- *Corresponding Author:
- T. Yang
Beijing Huilin Zegu Biotechnology Co., Ltd., Haidian, Beijing 100039, China
E-mail: yangtao686@163.com
| Date of Received | 24 June 2021 |
| Date of Revision | 11 December 2021 |
| Date of Acceptance | 21 July 2022 |
| Indian J Pharm Sci 2022;84(4):959-968 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
We herein aimed to evaluate the neuroprotective effects of resveratrol on a mouse model of Parkinson’s disease and to explore whether the Wnt/beta-catenin signaling pathway was involved. Thirty male C57 black 6 mice were randomly divided into a control group, a model group (1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine) and a treatment group (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine resveratrol) (n=10). The model and treatment groups were intraperitoneally injected with 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine (30 mg/kg in normal saline) to induce Parkinson’s disease. Then the treatment group was administered with resveratrol (20 mg/kg in 0.5 % sodium carboxymethylcellulose solution) on the 1st d of modeling. The motor coordination ability was studied by the pole climbing test. Astrocyte and microglia activations in the substantia nigra pars compacta were observed by immunohistochemical staining. 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced striatum dopamine release was measured by high performance liquid chromatography and dopamine transporter level was detected with Western blot. SH-SY5Y cell viability and dependence on the Wnt/beta-catenin signaling pathway were assessed by 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide assay and Western blot, respectively. Resveratrol relieved the motor dysfunction, death of dopaminergic neurons, activation of astrocytes and microglia, as well as reduction of striatum dopamine content induced by neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in vitro. It also alleviated the damage of SH-SY5Y cells induced by 1-methyl- 4-phenylpyridinium. Resveratrol increased the levels of proteins from the Wnt/beta-catenin signaling pathway in the mouse model of Parkinson’s disease and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine- treated SH-Y5Y cells. Resveratrol exerted neuroprotective effects on the Parkinson’s disease model predominantly by regulating the Wnt/beta-catenin signaling pathway.
Keywords
Parkinson’s disease, resveratrol, dopamine, Wnt/beta-catenin
Parkinson’s Disease (PD) is a common progressive neurodegenerative dyskinesia with the prevalence rate of about 1 % among the elderly aged over 65 y old. The main clinical manifestations include tremor, stiffness, bradykinesia and gait apraxia, as well as autonomic dysfunction, sleep disorders and Alzheimer’s disease in the later stage[1,2]. PD is pathologically typified by the progressive loss of dopaminergic neurons in the Substantia Nigra pars compacta (SNpc), but its exact pathogenesis remains unclear[3]. Levodopa replacement therapy is still the first-line treatment for PD, but the progression cannot be prevented, accompanied by many serious adverse reactions after long-term use, such as autonomic symptoms (nausea, orthostatic hypotension), daytime sleepiness and psychiatric complications (illusion, delusion)[4]. Therefore, researchers have endeavored to find new targets for developing drugs for PD, aiming to improve the quality of life of patients.
The Wnt protein family consists of 19 glycosylated proteins that regulate cell growth and differentiation[5]. It is also an important mediator of cell-to-cell communication and signal transduction during the development of the central nervous system. Wnt ligands are a class of secreted glycoproteins that work through paracrine or autocrine. Besides, the Wnt/Beta (β)-catenin signaling pathway (Wnt1 or Wnt3a) and Wnt signaling essentially participate in the development of midbrain dopaminergic neurons[6]. Wnt1 is involved in midbrain formation, progenitor cell differentiation in SNpc and survival of dopaminergic neurons.
Resveratrol is a natural polyphenolic chemical widely existing in grape skin and other plants, with biological characteristics such as antioxidation, anti-inflammation, anti-aging, antitumor and relief of the endoplasmic reticulum stress. At present, studies regarding the protective effects of resveratrol on dopaminergic neurons focus on resistance to oxidation and inflammation[7]. Nevertheless, the role of the Wnt/β-catenin signaling pathway has seldom been referred. Thereby motivated, we herein aimed to clarify the mechanism by which the Wnt/β-catenin signaling pathway participated in the neuroprotective effects of resveratrol on PD through establishing in vivo and in vitro models using SH-SY5Y cells treated with 1-Methyl-4-Phenylpyridinium (MPP+).
Materials and Methods
Animals and cell line:
This study has been approved by the animal ethics committee of Beijing Huilin Zegu Biotechnology Co., Ltd., and great efforts have been made to alleviate the suffering of animals. Thirty Specific Pathogen Free (SPF) male C57 Black 6 (C57BL/6) mice aged 12 w old and weighing 24-26 g were purchased from SPF (Beijing) Biotechnology Co., Ltd. (China, certificate of conformity: SCXK (jing) 2019-0010). They were fed in standard feeding boxes at 20°-26° with a 12 h/12 h light/ dark cycle.
Reagents and antibodies:
Resveratrol (purity: ≥98 %), 1-Methyl-4-Phenyl-1,2,3,6- Tetrahydropyridine (MPTP) and 3-(4,5-Dimethylthiazol- 2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) were obtained from Sigma (St. Louis, MO, USA). Dulbecco’s Modified Eagle Medium (DMEM), Opti-MEM, Non- Essential Amino Acids (NEAA) and penicillin were purchased from Gibco (Grand Island, NY, USA). Fetal Bovine Serum (FBS) was bought from Sciencell (Carlsbad, California (CA), United States of America (USA)). Wnt1 antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). P-Ser9- Glycogen Synthase Kinase 3 Beta (GSK3β) and β-catenin antibodies were provided by Cell Signaling Technology (Beverly, Massachusetts (MA), USA). Dopamine Transporter (DAT) and β-actin antibodies were purchased from Millipore (MA, USA). All other
drugs were obtained from Sigma (St. Louis, MO, USA).
Animal grouping and treatment:
Thirty mice were randomly divided into a control group, a model group (MPTP) and a treatment group (MPTP+resveratrol) (n=10). The model and treatment groups were intraperitoneally injected with MPTP (30 mg/kg in normal saline) to induce PD, once every 3.5 d for 5 w (a total of 10 injections). The control group was intraperitoneally injected with a corresponding volume of normal saline. The treatment group was administered with resveratrol (20 mg/kg in 0.5 % sodium carboxymethylcellulose solution) on the 1st d of modeling, once a day for 5 w. The control and model groups were intragastrically treated with corresponding volumes of 0.5 % sodium carboxymethylcellulose solution.
