- *Corresponding Author:
- F. Xu
Department of Brain Disorders, Beijing University of Chinese Medicine Third Affiliated Hospital, Beijing, Chaoyang 530021, China
E-mail: xufeng1666@163.com
| This article was originally published in a special issue, “Drug Discovery and Repositioning Studies in Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(4) Spl Issue “150-157” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This study aims to construct a rat model to observe the neuroprotective effect of tetramethylpyrazine during the process of ischemic stroke reperfusion and further explore the mechanism of nuclear factor erythroid 2 related factor 2 regulation of absent in melanoma 2 inflammasome on brain neurons, providing a basis for clinical treatment. Experimental rats were grouped into a model control group, tetramethylpyrazine group, nuclear factor erythroid 2 related factor 2 inhibitor (ML385) group, tetramethylpyrazine+ML385 group and healthy group. Neurological function and brain tissue water content were compared between the groups. Additionally, the infarct area in the brain, neuronal apoptosis, levels of B cell lymphoma-2 and B cell lymphoma-2-associated protein X were observed by triphenyltetrazolium chloride staining, terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick-end labeling and enzyme-linked immunosorbent assays respectively. Quantitative polymerase chain reaction and immunoblotting were adopted to study the levels of absent in melanoma 2 and nuclear factor erythroid 2 related factor 2 in brain tissues. On the 3rd and 7th d after modeling, modified neurological severity score behavioral scores denoted least in tetramethylpyrazine group and highest in ML385 group. Brain tissue water content analysis showed that tetramethylpyrazine rats had significantly higher brain tissue water content than the healthy group but significantly lower in other rats. ML385 group had the highest brain tissue water content. Triphenyltetrazolium chloride staining indicated that there was a white infarct area in the left cerebral cortex and striatum of controls, tetramethylpyrazine rats showed significantly less infarct area. ML385 group had a significantly increased infarct rate, while tetramethylpyrazine+ML385 group showed a drastically lower infarct rate than ML385 rats but higher than the tetramethylpyrazine group. Similarly, hippocampal cornu ammonis 1, quantitative polymerase chain reaction and immunoblotting results were also studied. Tetramethylpyrazine can promote nuclear factor erythroid 2-related factor 2 expression downregulates absent in melanoma 2 levels and reduce neuronal cell apoptosis in brain tissue, which is related to its inhibition of brain tissue inflammatory responses. This could indicate a novel therapeutic direction for treating cerebral ischemic reperfusion injury.
Keywords
Tetramethylpyrazine, nuclear factor erythroid 2-related factor 2, melanoma, ischemic stroke reperfusion, neuroprotection
Ischemic cerebral stroke with ischemia-reperfusion refers to the occurrence of perfusion disorders and reperfusion injury during cerebral ischemia. It is mainly caused by vascular obstruction or rupture, leading to insufficient blood supply to the brain and subsequent pathological changes[1]. Reperfused blood returning to the ischemic area causes rapid changes in the intra- and extracellular environment, resulting in cellular damage[2]. Currently, ischemic cerebral stroke has become a major global public health issue, with its incidence and mortality rates increasing year by year. Ischemic cerebral stroke often leads to cognitive impairments in patients[3] and severe ischemia-reperfusion can cause extensive necrosis of brain tissue, impair brain function and even result in death[4]. Therefore, timely treatment is required for ischemic cerebral stroke with ischemia-reperfusion. Currently, the main treatment methods include thrombolysis, intervention and medication, but they have certain limitations. For example, thrombolytic therapy increases the risk of cerebral hemorrhage and intervention requires specialized techniques and equipment, and medication therapy has variable effectiveness due to patient resistance and adverse reactions. Hence, it is necessary to explore and discover more effective and safe treatment methods.
Tetramethylpyrazine (TMP) as a bioactive alkaloid, possesses potent pharmacological activities such as vasodilation and improvement of microcirculation[5]. Previous studies have reported on the neuroprotective mechanisms of TMP, which are often associated with inflammatory responses[6,7]. Additionally, studies have found that the assembly and activation of the Absent in Melanoma 2 (AIM2) inflammasome can release large amount of inflammatory factors, leading to cell pyroptosis[8]. Furthermore, it has been indicated that AIM2 inflammasome-mediated pyroptosis plays a crucial role in cerebral ischemia-reperfusion injury[9]. Nuclear factor erythroid 2 related factor 2 (Nrf2) interacts with AIM2 inflammasome and other inflammatory bodies[10,11]. However, it remains to be explored whether TMP can exert an influence on the neuroprotection of ischemic cerebral stroke with ischemia-reperfusion through Nrf2 and AIM2. These questions are still worthy of investigation; based on this, the present study aims to establish an animal model to study how TMP modulates AIM2 inflammasome through Nrf2 in cerebral ischemiareperfusion injury, providing new insights for the treatment of such patients in clinical settings.
