- *Corresponding Author:
- Xiaoyu Hu
Department of Critical Care Medicine, Zhongxian People’s Hospital of Chongqing City, Zhongxian, Chongqing 404300, China
E-mail: 284567058@qq.com
| Date of Received | 29 October 2022 |
| Date of Revision | 08 May 2023 |
| Date of Accepted | 29 January 2024 |
| Indian J Pharm Sci 2024;86(1):376-380 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To examine the potential protective impact and underlying mechanism of nerve growth factor on brain tissue in hyperbilirubinemia rats through the downregulation of Fas/Fas ligand expression. Thirty six healthy Sprague-Dawley rats were selected as normal control group, and 0.5 ml saline was injected intraperitoneally. The remaining 24 rats were injected intraperitoneally with 50 μg/g bilirubin to establish the model of hyperbilirubinemia. After the model was established successfully, they were divided into hyperbilirubinemia group and nerve growth factor intervention group. The rats in the nerve growth factor intervention group were intraperitoneally injected with 50 μg/kg/d nerve growth factor once a day for 3 d. Rats in each group were killed and their brain tissues were taken to wait for detection. The changes of pathological morphology and the Fas and Fas ligand were compared between the normal control group at 72 h and the hyperbilirubinemia group at 6 h, 24 h, 72 h and the nerve growth factor intervention group. In hyperbilirubinemia group and nerve growth factor intervention group, the brain tissue of rats had different degrees of neurocyto edema. After 6 h, the brain tissue of both groups had degeneration. After 24 h, the neuron necrosis was obvious. After 72 h, the brain tissue of both groups had glial cell proliferation, nuclear pyknosis and fragmentation. However, the severity of brain tissue in nerve growth factor intervention group was less than that in hyperbilirubinemia group, while that in normal control group was not obvious change. At 6 h, 24 h and 72 h, the Fas in the hyperbilirubinemia group was raised than that of the normal control group; while this in the nerve growth factor intervention group was reduced than that of hyperbilirubinemia. At 6 h, 24 h and 72 h, the Fas ligand in hyperbilirubinemia group was raised than that of normal control group; and it in nerve growth factor intervention group was reduced than that of hyperbilirubinemia group. The expression of Fas/Fas ligand can be downregulated by nerve growth factor, thereby providing protection to the brain tissue of rats with hyperbilirubinemia.
Keywords
Nerve growth factor, Fas, Fas-ligand, hyperbilirubinemia, apoptosis
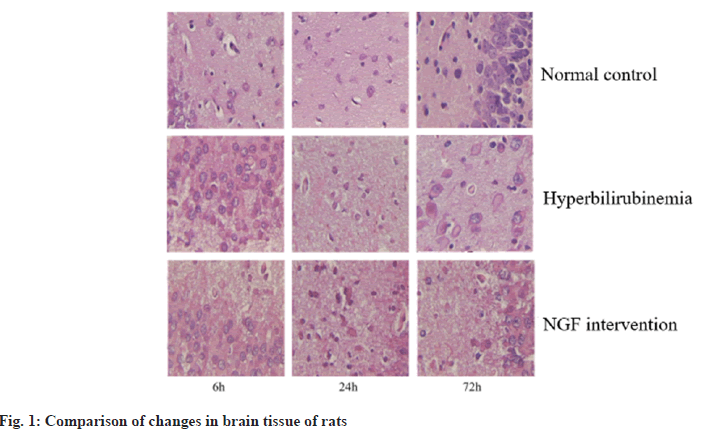
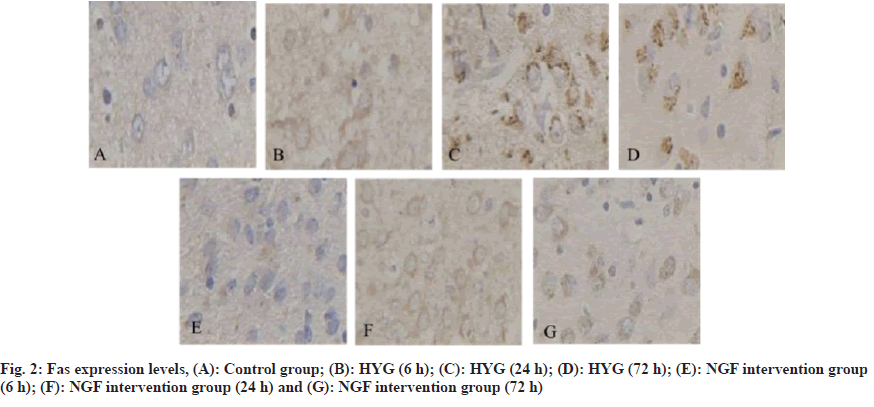
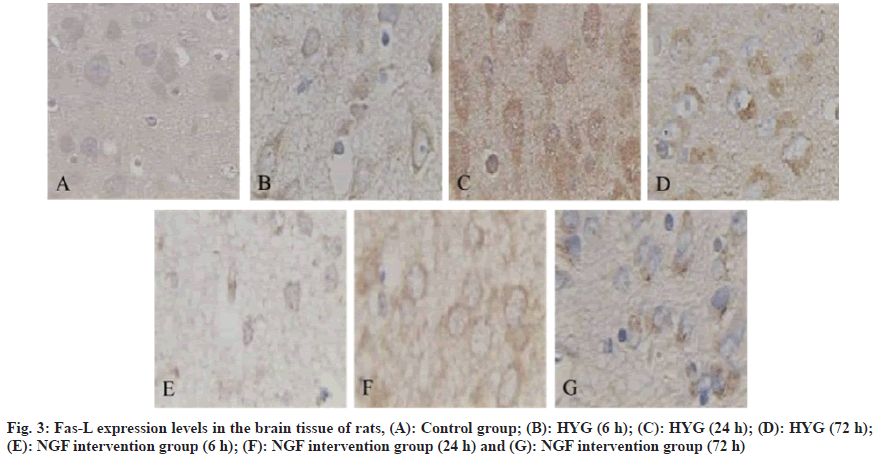
The incidence of neonatal hyperbilirubinemia is high, mainly manifested as jaundice, and even complicated with bilirubin encephalopathy in severe cases, which leads to irreversible brain damage, neurological sequelae, and eventually neonatal death[1]. Relevant surveys show that the incidence of hyperbilirubinemia in full-term newborns is 25/100 000. It has been reported that high levels of free bilirubin can be deposited in brain tissue after opening the Blood-Brain Barrier (BBB). After the onset of hyperbilirubinemia in children, it can lead to metabolic disorders that reduce brain energy and vitality, and form abnormally active Na+-K+-ATPase, excitatory tyrosine that continues to increase, and intracellular overload. Calcium ions (Ca2+) can cause mitochondrial structure damage and nerve cell membrane dysfunction, which in turn leads to brain cell edema and even apoptosis[2,3]. Clinical reports have shown that there are temporal changes involved in the onset and progression of apoptotic nerve and brain injury. Endogenous and exogenous apoptotic pathways can lead to the