Animal behaviors:
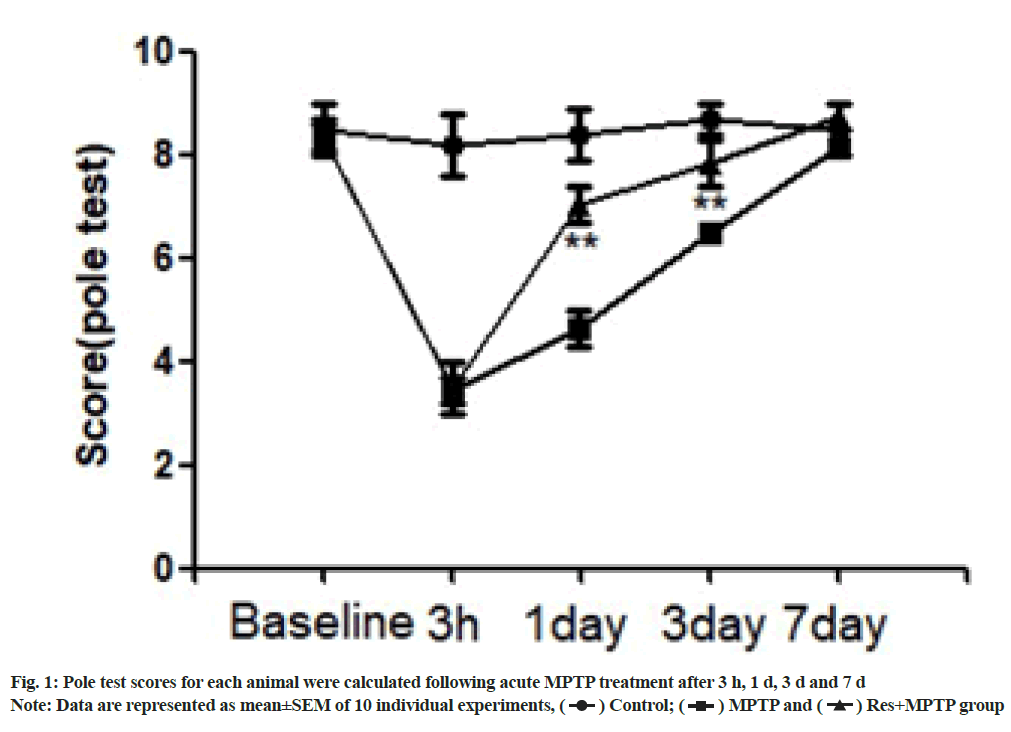
The pole climbing test was used to investigate the motor coordination ability of a mouse[8]. A straight wooden rod with a diameter of 10 mm and a height of 500 mm was used, and a cork ball with a diameter of 25 mm was placed on the top of the rod. The mouse head was placed upside down on the top of the rod and the following three time points were recorded; T1 for the time from the start of movement to the time when the head was turned downward; T2 for the time of climbing down to the middle of pole and T3 for the time of climbing to the bottom of pole from the middle. The above activities were scored as follows, 3 points for shorter than 3 s; 2 points for shorter than 6 s but longer than 3 s and 1 point for over 6 s. Finally, the sum of them (Tl+T2+T3) was recorded. The mice were trained for 3 d before the test. Detection was conducted three times during the test, with the interval of 1 min each time and the shortest time was recorded. If the mouse failed to completely flip, slip or drop, the score was 3 points. The pole climbing ability was evaluated 1 w before establishment of the MPTP model and 3 h, 1 d, 3 d and 7 d after it.
Immunohistochemical staining:
The mice were anesthetized through intraperitoneal injection with 0.004 ml/g of 10 % chloral hydrate. After anesthesia, the heart was exposed. A scalp needle was inserted into the left ventricle and the auricula dextra was cut open, into which normal saline and 4 % paraformaldehyde were perfused. The mouse brain was taken out immediately and fixed in 4 % paraformaldehyde overnight. Afterwards, the brain was dehydrated in 20 % saccharose solution for 3 d and in 30 % saccharose solution for another 3 d and stored at -70°. Frozen tissue was sectioned into 30 μm thick. The brain sections were collected in Phosphate-Buffered Saline (PBS) and stored at 20° prior to use. After being washed with PBS at room temperature, the sections were incubated with 3 % Hydrogen peroxide (H O ) for 30 min to eliminate endogenous horseradish peroxidase and then washed three times with PBS on a shaker, 5 min each time. At room temperature, the sections were blocked by using 5 % Bovine Serum Albumin (BSA) (prepared with PBS/0.1 % Triton X-100) for 1 h, incubated with mouse anti-Tyrosine Hydroxylase (TH) (1:3000), anti- Glial Fibrillary Acidic Protein (GFAP) (1:800) or anti- Macrophage-1 (MAC-1) (1:100) primary antibody overnight at 4° and washed with 0.01 M PBS 3 times, 5 min each time. Subsequently, they were incubated with corresponding horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature and color-developed with diaminobenzidine. Stereoscopic counting was performed for TH-positive cells in SNpc, GFAP-positive astrocytes and MAC-1-positive microglia.
Measurement of dopamine levels in the striatum:
Dopamine level was measured using High Performance Liquid Chromatography (HPLC) with LC-20A pump (Shimadzu, Kyoto, Japan) and RF-20A fluorescence detector (Shimadzu, Kyoto, Japan). Chromatographic separation of dopamine was conducted using Thermo Hypersil GOLDTM C18 column (4.6×150 mm, i.d., 5 μm; Thermo Fisher Scientific Inc., MA, USA). Mobile phase A consisted of 30 mM citric acid, 40 mM sodium acetate anhydrous, 0.2 mM Ethylenediaminetetraacetic Acid Disodium (EDTA-2Na) salt and 0.4 mM sodium octane sulfonate (pH=3.9) and phase B was methanol. The ratio of phase A to phase B was 86:14 and the flow rate was 1.0 ml/min. Dopamine level was calculated based on the peak area of standard curve using Lab Solutions LC-solution Version 1.2 Workstation (Shimadzu, Kyoto, Japan).