Materials and Methods
Reagents and materials:
Specific Pathogen Free (SPF) adult Sprague-Dawley (SD) rats were obtained from the Experimental Animal Center of Peking University. Lipofectamine 3000, cell lysis buffer, protease inhibitor, fetal bovine serum, Reverse Transcription Polymerase Chain Reaction (RT-PCR) kit, Total Ribonucleic Acid (RNA) Isolation (TRIzol) reagent, fluorescent quantitation reagent kit (Thermo Fisher Scientific); complementary Deoxyribonucleic Acid (cDNA) synthesis kit (Tiangen Biotech, Co., Ltd.); Nrf2 inhibitor (ML385, HY-100523, MedChemExpress); TMP of purity ≥98 % and with batch number 200809E2 (Harbin Sanlian Pharmaceutical, Co., Ltd.); AIM2 rabbit polyclonal antibody, Nrf2 rabbit polyclonal antibody (Abcam); 2,3,5-Triphenyltetrazolium Chloride (TTC) (MP Biomedicals, Pvt., Ltd.).
Animal model preparation:
120 SD rats were kept in a 22°-28° environment (12 h light/dark cycle) with free access to food and water. Brain ischemia-reperfusion model was constructed using the suture method. After anesthesia, a midline incision was made, muscles and left Common Carotid Artery (CCA), External (E) CA, and Internal (I) CA were separated. The distal and proximal ends of CCA and ECA were clamped and the proximal ends of CCA and ECA were ligated. A small incision was made 4 mm distal to CCA bifurcation and a suture thread was inserted. The thread was then pulled out from the CCA using a fine silk thread and fixed. After suturing the incision, the rat was placed in a separate cage for observation. The neurological deficits of all rats were scored according to the Longa scale. A score of 0 indicated no significant neurological damage; 1 indicated inability of the contralateral forelimb to extend; 2 indicated circling towards the contralateral side during spontaneous movement; 3 indicated leaning towards the contralateral side and 4 indicated inability to walk with impaired consciousness. Rat models with cumulative scores between 1 and 3 were considered successful. A total of 100 models were successfully established.
Animal grouping:
Rats with successful modeling were randomly separated into model control group, TMP group, Nrf2 inhibitor (ML385) group and TMP+ML385 group (n=25). Additionally, 25 healthy rats were included as healthy controls.
Group intervention:
All the rats were fed normally after surgery. The daily dosage for rats was calculated based on the principle of equivalent dose calculated by body surface area for humans and animals. Model controls received a daily intraperitoneal injection of 1 ml/100 g of normal saline in the morning. TMP rats received 252 mg/ kg of TMP with concentration of 0.72 mg/ml). Nrf2 inhibitor (ML385) group rats received 1 ml/100 g of ML385 while TMP+ML385 rats received ML385 and TMP solution at the same doses as mentioned above; the injections were given continuously for 1 w.
Neurological deficit scoring:
On 1st, 3rd and 7th d after modeling, neurological deficits in each group were assessed using the modified Neurological Severity Score (mNSS)[12]. The sum of the scores from various neurological function tests represented the mNSS behavioral score for each group of rats, with higher scores indicating more severe damage.
Measurement of brain water content:
Brains were extracted from the rats after removing the cerebellum and olfactory bulb. The right brain was washed with physiological saline, dried and weighed. Then, it was placed in an electric heating incubator maintained at 60° and air-dried for 3 d. Percentage (%) of brain water content was calculated.
Brain water content=wet weight-dry weight/wet weight×100 %
TTC staining of brain tissue slices and cerebral infarction:
Coronal sections of rat brain tissue were made into 2 mm thick, soaked in 2 % TTC and kept in a light-protected room at 37° for 20 min, fixed in 4 % Paraformaldehyde (PFA) solution for 1 d, and photographed. TTC-stained images were analyzed, where red or white area represented normal infarcted brain tissue. The percentage of cerebral infarction was calculated.