production of apoptotic cells. Fas Ligand (Fas-L) is a wellstudied pathway[4,5]. Nerve Growth Factor (NGF) has a small molecular weight and can play important biological activities in the nervous system[6]. It has been reported that NGF has the function of protecting the damaged brain tissue of the hand, and its mechanism of action is achieved through its own anti-apoptotic effect[7,8]. Therefore, by establishing a hyperbilirubinemia rat model, this research was to exam the protective effect and underlying mechanism of NGF in down-regulating the Fas/Fas-L on the brain tissue of rats with hyperbilirubinemia. 36 cases of clean-grade healthy Sprague-Dawley (SD) rats were purchased from Guangzhou Forbo Biotechnology Co., Ltd., 1 w old, body weight (16±2) g, production batch number: SCXK (Guangdong) 2017-0018, all rats were collected before the study 1 w line of adaptive feeding. Crystal bilirubin was purchased from Linyi Azeroth Biotechnology Co., Ltd.; NGF preparation was purchased from Fuyinde Technology (Wuhan) Co., Ltd.; rabbit anti-Fas and Fas-L antibodies were purchased from Jiangxi Jianglan Pure Biological Reagent Co., Ltd.; Strept Avidin-Biotin Complex (SABC) immunohistochemical kit was purchased from Rhenium Bo (Shanghai) Biochemical Technology Co., Ltd.; 3,3′-Diaminobenzidine (DAB) chromogenic reagent was purchased from Beijing Kairuiji Biotechnology Co., Ltd.; ether was purchased from Boster Biotechnology Co., Ltd.; aniline blue was purchased from Jinghuamaike Biotechnology Co., Ltd.; 10 % paraformaldehyde was purchased from Guangzhou Peiyu Biotechnology Co., Ltd. products Co., Ltd. The electronic analytical balance was purchased from Beijing Xiangsheng Xingye Technology Co., Ltd.; Ltd.; 450 nm Ultraviolet (UV) spectrophotometer was purchased from Wuhan Heyan Biomedical Technology Co., Ltd.; automatic tissue dehydrator purchased from Beijing Delica Biotechnology Co., Ltd.; -20° ultra-low temperature refrigerator was purchased from Shanghai Shiwei Experimental Instrument Technology Co., Ltd.; paraffin embedding machine was purchased from Dongguan Spectrum Experimental Equipment Technology Co., Ltd. and Shenzhen Reward Life Technology Co., Ltd.; electron microscope was purchased from Fusen (Shanghai) International Trading Co., Ltd.; electric heating constant temperature drying box was purchased from Beijing Taize Jiaye Technology Development Co., Ltd. and medical image analysis system was purchased from Beijing Zhongshidi Chuang Technology Development Co., Ltd. 12 SD rats were randomly selected as the Normal Control Group (NCG), and 0.5 ml of normal saline was injected intraperitoneally, and the remaining 24 rats were intraperitoneally injected with 50 μg/g bilirubin to establish hyperbilirubinemia rats model, after successful modeling, were divided into Hyperbilirubinemia Group (HYG) and NGF intervention group (NIG). The rats in the HYG were not given any treatment, and the rats in the NIG were given 50 μg/kg of NGF/d. The rats in the NCG, HYG, and NIG were all sacrificed at 6 h, 24 h, and 72 h, 4 rats each time. The cerebral tissue of the rats in each experimental group was examined for pathological and morphological alterations using Hematoxylin and Eosin (H&E) staining at 6 h, 24 h, and 72 h. The Fas and Fas-L in the brain tissue of rats in NCG, 6 h, 24 h, 72 h HYG and NIG were detected by immunohistochemically method at 72 h. The Statistical Package for the Social Sciences (SPSS) 23.0 was employed to conduct the analysis, specifically utilizing the Chi-Square (χ2) test for count data, and was used for measurement data. The t-test was employed to conduct a comparison between two distinct groups, a statistically significant difference was observed, as indicated by ap<0.05, compared with the NCG and bp<0.05, compared with the HYG. Different degrees of neuronal edema