Cell culture and grouping:
SH-SY5Y cells were cultured in DMEM containing 10% FBS, 1 % NEAA, 100 U/ml penicillin and 0.1 U/ml streptomycin at 37° with 5 % Carbon dioxide (CO2). The cells were cultured in sterile plastic culture flasks at the density of 1×105/cm2 and trypsinized and passaged every 3 d. Then the cells were seeded into 96 or 6-well plates and cultured by DMEM containing 10 % FBS for 24 h. Drug treatment was started after the culture medium was replaced by serum-free one.
MTT assay:
The cell viability was tested by the MTT assay. Wellgrown cells were digested, diluted into a suspension by DMEM containing 10 % FBS, seeded into 96-well plates (200 μl each well) and incubated at 37° with 5 % CO2 for 24 h. After the culture medium was replaced by serumfree medium, the cells were treated for 24 h as mentioned above, from which the medium was discarded. After addition of 200 μl of 0.5 mg/ml MTT solution prepared by PBS, the cells were further cultured at 37° with 5 % CO2 for 4 h and MTT solution was removed. Subsequently, 200 μl of Dimethyl Sulfoxide (DMSO) was added and the plates were shaken at room temperature for 15 min. The Optical Density (OD) of each well was measured at 450 nm by a micro plate reader. Cell viability was determined by using the percentage of OD of drug treatment group to that of the control group.
Western blotting:
1 w after MPTP administration, brain tissues were rapidly removed onto ice, added in a proportion of 1:10 (v/v) to buffer containing protease inhibitor cocktail (Life Technologies Corp., Carlsbad, CA, USA), ultrasonicated and incubated on ice for 40 min. Cell lysates were centrifuged at 12 000 ×g for 15 min, and the supernatants were collected as whole-cell protein samples. A 1 μl sample of lysate was used to measure the total protein concentration. The samples were mixed with 5× loading buffer and denatured in boiling water for 5 min before storage at -20° for future use.
According to the Bicinchoninic Acid (BCA) quantification results, 20 μg protein was loaded on each lane of 10 % polyacrylamide gel and separated by Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) (80 V). Separated proteins were electronically transferred onto polyvinylidene fluoride membranes at 300 mA constant current for 90 min. Blotted membranes were incubated in Tris Buffered Saline+Tween 20 (TBST) (pH 7.4, 10 mM Tris-HCl, 150 mM Sodium chloride (NaCl), 0.1 % Tween-20) containing 5 % non-fat milk for 1 h and then in 5 % BSA-TBST containing one of the following primary antibodies overnight at 4° anti-Wnt1 (1:200), anti-p- Ser9-GSK3β (1:1000), anti-β-catenin (1:1000) or anti-β- actin (1:1000). Immunolabeled membranes were washed three times in TBST and incubated with secondary antibodies for 1 h, followed by three washes with TBST. Finally, immunoblots were scanned by Gel Max imager (Ultra-Violet Products Ltd., Upland, CA, USA). Band density was measured by Gel Pro Analyzer software (Media Cybernetics, Inc., Bethesda, MD, USA).
Statistical analysis:
All data are represented as mean±Standard Error of the Mean (SEM). Treatment group means were compared by one-way or two-way analysis of variance. When analysis of variance showed significant differences among groups, pairwise comparisons were carried out by the Newman-Keuls post hoc test. For all analyses, p<0.05 was considered statistically significant. All statistical analyses were performed with IBM Statistical Package for the Social Sciences (SPSS) software (version 21.0, USA).
Results and Discussion
A mouse model of MPTP was established to evaluate the protective effects of resveratrol on neurological and motor deficits. The mice showed obvious acute reaction after a few min of intraperitoneal injection of MPTP, such as tail stiffness, vertical hair, decreased activity, etc., the symptoms were alleviated after 12 h, but the activity of the mice was still significantly reduced. In contrast, the control group did not undergo motor dysfunction. At 3 h as well as on the 1st, 3rd and 7th d after intraperitoneal injection of MPTP, the behavior of climbing rods was observed. The rats showed obvious motor dysfunction 3 h and 1 d after intraperitoneal injection of MPTP. The time required for climbing was significantly increased. Resveratrol pretreatment significantly increased the motor function scores of mice on 1 d and 3 d after intraperitoneal injection of MPTP compared with MPTP as shown in fig. 1.
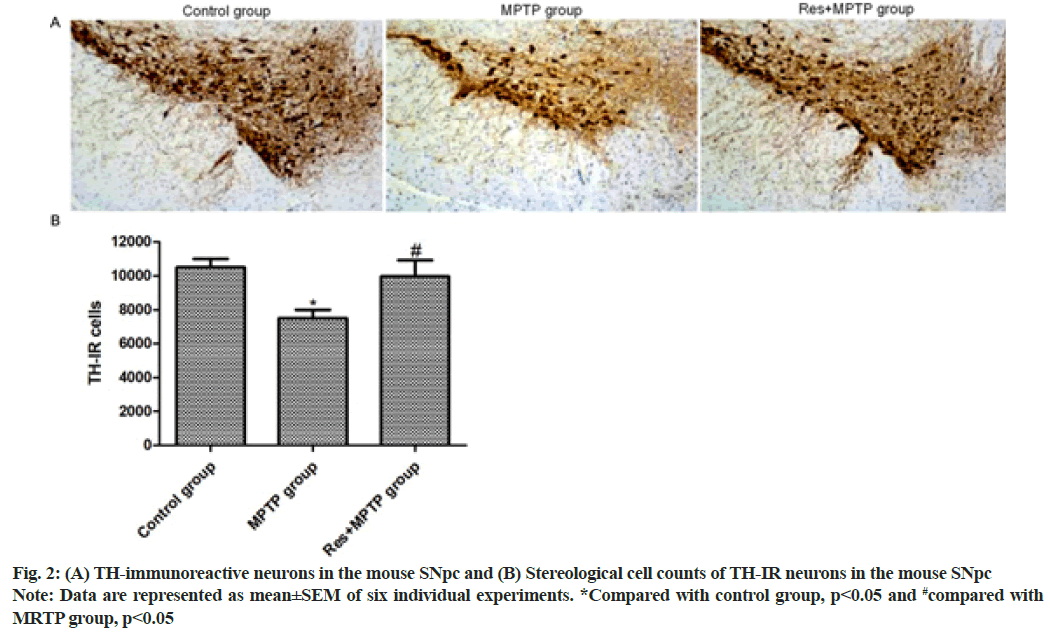
In the pole climbing test, resveratrol significantly alleviated the motor dysfunction of PD mice induced by MPTP. Consistent with behavioral testing, resveratrol attenuated the neurotoxicity of MPTP and protected dopaminergic neurons. Immunohistochemical staining was used to observe the dopaminergic neurons in SNpc. Intraperitoneal injection of MPTP reduced the number of TH positive cells in SNpc. Compared with the MPTP group; pretreatment with resveratrol significantly increased the number of TH positive cells in SNpc as shown in fig. 2.