Infarct area percentage=contralateral hemisphereinfarcted hemisphere/brain tissue area of the contralateral hemisphere×100 %
In situ observation of neuronal apoptosis using Terminal deoxynucleotidyl transferase deoxyUridine Triphosphate (dUTP) Nick-End Labeling (TUNEL) assay:
The removed rat brain tissue was paraffin-embedded and sections were deparaffinized. The sections were then soaked in 3 % Hydrogen peroxide (H2O2) for 10 min, rinsed and soaked with 0.1 % Triton X-100 at 4° overnight. Afterwards, the tissue was heated at 37° for 1 h, treated with the TUNEL reaction mixture, sealed with a coverslip and kept in a dark humid chamber for 1 h. The reacted sections were rinsed with Phosphate Buffered Saline (PBS), dehydrated, cleared and sealed. Sections were observed under a fluorescence microscope to assess neuronal apoptosis. The apoptotic rate was calculated.
Detection of B cell lymphoma-2 (Bcl-2) and Bcl-2- Associated protein X (BAX) using Enzyme-Linked Immunosorbent Assay (ELISA):
Rat brain tissue was added to reaction wells and supernatant was added. After incubation, at room temperature for 24 h the reaction wells were washed with PBS. Then, corresponding enzymelabeled antibodies were used for 1 h incubation. After washing the reaction wells, 0.1 ml of 3,3’,5,5’-tetramethylbenzidine and 0.05 ml of 2 mmol/l sulfuric acid were added and absorbance was recorded.
Real-time PCR experiment for detection of AIM2 and Nrf2:
Tissue samples were thawed and 0.1 g of the sample was taken into a centrifuge tube. Trizol reagent and sterile water were added. cDNA double-strands were prepared and used as a template. PCR reaction system consisted of 10 μl 2X PCR buffer, 6 μl Double Distilled Water (DDH2O), 2 μl cDNA and 1 μl primers. Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) was included as an internal control and relative expression of messenger RNA (mRNA) was determined using the 2-ΔΔCt formula (Table 1).
| Primer | Primer sequence | |
|---|---|---|
| AIM2 | F | 5'-TTCCTCAGTTGTGGTTGATGTT-3' |
| R | 5'-ATTTTGTTTTGCTCTGGTTTCC-3' | |
| Nrf2 | F | 5'-TGGAGACGGCCATGACTGATT-3' |
| R | 5'-GGTGGAAGAATGGGAGTTGCT-3' | |
| GAPDH | F | 5'-ACCCCACTATCTC-3’ |
| R | 5'-TGCCTGCTTCACCACCTTCT-3' | |
Table 1: qPCR primer sequencing
Detection of AIM2 and Nrf2 using Western blot:
Total protein was extracted from the corresponding anatomical site of the brain tissue in the arterial supply cortex of the ipsilateral hemisphere of rats with cerebral infarction. Protein was quantified by Bicinchoninic Acid (BCA) kits, resolved by Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) and immunoblotted to a Poly Vinylidene Fluoride (PVDF) membrane. The membrane was blocked with 5 % Bovine Serum Albumin (BSA) for 1 h, kept with 1:400 1st antibody overnight, washed by Tris-Buffered saline with 0.1 % Tween® (TBST), incubated with 1:5000 2nd antibody, washed by TBST and exposed for detection.
Statistical analysis:
Data collected was measured in terms of mean±standard deviation and was analyzed by Standard Package for Social Sciences (SPSS) version 26.0. Independent sample t-tests were used to compare inter-group quantitative data. For multiple groups, one-way Analysis of Variance (ANOVA) followed by Least Significant Difference (LSD) test was applied, p<0.05 was defined to be significant.
Results and Discussion
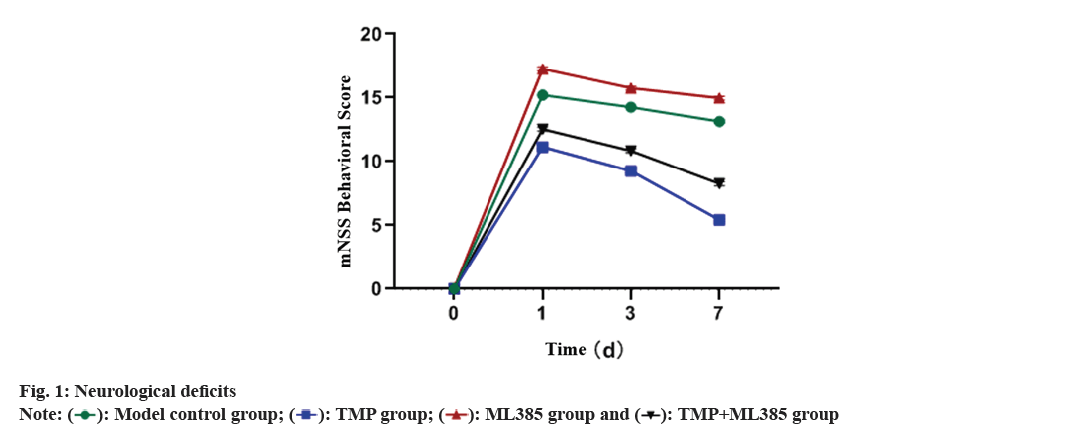
Neurological deficits of the rats were studied. 100 out of 120 rats were successfully modeled, and no deaths occurred during the experimental process. No significant difference was found in mNSS behavioral scores on the 1st d after modeling. On 3rd and 7th d after modeling, mNSS behavioral scores in TMP rats were significantly lower than other groups, while the mNSS behavioral score in ML385 rats was sharply higher than other groups (fig. 1).