occurred in the HYG and NIG. After 6 h, the brain tissues of both groups were degenerated. After 24 h, neuronal necrosis was obvious. After 72 h, glial tissue appeared in the brain tissues of both groups. Cell proliferation, nuclear pyknosis and fragmentation, but the severity of brain tissue in the NIG was less severe than that the HYG (fig. 1). At 6 h, 24 h and 72 h, the Fas in the brain tissue in the HYG was raised than the NCG; while it in the brain tissue in the NIG was reduced in hyperbilirubinemia (Table 1 and fig. 2). At 6 h, 24 h and 72 h, the Fas-L in the brain tissue in the HYG was raised than the NCG; while it in the brain tissue of the rats in the NIG was reduced than those in the HYG (Table 2 and fig. 3). Hyperbilirubinemia is caused by increased bilirubin release and decreased bilirubin excretion, so jaundice is a sign of many diseases[9]. Severe bilirubin encephalopathy in the neonatal period can lead to permanent damage to the nervous system, heart and kidneys, and even death in severe cases. A new method that can effectively treat hyperbilirubinemia has become a hot research topic in the current clinical professional field. The biological activity of NGF is extremely important. Among them, the most classic is glycoprotein, whose expression level in normal brain tissue is low, and the expression period cannot protect damaged neurons for a long time[10]. It has been reported that after intraperitoneal injection of radiolabeled NGF in SD rats, the NGF in rat brain tissue was increased, revealing that NGF can pass through the damaged BBB, and then enter the brain tissue and ultimately play a protective role in brain tissue[11]. Studies related to cerebral hemorrhage have confirmed that NGF can effectively protect the nervous system of rats with cerebral hemorrhage[12]. Fas plays a crucial regulatory role in the development of nerve cells. Some studies have analyzed the levels of Fas in the serum of asphyxia neonates. The results show that Fas may be involved in cell apoptosis. Pathological process of brain injury after asphyxia[13]. Another study found that bilirubin can trigger neuronal apoptosis by establishing a model of hyperbilirubinemia, and its mechanism of action may be achieved by inducing the Fas system to activate N-methyl-D-aspartate receptors[14]. Another report found that the detection of Fas and Fas-L in the serum of patients with traumatic brain injury is crucial in understanding the significant contribution of Fas/ Fas-L in secondary brain injury. The above experimental results indicate that Fas/Fas-L can lead to hyperbilirubinemia model cell apoptosis[15]. Relevant foreign studies have shown that when NGF in the neural culture medium is discarded, it can be found that the apoptosis rate is as high as 50 %. Here, we collected 1 w old rats as the research objects and injected 50 μg/g bilirubin into the model group by intraperitoneal injection. Nerve cells were edematous, but the severity of brain tissue in the NGF intervention group was less severe than that in the HYG. It is suggested that NGF can alleviate the degree of brain tissue damage in hyperbilirubinemia rats. Next, we detected the Fas and Fas-L in the brain tissue. At 6 h, 24 h, and 72 h, the Fas and Fas-L in the brain tissue in the HYG were raised than those in the NCG; the Fas and Fas-L in the brain tissue in the NGF intervention group were reduced than the hyperbilirubinemia. It is suggested that NGF can reduce the Fas/Fas-L. In conclusion, NGF can protect the brain tissue of hyperbilirubinemia rats, and its mechanism of action is achieved by downregulating the expression of Fas/Fas-L. However, whether NGF can be used for long-term high-dose clinical treatment of patients with hyperbilirubinemia and whether it has a certain degree of adverse reactions remains to be further explored.