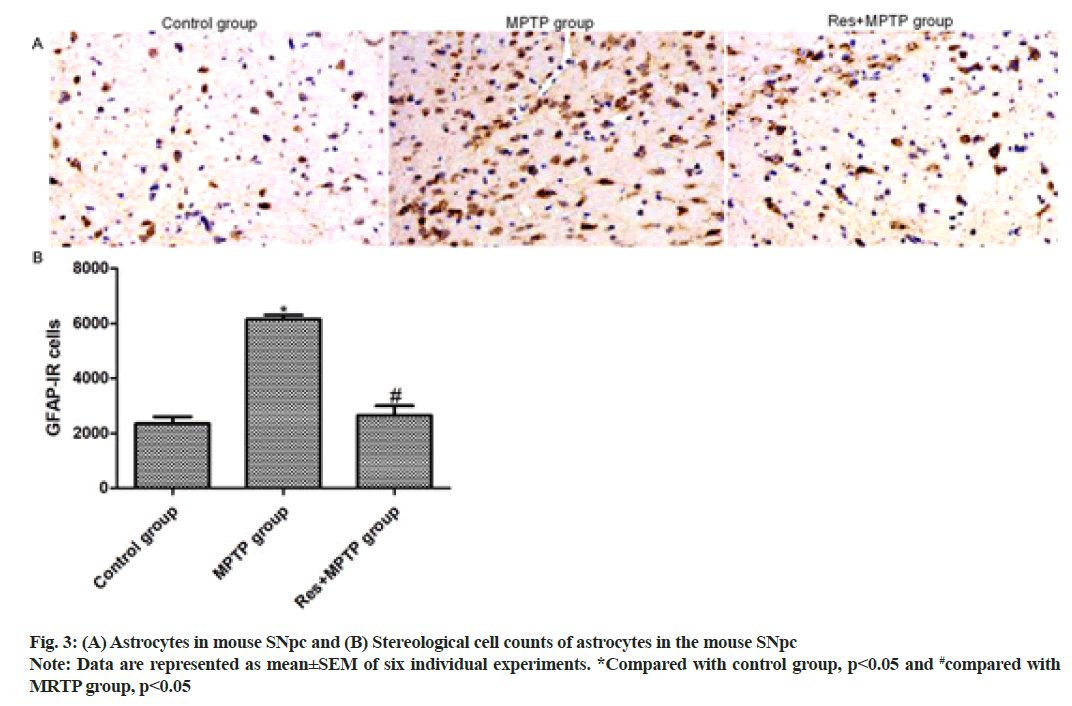
To further investigate the effects of resveratrol on MPTP-induced activation of mouse astrocytes. GFAP staining showed the number of GFAP-positive astrocytes in SNpc. Only a weak GFAP-positive astrocyte immune response was observed in SNpc of the control mice. Intraperitoneal injection of MPTP increased the number of GFAP-positive astrocytes in SNpc 3.12-fold compared with that of the control group. Resveratrol pretreatment inhibited MPTP-induced activation of astrocytes (p<0.001, compared with MPTP group) as shown in fig. 3.
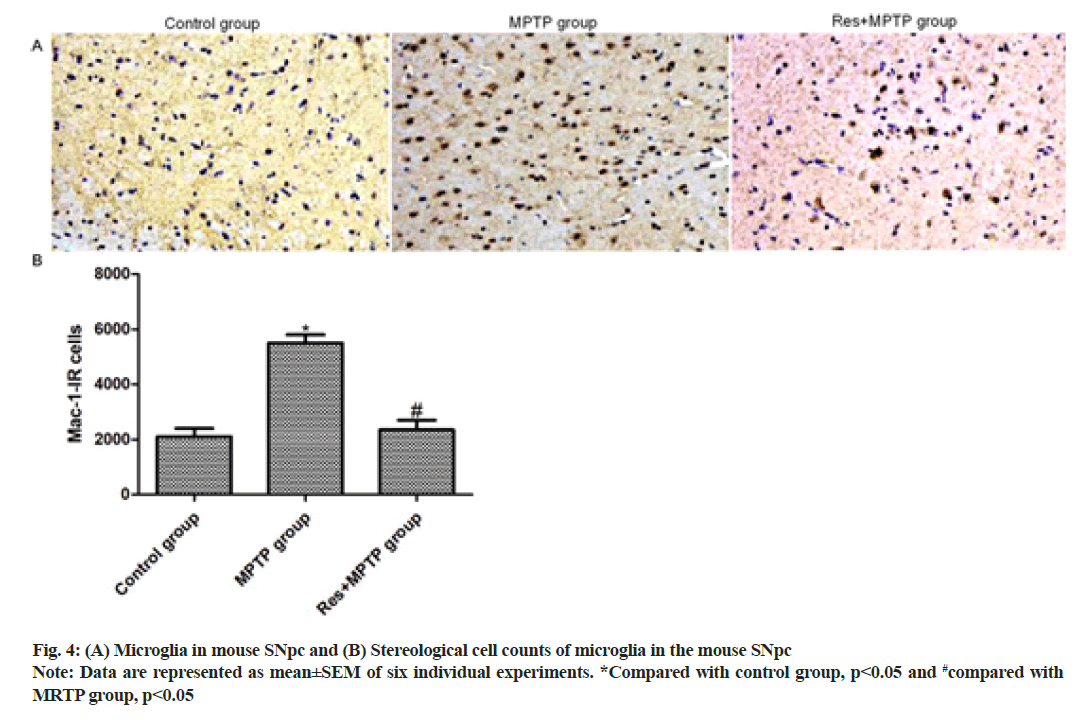
In this study, MAC-1 staining was used to label activated microglia to study the effect of resveratrol on MPTP-induced mouse microglia activation. MAC-1 staining showed that only weak MAC-1 positive microglial immune response was observed in SNpc of the control group. The intraperitoneal injection of MPTP increased the number of MAC-1 positive microglia in SNpc 2.85- fold compared with that of the control group (p<0.001). Resveratrol pretreatment inhibited MPTP-induced activation of microglia (p<0.001, compared to MPTP group) as shown in fig. 4.