Brain tissue water content was observed. Healthy rats had lowest brain tissue water content. TMP rats had significantly higher brain tissue water content than the healthy group, but significantly lower than the other groups. ML385 rats had the highest brain tissue water content (fig. 2).
TTC staining and cerebral infarction results was evaluated. TTC staining revealed that model control group had white infarct areas in the left hemisphere cortex and striatum. TMP group showed significant reduction in infarct area, but still exhibited noticeable white spots at the site of infarction while ML385 group had a significantly increased infarction rate. TMP+ML385 rats showed a lower infarction rate compared with ML385 rats but higher in TMP rats (fig. 3).
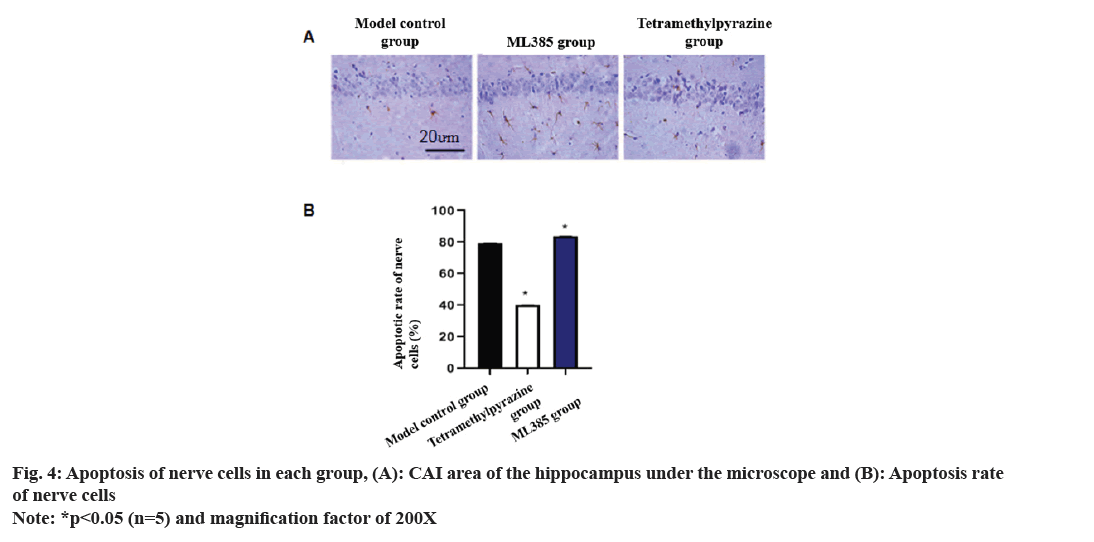
Comparison of neuronal apoptosis rate was carried out. Staining observations of the hippocampal Cornu Ammonis (CA) 1 region showed a sharp suppression in neuronal apoptosis in TMP rat brains. On the other hand, ML385 rats showed a sharp increase in neuronal apoptosis in the damaged brains of the rats (fig. 4).
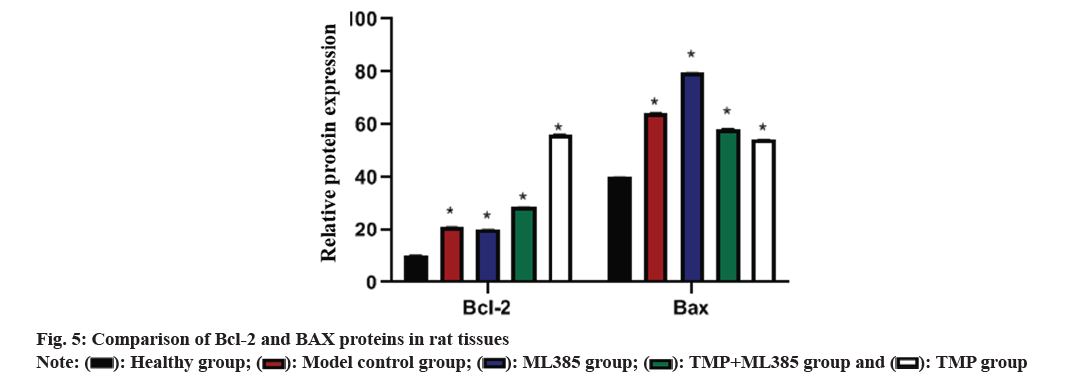
Further, Bcl-2 and BAX protein in rat tissues was compared. ELISA results revealed that Bcl-2 and BAX in model rats were higher than in the healthy rats. In TMP rats, BAX was drastically reduced, while Bcl-2 increased (fig. 5).
