52(3):1513-22.
[Crossref] [Google Scholar] [PubMed]
- Abanoz HS, Kunduhoglu B. Antimicrobial activity of a bacteriocin produced by Enterococcus faecalis KT11 against some pathogens and antibiotic-resistant bacteria. Korean J Food Sci Anim Resour 2018;38(5):1064-79.
[Crossref] [Google Scholar] [PubMed]
- Molinero N, Conti E, Sánchez B, Walker AW, Margolles A, Duncan SH, et al. Ruminococcoides bili gen. nov., sp. nov., a bile-resistant bacterium from human bile with autolytic behavior. Int J Syst Evol Microbiol 2021;71(8):1-14.
[Crossref] [Google Scholar] [PubMed]
- Kim JS, Park JE, Lee KC, Choi SH, Oh BS, Yu SY, et al. Blautia faecicola sp. nov., isolated from faeces from a healthy human. Int J Syst Evol Microbiol 2020;70(3):2059-65.
[Crossref] [Google Scholar] [PubMed]
- La Reau AJ, Suen G. The Ruminococci: Key symbionts of the gut ecosystem. J Microbiol 2018;56(3):199-208.
[Crossref] [Google Scholar] [PubMed]
- Jiang S, Cai L, Lv L, Li L. Pediococcus pentosaceus, a future additive or probiotic candidate. Microb Cell Fact 2021;20(1):1-14.
[Crossref] [Google Scholar] [PubMed]
- Adesulu-Dahunsi AT, Sanni AI, Jeyaram K. Diversity and technological characterization of Pediococcus pentosaceus strains isolated from Nigerian traditional fermented foods. LWT 2021;140:1-11.
- Qi Y, Huang L, Zeng Y, Li W, Zhou D, Xie J, et al. Pediococcus pentosaceus: Screening and application as probiotics in food processing. Front Microbiol 2021;12:1-7.
[Crossref] [Google Scholar] [PubMed]
- Pan S, Wei H, Yuan S, Kong Y, Yang H, Zhang Y, et al. Probiotic Pediococcus pentosaceus ameliorates MPTP-induced oxidative stress via regulating the gut microbiota–gut–brain axis. Front Cell Infect Microbiol 2022;12:1-10.
[Crossref] [Google Scholar] [PubMed]
- Jafari-Nasab T, Khaleghi M, Farsinejad A, Khorrami S. Probiotic potential and anticancer properties of Pediococcus sp. isolated from traditional dairy products. Biotechnol Rep 2021;29:1-9.
[Crossref] [Google Scholar] [PubMed]
- Dong F, Xiao F, Li X, Li Y, Wang X, Yu G, et al. Pediococcus pentosaceus CECT 8330 protects DSS-induced colitis and regulates the intestinal microbiota and immune responses in mice. J Transl Med 2022;20(1):1-13.
[Crossref] [Google Scholar] [PubMed]
- Wang R, Deng Y, Zhang Y, Li X, Sun L, Deng Q, et al. Modulation of intestinal barrier, inflammatory response, and gut microbiota by Pediococcus pentosaceus zy-B alleviates Vibrio parahaemolyticus infection in C57BL/6J mice. J Agric Food Chem 2022;70(6):1865-77.
[Crossref] [Google Scholar] [PubMed]
- Bhukya KK, Bhukya B. Exploration of antidiabetic, cholesterol-lowering, and anticancer upshot of probiotic bacterium Pediococcus pentosaceus OBK05 strain of buttermilk. Probiotics Antimicrob Proteins 2023;15(6):1484-500.
[Crossref] [Google Scholar] [PubMed]
- Eeckhaut V, Wang J, Van Parys A, Haesebrouck F, Joossens M, Falony G, et al. The probiotic Butyricicoccus pullicaecorum reduces feed conversion and protects from potentially harmful intestinal microorganisms and necrotic enteritis in broilers. Front Microbiol 2016;7:1416.
[Crossref] [Google Scholar] [PubMed]
- Geirnaert A, Steyaert A, Eeckhaut V, Debruyne B, Arends JB, Van Immerseel F, et al. Butyricicoccus pullicaecorum, a butyrate producer with probiotic potential, is intrinsically tolerant to stomach and small intestine conditions. Anaerobe 2014;30:70-4.
[Crossref] [Google Scholar] [PubMed]
- Chang SC, Shen MH, Liu CY, Pu CM, Hu JM, Huang CJ. A gut butyrate-producing bacterium Butyricicoccus pullicaecorum regulates short-chain fatty acid transporter and receptor to reduce the progression of 1, 2-dimethylhydrazine-associated colorectal cancer. Oncol Lett 2020;20(6):1-12.
[Crossref] [Google Scholar] [PubMed]
- Lucas N, Legrand R, Deroissart C, Dominique M, Azhar S, Le Solliec MA, et al. Hafnia alvei HA4597 strain reduces food intake and body weight gain and improves body composition, glucose, and lipid metabolism in a mouse model of hyperphagic obesity. Microorganisms 2019;8(1):1-10.
[Crossref] [Google Scholar] [PubMed]
- Legrand R, Lucas N, Dominique M, Azhar S, Deroissart C, Le Solliec MA, et al. Commensal Hafnia alvei strain reduces food intake and fat mass in obese mice-a new potential probiotic for appetite and body weight management. Int J Obes 2020;44(5):1041-51.
[Crossref] [Google Scholar] [PubMed]
- Dechelotte P, Breton J, Trotin-Picolo C, Grube B, Erlenbeck C, Bothe G, et al. The probiotic strain H. alvei HA4597® improves weight loss in overweight subjects under moderate hypocaloric diet: A proof-of-concept, multicenter randomized, double-blind placebo-controlled study. Nutrients 2021;13(6):1-14.
[Crossref] [Google Scholar] [PubMed]
- Vinot N, Gehring J. Portrait of Hafnia alvei HA4597®-the first precision probiotic changing the game and paradigm in probiotic developments. Int J Nutra Func Foods and Novel Foods 2023;512-17.