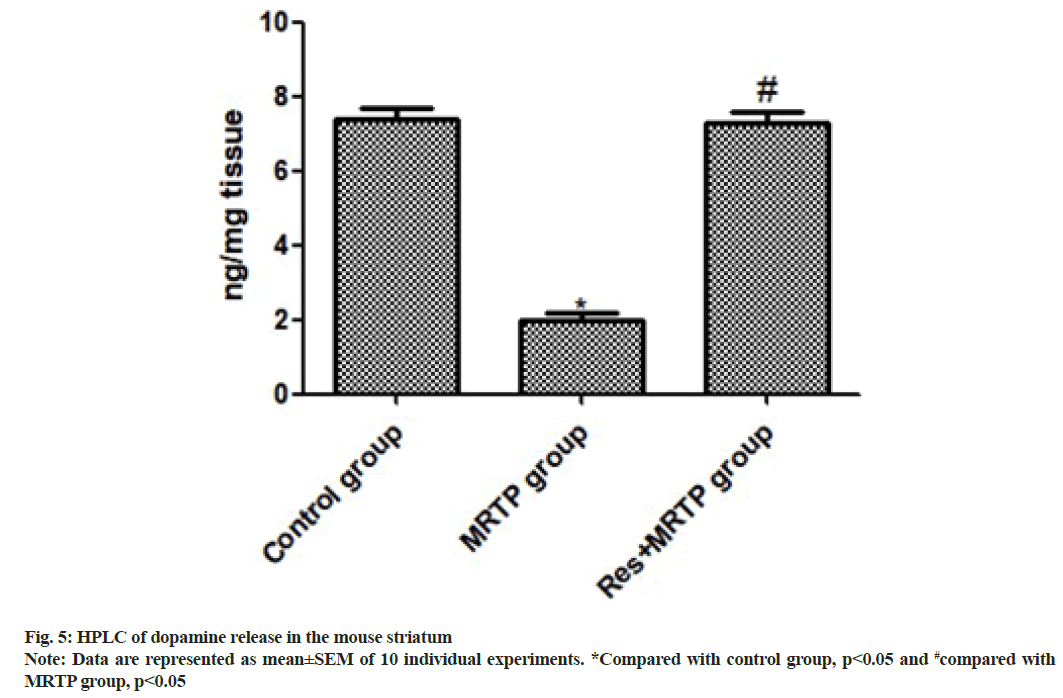
We determined the release of striatal dopamine by HPLC. The striatum dopamine level was significantly decreased after MPTP administration. Compared with the MPTP group, the MPTP+ resveratrol group had significantly higher level of dopamine in the striatum (p<0.001) as shown in fig. 5.
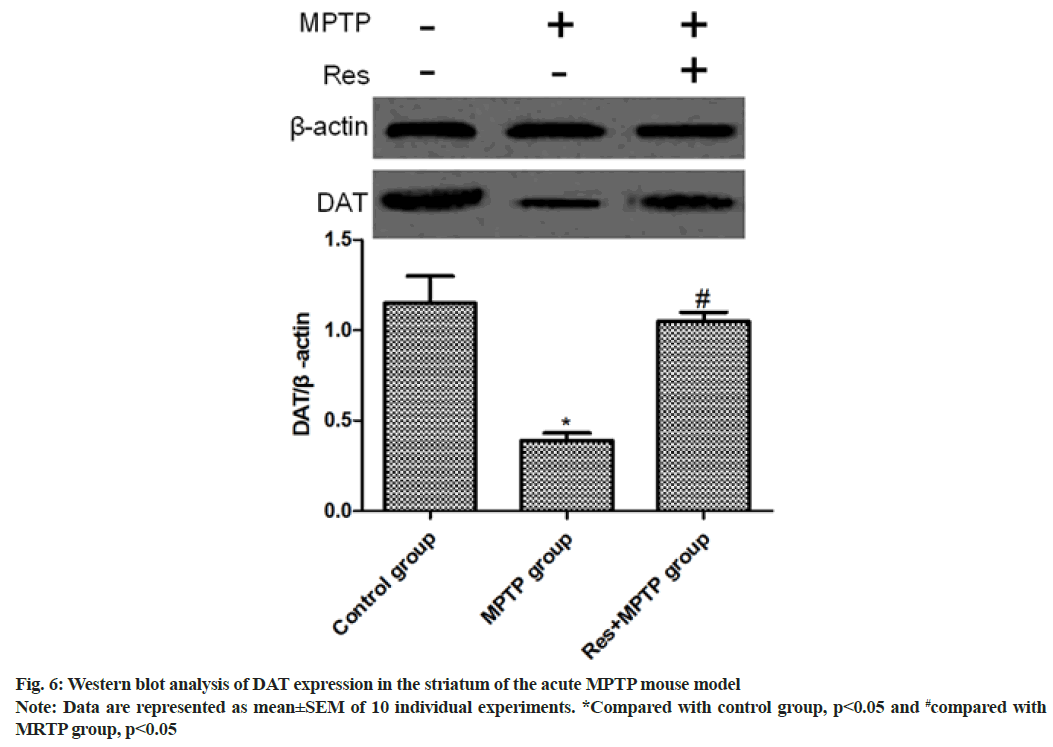
Western blotting was used to determine the level of DAT in the striatum. Intraperitoneal injection of MPTP significantly decreased the expression of DAT (p<0.01, compared with control group). Resveratrol was able to reverse the decrease in DAT level (p<0.01, compared with MPTP group) as shown in fig. 6.