Nrf2 and AIM2 expression in each group was compared. Results indicated that Nrf2 and AIM2 were up-regulated in model rats compared to healthy rats, but were lower in ML385 rats. In TMP+ML385 rats group, Nrf2 was higher than that in the ML385 rats but was low in TMP rats, while AIM2 was drastically lower than that of ML385 rats but higher than that of TMP rats (fig. 6).
According to statistics, approximately 87 % of stroke patients have ischemic stroke[13]. However, the restoration of blood flow (reperfusion) exacerbates the damage, leading to Cerebral Ischemia-Reperfusion Injury (CIRI) which is the result of combined action of inflammatory reactions and cell necrosis, amoalt="Equation"ng other factors, which trigger the pathological cascade reactions and directly or indirectly lead to neuronal death[14,15]. Inflammation is an important pathological process in CIRI, and recent research has reported that TMP has functions such as reducing blood-brain barrier damage, dilating cerebral blood vessels and exerting anti-inflammatory effects[16,17]. Lijuan et al.[18] found that TMP inhibits the neuronal apoptosis in PC12 cell line by upregulating Bcl-2 and inhibiting caspase-3. However, the mechanism of neuroprotective action of TMP is still unknown. Therefore, we created a rat model of CIRI to observe the severity of neuronal functional impairment in each group of rats. Data demonstrated that mNSS of TMP rats was remarkably lower than that of control model, indicating that TMP could improve neuronal damage, consistent with previous report[19].
Nrf2 signaling has antioxidant effect and plays a key role in regulating Reactive Oxygen Species (ROS)-related apoptosis and CIRI[20,21]. Nrf2 controls many antioxidant genes’ expression, and its induced endogenous antioxidant enzymes are involved in various diseases. To further clarify whether TMP can exert its effects through Nrf2 in brain protection, the ML385 group was established in this study. Data indicated that the water content of brain tissue in TMP rats was drastically lower than that of control model, with the highest water content in the ML385 group. TTC staining demonstrated that infarct rate of ML385 rats enhanced sharply, while the infarct rate in TMP rats decreased significantly. Meanwhile, TUNEL staining results revealed a sharp decrease of neuronal apoptosis in TMP group, but a significant increase in ML385 group. Compared to control model, BAX in TMP rats down-regulated, while Bcl- 2 up-regulated. ML385 rats had the highest BAX level but least Bcl-2 level. When TMP and ML385 were used in combination, relative improvements in Bcl-2 and BAX protein levels were observed. This suggests that TMP may exert its neuroprotective effects by regulating the Nrf2 pathway.
It has been reported that AIM2 inflammasome absence leads to ischemic brain injury through Apoptosis-associated Speck-like protein containing Caspase (ASC) recruitment domain, leading to caspase-1 activation[22,23]. This study showed that AIM2 is an important component of innate immunity and enhances Interleukin (IL)-18 and IL-1 Beta (β) production, exacerbating the inflammatory response[24]. In addition, Gong et al.[25] found that after AIM2 gene deletion, AIM2 and IL-1β in mouse brain decreased and cerebral infarction and symptoms of neurological dysfunction were sharply alleviated. Based on these findings, this study further explored the connection between Nrf2 and AIM2 and investigated the mechanism of action in TMP-induced brain protection; quantitative PCR (qPCR) and immunoblotting demonstrated that Nrf2 and AIM2 in model intervention group was up-regulated with the highest expression in ML385 group while TMP group tended to be within the normal range. Therefore, it is speculated that the potential mechanism of TMP in brain protection involves the activation of Nrf2 via the Nrf2 pathway, downregulating AIM2 expression, controlling the entry of AIM2 into brain tissue, regulating Bcl-2 and BAX expression, thereby reducing brain tissue water content to some extent, inhibiting neuronal apoptosis, reducing cerebral infarction and ultimately exerting neuroprotective effects.
In summary, TMP can promote Nrf2 expression and downregulate AIM2, thus reducing neuronal apoptosis in brain tissue. This provides a new mechanism for drug treatment of CIRI. However, this study was conducted in animal models and did not investigate the therapeutic effects of TMP in combination with other drugs. The protective effects of TMP on patients with ischemic reperfusion injury and its synergistic effects with other drugs need further experimental analysis.
Conflict of interests:
The authors declared no conflict of interests.
References
- Jurcau A, Simion A. Neuroinflammation in cerebral ischemia and ischemia/reperfusion injuries: From pathophysiology to therapeutic strategies. Int J Mol Sci 2021;23(1):1-14.
[Crossref] [Google Scholar] [PubMed]
- Tuo QZ, Zhang ST, Lei P. Mechanisms of neuronal cell death in ischemic stroke and their therapeutic implications. Med Res Rev 2022;42(1):259-305.
[Crossref] [Google Scholar] [PubMed]
- Jurcau A, Ardelean AI. Oxidative stress in ischemia/reperfusion injuries following acute ischemic stroke. Biomedicines 2022;10(3):1-15.
[Crossref] [Google Scholar] [PubMed]
- Mao R, Zong N, Hu Y, Chen Y, Xu Y. Neuronal death mechanisms and therapeutic strategy in ischemic stroke. Neurosci Bull 2022;38(10):1229-47.
[Crossref] [Google Scholar] [PubMed]
- Zhang Q, Wang J, Zhu L, Jiang SJ, Liu J, Wang LX, et al. Tetramethylpyrazine attenuates hyperhomocysteinemia-induced Alzheimer-like pathologies in rats. Curr Med Sci 2021;41(3):548-54.
[Crossref] [Google Scholar] [PubMed]
- Du HY, Wang R, Li JL, Luo H, Xie XY, Yan R, et al. Tetramethylpyrazine induces viability, suppresses apoptosis and autophagy of retinal ganglion cells with ischemia/reperfusion injury through the PI3K/Akt/mTOR signaling pathway. Bioengineered 2021;12(1):507-15.
[Crossref] [Google Scholar] [PubMed]
- Zhao T, Fu Y, Sun H, Liu X. Tetramethylpyrazine suppresses neuron apoptosis via the Bax/Bcl-2 and caspase-3 pathway in PC12 cells and in rats with vascular dementia. IUBMB Life 2018;70(1):60-70.
[Crossref] [Google Scholar] [PubMed]
- Joshi B, Singh D, Wasan H, Sharma U, Reeta KH. Tideglusib ameliorates ischemia/reperfusion damage by inhibiting GSK-3β and apoptosis in rat model of ischemic stroke. J Stroke Cerebrovasc Dis 2022;31(4):1-10.
[Crossref] [Google Scholar] [PubMed]
- Oztanir MN, Dogan MF, Turkmen BN, Taslidere A, Sahin Y, Ciftci O. Secukinumab ameliorates oxidative damage induced by cerebral ischemia-reperfusion in rats. Turk Neurosurg 2022;32(5):732-9.
[Crossref] [Google Scholar] [PubMed]
- Zeng J, Chen Y, Ding R, Feng L, Fu Z, Yang S, et al. Isoliquiritigenin alleviates early brain injury after experimental intracerebral hemorrhage via suppressing ROS- and/or NF-κB-mediated NLRP3 inflammasome activation by promoting Nrf2 antioxidant pathway. J Neuroinflammation 2017;14(1):1-11.
[Crossref] [Google Scholar] [PubMed]
- Vahidinia Z, Tameh AA, Karimian M, Zare-Dehghanani Z, Moradi F, Joghataei MT. Calcitriol ameliorates brain injury in the rat model of cerebral ischemia-reperfusion through Nrf2/HO-1 signalling axis: An in silico and in vivo study. J Stroke Cerebrovasc Dis 2022;31(6):106331.
[Crossref] [Google Scholar] [PubMed]
- Sha R, Zhang B, Han X, Peng J, Zheng C, Zhang F, et al. Electroacupuncture alleviates ischemic brain injury by inhibiting the miR-223/NLRP3 pathway. Med Sci Monit 2019;25:4723-33.
[Crossref] [Google Scholar] [PubMed]
- Jurcau A, Ardelean IA. Molecular pathophysiological mechanisms of ischemia/reperfusion injuries after recanalization therapy for acute ischemic stroke. J Integr Neurosci 2021;20(3):727-44.
[Crossref] [Google Scholar] [PubMed]
- Franke M, Bieber M, Kraft P, Weber AN, Stoll G, Schuhmann MK. The NLRP3 inflammasome drives inflammation in ischemia/reperfusion injury after transient middle cerebral artery occlusion in mice. Brain Behav Immun 2021;92:223-33.