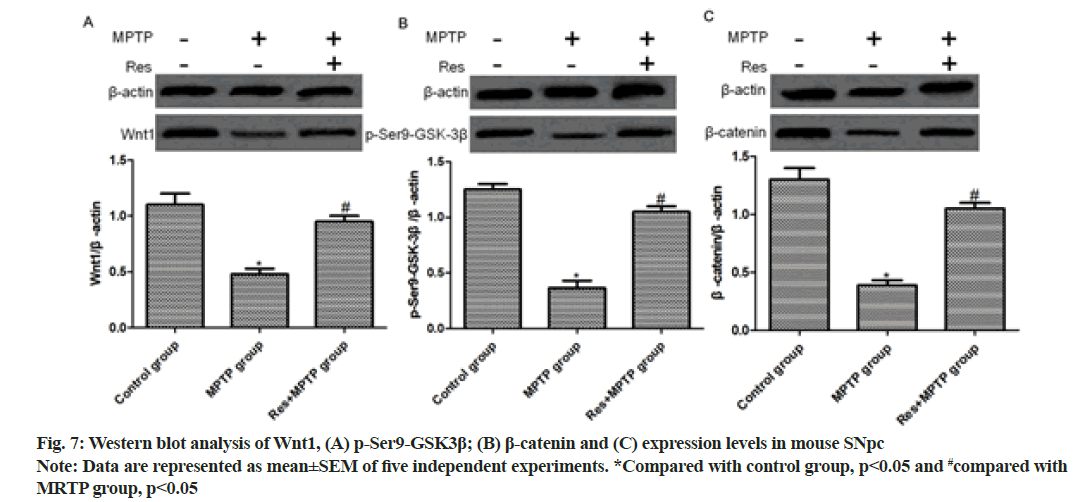
Intraperitoneal injection of MPTP significantly reduced the levels of Wnt1, p-Ser9-GSK-3β and β-catenin, but the resveratrol+MPTP group had significantly elevated levels (p<0.01, compared with MPTP group) as shown in fig. 7.
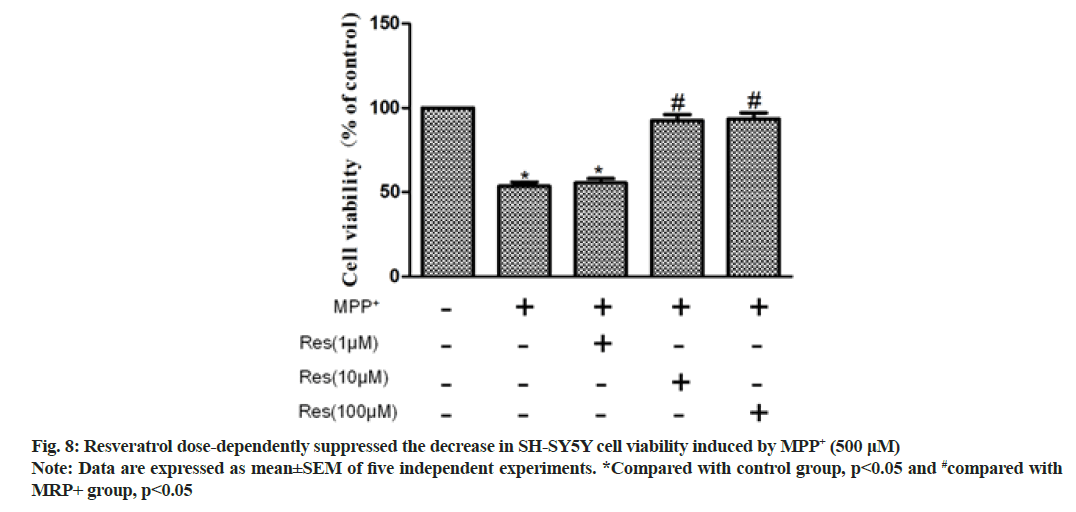
SH-SY5Y cells were pretreated with resveratrol (1 μM, 10 μM or 100 μM) for 24 h before administration of 500 μM MPP+. Cell viability was measured by MTT assay. Compared with the MPP+ group, the MPP++resveratrol group showed significant resveratrol dose dependence. Specifically, 10 μM and 100 μM resveratrol pretreatment significantly augmented cell viability (p<0.01) as shown in fig. 8.
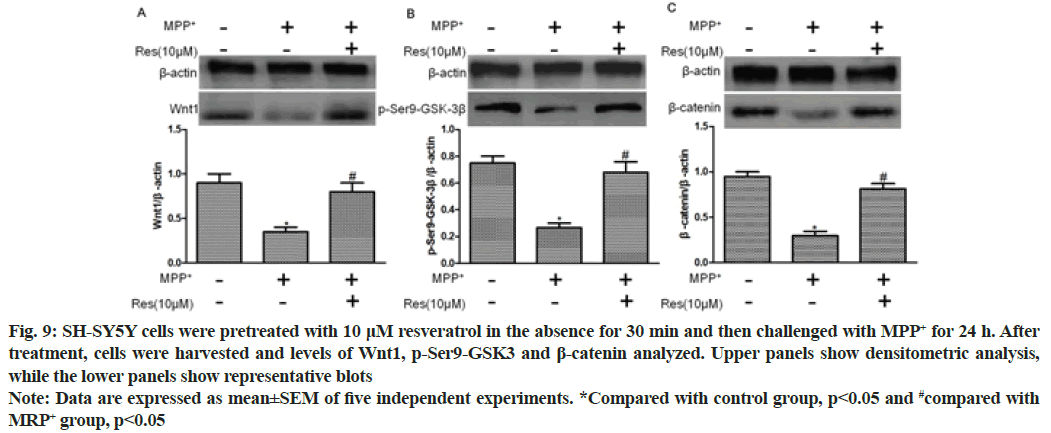
Western blotting exhibited that MPP+ treatment significantly reduced the expression levels of Wnt1, p-Ser9-GSK-3β and β-catenin (p<0.01, compared with control group). In addition, 10 μM resveratrol pretreatment for 30 min significantly elevated their levels (p<0.01, compared with MPP+ group) as shown in fig. 9.