[Crossref] [Google Scholar] [PubMed]
- Chen H, He Y, Chen S, Qi S, Shen J. Therapeutic targets of oxidative/nitrosative stress and neuroinflammation in ischemic stroke: Applications for natural product efficacy with omics and systemic biology. Pharmacol Res 2020;158:1-10.
[Crossref] [Google Scholar] [PubMed]
- Almatroudi A, Alsahli MA, Alrumaihi F, Allemailem KS, Rahmani AH. Ginger: A novel strategy to battle cancer through modulating cell signalling pathways: A review. Curr Pharm Biotechnol 2019;20(1):5-16.
[Crossref] [Google Scholar] [PubMed]
- Dou J, Wang Z, Ma L, Peng B, Mao K, Li C, et al. Baicalein and baicalin inhibit colon cancer using two distinct fashions of apoptosis and senescence. Oncotarget 2018;9(28):20089-102.
[Crossref] [Google Scholar] [PubMed]
- Lijuan T, Xiaolu C, Xin W, Yuying H, Xi L, Xiliang Y, et al. Identification of Tetramethylpyrazine-based analogs of piperlongumine as potential anti-ischemic stroke agents. Fitoterapia 2023;165:1-10.
[Crossref] [Google Scholar] [PubMed]
- Ding Y, Du J, Cui F, Chen L, Li K. The protective effect of tetramethylpyrazine on rats with cerebral ischemia-reperfusion injury via activating PI3K/Akt pathway. Hum Exp Toxicol 2019;38(10):1168-77.
[Crossref] [Google Scholar] [PubMed]
- Xiao L, Dai Z, Tang W, Liu C, Tang B. Astragaloside IV alleviates cerebral ischemia-reperfusion injury through NLRP3 inflammasome-mediated pyroptosis inhibition via activating Nrf2. Oxid Med Cell Longev 2021;1-19.
[Crossref] [Google Scholar] [PubMed]
- Liu Q, Ci X, Wen Z, Peng L. Diosmetin alleviates lipopolysaccharide-induced acute lung injury through activating the Nrf2 pathway and inhibiting the NLRP3 inflammasome. Biomol Ther 2018;26(2):157-66.
[Crossref] [Google Scholar] [PubMed]
- Wang M, Chen Z, Yang L, Ding L. Sappanone A protects against inflammation, oxidative stress and apoptosis in cerebral ischemia-reperfusion injury by alleviating endoplasmic reticulum stress. Inflammation 2021;44(3):934-45.
[Crossref] [Google Scholar] [PubMed]
- Hennig P, Garstkiewicz M, Grossi S, di Filippo M, French LE, Beer HD. The crosstalk between Nrf2 and inflammasomes. Int J Mol Sci 2018;19(2):1-12.
[Crossref] [Google Scholar] [PubMed]
- Xu Q, Zhao B, Ye Y, Li Y, Zhang Y, Xiong X, et al. Relevant mediators involved in and therapies targeting the inflammatory response induced by activation of the NLRP3 inflammasome in ischemic stroke. J Neuroinflammation 2021;18(1):1-12.
[Crossref] [Google Scholar] [PubMed]
- Gong S, Ma H, Zheng F, Huang J, Zhang Y, Yu B, et al. Inhibiting YAP in endothelial cells from entering the nucleus attenuates blood-brain barrier damage during ischemia-reperfusion injury. Front Pharmacol 2021;12:1-17.
[Crossref] [Google Scholar] [PubMed]
 ): Model control group; (
): Model control group; (  ): TMP group; (
): TMP group; ( ): ML385 group and (
): ML385 group and ( ): TMP+ML385 group
): TMP+ML385 group 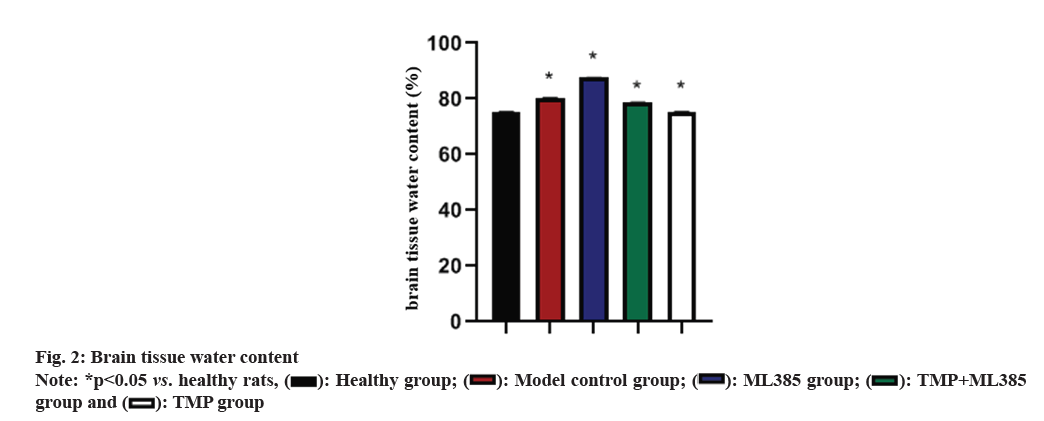
 ): Healthy group; (
): Healthy group; ( ): Model control group; (
): Model control group; ( ): ML385 group; (
): ML385 group; ( ): TMP+ML385 group and (
): TMP+ML385 group and ( ): TMP group
): TMP group 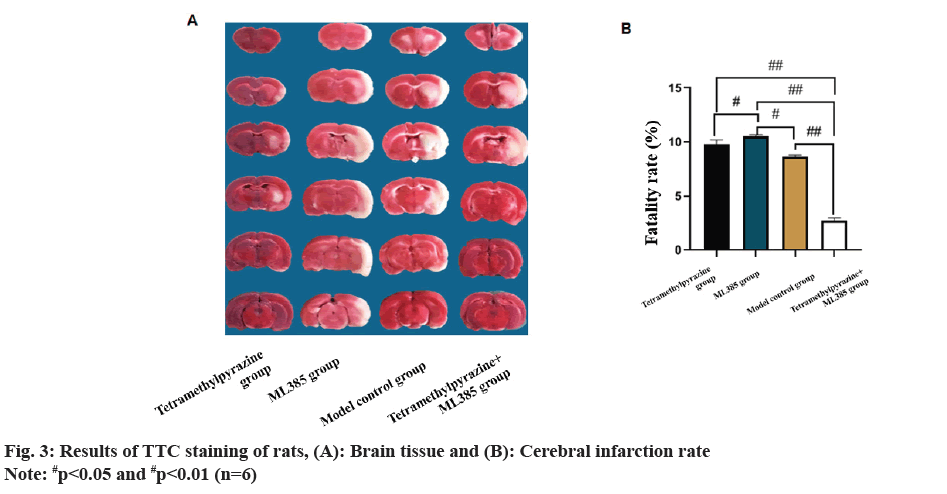
 ): Healthy group; (
): Healthy group; ( ): Model control group; (
): Model control group; ( ): ML385 group; (
): ML385 group; ( ): TMP+ML385 group and (
): TMP+ML385 group and ( ): TMP group
): TMP group 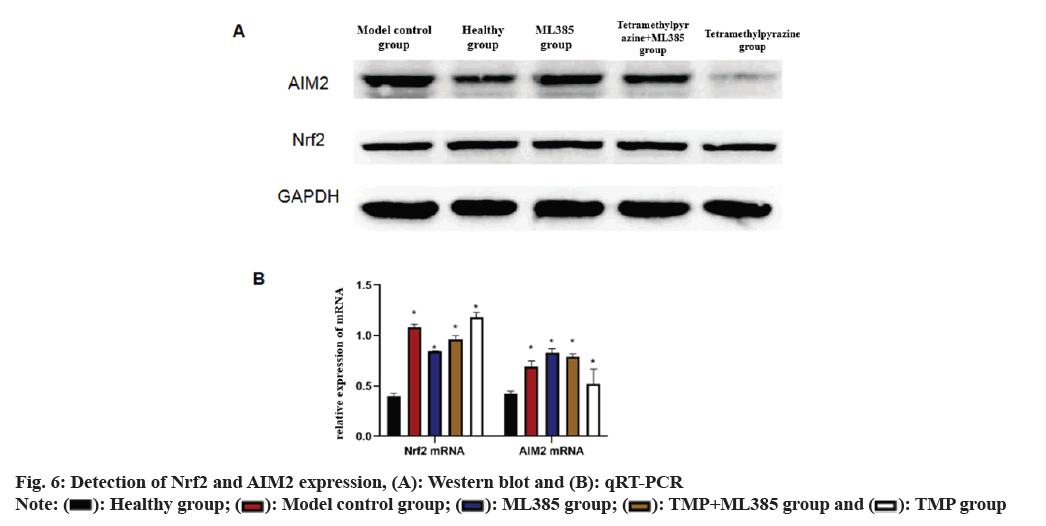
 ): Healthy group; (
): Healthy group; (  ): Model control group; (
): Model control group; ( ): ML385 group; (
): ML385 group; (  ): TMP+ML385 group and (
): TMP+ML385 group and (  ): TMP group
): TMP group 