Fig. 9: SH-SY5Y cells were pretreated with 10 μM resveratrol in the absence for 30 min and then challenged with MPP+ for 24 h. After treatment, cells were harvested and levels of Wnt1, p-Ser9-GSK3 and β-catenin analyzed. Upper panels show densitometric analysis, while the lower panels show representative blots
Note: Data are expressed as mean±SEM of five independent experiments. *Compared with control group, p<0.05 and #compared with MRP+ group, p<0.05
With the deepening of the research on the pathogenesis and pathophysiological process of PD, it is a hot research topic of PD to find neuroprotective drugs that can prevent or delay the course of the disease. For the treatment of PD, neuroprotective therapy can bring long-term benefits by affecting the etiology and pathogenesis. It should delay the onset or delay the progression of the disease.
Resveratrol has a wide range of pharmacological effects, including anti-oxidative stress, anti-inflammatory, anti-tumor, liver protection, liver cirrhosis, immune regulation, sedative and estrogen-like effects, antiviral, etc.[9,10]. There have been no reports of its prevention or research on PD. Because of its anti-oxidative stress, inhibition of cell apoptosis and immune regulation, combined with the known factors involved in PD pathogenesis, it is envisaged that it may inhibit or even prevent oxidative stress and apoptosis in the process of PD pathogenesis. Estrogen may play a protective role in PD dopaminergic neurons, so this experiment used C57BL/6 male mice[11]. The mouse PD model induced by intraperitoneal injection of MPTP is more suitable for the study of the therapeutic effect of drugs on PD.
In this study, mice were found to have obvious acute reactions in mice a few min after intraperitoneal injection of MPTP, such as tail stiffness, vertical hair, decreased activity, etc., the symptoms were alleviated after 12 h, but the activity of the mice was still significantly reduced. Significant motor dysfunction occurred at 3 h and 1 d after intraperitoneal injection of MPTP and the time required for climbing was significantly increased, while resveratrol pretreatment significantly increased mice on 1 d and 3 d after MPTP intraperitoneal injection. Resveratrol significantly mitigated dyskinesia in MPTP model mice. In accordance with the behavioral test, intraperitoneal injection of MPTP reduced the number of TH-positive cells in SNpc of mice. Compared with the mouse MPTP group, the pretreatment with resveratrol significantly increased TH-positive SNpc in the mouse MPTP+resveratrol group. The number of cells suggests that resveratrol can inhibit the neurotoxicity of MPTP and protect dopaminergic neurons.
Astrocytes are the most abundant cell types in the brain and play a vital role in the central nervous system. Astrocytes are involved in the innate immune response and are involved in the regulation of immune inflammation[12-14]. Since neuroinflammatory response plays a key role in the pathogenesis of PD, the effect of resveratrol on mouse astrocyte activation induced by MPTP was further explored. GFAP staining showed the number of GFAP-positive astrocytes in SNpc of mice. Only a weak GFAP-positive astrocyte immune response was observed in SNpc of the control mice. Intraperitoneal injection of MPTP increased the number of GFAP-positive astrocytes in SNpc 3.12-fold compared with the control group. Resveratrol pretreatment inhibited MPTP-induced activation of astrocytes (p<0.001, compared with MPTP group). In the brain tissue, microglia can regulate immune inflammation and MPTP can promote the occurrence of neuroinflammation. Herein, the number of MAC-1 positive microglia in SNpc of mice was observed by MAC-1 staining. Intraperitoneal injection of MPTP increased the number of MAC-1 positive microglia in the SNpc area 2.85-fold compared with the control group (p<0.001). Resveratrol pretreatment inhibited MPTPinduced activation of microglia (p<0.001, compared with MPTP group), suggesting that resveratrol significantly suppressed MPTP-induced astrocyte and microglia activation.
To further validate the inhibitory effect of resveratrol on the function of MPTP-damaging dopaminergic neurons, we measured the release of striatal dopamine by HPLC. The dopamine level in the striatum was significantly reduced after MPTP administration in mice. Compared with the MPTP group, the striatum dopamine level was significantly increased in the MPTP+resveratrol group (p<0.001). DAT is a transmembrane protein that reabsorbs dopamine from the synaptic cleft to presynaptic neurons, controls dopamine transport and maintains a balanced dopamine level[15]. To further investigate whether resveratrol plays an important role in the stable expression of DA, the level of DAT in mouse striatum was determined by Western blotting. The results showed that intraperitoneal injection of MPTP in mice can significantly reduce the expression of DAT (p<0.01, compared with control group), resveratrol could reverse the decrease of DAT level induced by MPTP in mice (p<0.01, compared with MPTP group).
Accumulating evidence has proven that the dysregulation of Wnt signaling plays a crucial role in the pathogenesis of PD[16] and Wnt are crucial regulators of inflammatory response[17-19]. In the brain, both astrocytes and microglia express Wnt receptors and respond to Wnt proinflammatory or anti-inflammatory activity[20,21]. However, the relationship between the resveratrol antiinflammatory pathway involved in the damage and repair of nigrostriatal dopaminergic neurons and the Wnt/β- catenin signaling pathway remains unclear. In this study, intraperitoneal injection of MPTP in mice significantly reduced Wnt1, p-Ser9-GSK-3β and β-catenin, and resveratrol+MPTP group Wnt1, p-Ser9-GSK-3β and β-catenin were significantly elevated (p<0.01, compared to the MPTP group). To evaluate the direct effects, we investigated the protective effects of resveratrol on dopaminergic neurons in inhibiting MPP+ neurotoxicity. SH-SY5Y was pretreated with resveratrol (1 μM, 10 μM or 100 μM) for 24 h before administration of 500 pM MPP+. Cell viability was measured by MTT assay. Compared with the MPP+ group, the MPP++resveratrol group had significant dose dependence on resveratrol, and 10 μM and 100 μM resveratrol pretreatment significantly increased cell viability (p<0.01). We further evaluated the role of the Wnt/β-catenin signaling pathway in the involvement of resveratrol in neuroprotection. Moreover, Western blotting exhibited that MPP+ treatment significantly reduced the expression levels of Wnt1, p-Ser9-GSK-3β and β-catenin (p<0.01, compared with control group) and 10 μM resveratrol pretreatment for 30 min significantly raised the levels (p<0.01, compared with MPP+ group).
In summary, resveratrol relived the neurotoxicity of MPTP against dopaminergic neurons in SNpc and maintained the function of dopaminergic neurons and the reuptake of striatal dopamine. Meanwhile, resveratrol significantly alleviated the toxicity of SH-SY5Y neurons induced by MPP+. Furthermore, the levels of Wnt1, p-Ser9-GSK-3β and β-catenin in SNpc of PD model mice and SH-SY5Y cells decreased, whereas resveratrol significantly elevated such levels. Resveratrol exerted neuroprotective effects on the PD model predominantly by regulating the Wnt/β-catenin signaling pathway.
Author’s contributions:
Yiying Dong and Zhonghai Wang have contributed equally to this work.
Conflict of interests:
The authors declared no conflict of interest.
References
- Chaudhuri KR, Healy DG, Schapira AH. Non-motor symptoms of Parkinson's disease: Diagnosis and management. Lancet Neurol 2006;5(3):235-45.
[Crossref] [Google Scholar] [PubMed]
- Chang D, Nalls MA, Hallgrímsdóttir IB, Hunkapiller J, Van Der Brug M, Cai F, et al. A meta-analysis of genome-wide association studies identifies 17 new Parkinson's disease risk loci. Nat Genet 2017;49(10):1511-6.
[Crossref] [Google Scholar] [PubMed]
- Mandel S, Grunblatt E, Riederer P, Amariglio N, Hirsch JJ, Rechavi G, et al. Gene expression profiling of sporadic Parkinson's disease substantia nigra pars compacta reveals impairment of ubiquitin-proteasome subunits, SKP1A, aldehyde dehydrogenase and chaperone HSC-70. Ann N Y Acad Sci 2005;1053(1):356-75.
[Crossref] [Google Scholar] [PubMed]
- Whitfield AC, Moore BT, Daniels RN. Classics in chemical neuroscience: Levodopa. ACS Chem Neurosci 2014;5(12):1192-7.
[Crossref] [Google Scholar] [PubMed]
- Zhou T, He X, Cheng R, Zhang B, Zhang RR, Chen Y, et al. Implication of dysregulation of the canonical wingless-type MMTV integration site (WNT) pathway in diabetic nephropathy. Diabetologia 2012;55(1):255-66.
[Crossref] [Google Scholar] [PubMed]
- Arenas E. Wnt signaling in midbrain dopaminergic neuron development and regenerative medicine for Parkinson's disease. J Mol Cell Biol 2014;6(1):42-53.
[Crossref] [Google Scholar] [PubMed]
- Yang T, Li S, Zhang X, Pang X, Lin Q, Cao J. Resveratrol, sirtuins and viruses. Rev Med Virol 2015;25(6):431-45.
[Crossref] [Google Scholar] [PubMed]
- Schaale K, Neumann J, Schneider D, Ehlers S, Reiling N. Wnt signaling in macrophages: Augmenting and inhibiting mycobacteria-induced inflammatory responses. Eur J Cell Biol 2011;90(6):553-9.
[Crossref] [Google Scholar] [PubMed]
- Moussa C, Hebron M, Huang X, Ahn J, Rissman RA, Aisen PS, et al. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer’s disease. J Neuroinflammation 2017;14(1):1-0.
[Crossref] [Google Scholar] [PubMed]
- Cilibrasi C, Riva G, Romano G, Cadamuro M, Bazzoni R, Butta V, et al. Resveratrol impairs glioma stem cells proliferation and motility by modulating the Wnt signaling pathway. PLoS One 2017;12(1):e0169854.
[Crossref] [Google Scholar] [PubMed]
- Sarkar S, Chigurupati S, Raymick J, Mann D, Bowyer JF, Schmitt T, et al. Neuroprotective effect of the chemical chaperone, trehalose in a chronic MPTP-induced Parkinson's disease mouse model. Neurotoxicology 2014;44:250-62.
[Crossref] [Google Scholar] [PubMed]
- Pfefferkorn C, Kallfass C, Lienenklaus S, Spanier J, Kalinke U, Rieder M, et al. Abortively infected astrocytes appear to represent the main source of interferon beta in the virus-infected brain. J Virol 2016;90(4):2031-8.
[Crossref] [Google Scholar] [PubMed]
- Yi C, Teillon J, Koulakoff A, Berry H, Giaume C. Monitoring gap junctional communication in astrocytes from acute adult mouse brain slices using the gap-FRAP technique. J Neurosci Methods 2018;303:103-13.
[Crossref] [Google Scholar] [PubMed]
- Kudabayeva M, Kisel A, Chernysheva G, Smol’Yakova V, Plotnikov M, Khodanovich M. The increase in the number of astrocytes in the total cerebral ischemia model in rats. J Phys Conf Ser 2017;886(1):012009.
- Henríquez BH, Henríquez HM, Rothhammer AP, Llop RE, Aboitiz F, Rothhammer EF. Combination of DRD4 and DAT1 genotypes is an important risk factor for attention deficit disorder with hyperactivity families living in Santiago, Chile. Rev Med Chil 2008;136(6):719-24.
[Google Scholar] [PubMed]
- Salinas PC, Zou Y. Wnt signaling in neural circuit assembly. Ann Rev Neurosci 2008;31(1):339-58.
[Crossref] [Google Scholar] [PubMed]
- Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: From human mutations to treatments. Nat Med 2013;19(2):179-92.
[Crossref] [Google Scholar] [PubMed]
- Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer 2013;13(1):11-26.
[Crossref] [Google Scholar] [PubMed]
- Nusse R, Varmus H. Three decades of Wnts: A personal perspective on how a scientific field developed. EMBO J 2012;31(12):2670-84.
[Crossref] [Google Scholar] [PubMed]
- Oliva CA, Vargas JY, Inestrosa NC. Wnt signaling: Role in LTP, neural networks and memory. Ageing Res Rev 2013;12(3):786-800.
[Crossref] [Google Scholar] [PubMed]
- Maruotti N, Corrado A, Neve A, Cantatore FP. Systemic effects of Wnt signaling. J Cell Physiol 2013;228(7):1428-32.
[Crossref] [Google Scholar] [PubMed]
 Res+MPTP group
Res+MPTP group