| Group | n | 6 h | 24 h | 72 h |
|---|---|---|---|---|
| NCG | 12 | 162.90±3.76 | 162.90±3.76 | 162.90±3.76 |
| HYG | 12 | 172.53±2.87a | 169.47±3.12a | 174.18±2.54a |
| NIG | 12 | 165.97±2.42b | 156.37±2.71b | 164.48±2.72b |
Note: ap<0.05, compared with the HYC and bp<0.05, compared with the NIG
Table 1: Fas Expression Levels (X±S)
| Group | n | 6 h | 24 h | 72 h |
|---|---|---|---|---|
| NCG | 12 | 158.53±3.21 | 158.53±3.21 | 158.53±3.21 |
| HYG | 12 | 176.02±2.92a | 166.27± 2.83a | 170.23±0.18a |
| NIG | 12 | 166.90±2.21b | 155.88±1.41b | 165.48±2.98b |
Note: ap<0.05, compared with the HYC and bp<0.05, compared with the NIG
Table 2: Comparison of Fas-L Expression Levels (X̄±S)
Author’s contributions:
Chuanchun Liu and Xun Zhang have contributed equally to this work.
Conflict of interests:
The authors declared no conflict of interests.
References
- Kanmaz HG, Okur N, Dilli D, Yesilyurt A, Oguz SS. The effect of phototherapy on sister chromatid exchange with different light density in newborn hyperbilirubinemia. Turk Arch Pediatr 2017;52(4):202-7.
[Crossref] [Google Scholar] [PubMed]
- Hu HJ, Jin YW, Zhou RX, Ma WJ, Yang Q, Wang JK, et al. Clinical value of inflammation-based prognostic scores to predict the resectability of hyperbilirubinemia patients with potentially resectable hilar cholangiocarcinoma. J Gastrointest Surg 2019;23:510-7.
[Crossref] [Google Scholar] [PubMed]
- Zhang M, Wang L, Wang Y, Tang J. The influence of massage on neonatal hyperbilirubinemia: A meta-analysis of randomized controlled trials. J Matern Fetal Neonatal Med 2019;32(18):3109-14.
[Crossref] [Google Scholar] [PubMed]
- Cortesi F, Delfanti G, Grilli A, Calcinotto A, Gorini F, Pucci F, et al. Bimodal CD40/Fas-dependent crosstalk between iNKT cells and tumor-associated macrophages impairs prostate cancer progression. Cell Rep 2018;22(11):3006-20.
[Crossref] [Google Scholar] [PubMed]
- Ta NL, Chakrabandhu K, Huault S, Hueber AO. The tyrosine phosphorylated pro-survival form of Fas intensifies the EGF-induced signal in colorectal cancer cells through the nuclear EGFR/STAT3-mediated pathway. Sci Rep 2018;8(1):12424.
[Crossref] [Google Scholar] [PubMed]
- Indo Y. NGF-dependent neurons and neurobiology of emotions and feelings: Lessons from congenital insensitivity to pain with anhidrosis. Neurosci Biobehav Rev 2018;87:1-6.
[Crossref] [Google Scholar] [PubMed]
- Xia B, Lv Y. Dual-delivery of VEGF and NGF by emulsion electrospun nanofibrous scaffold for peripheral nerve regeneration. Mater Sci Eng C 2018;82:253-64.
[Crossref] [Google Scholar] [PubMed]
- Gao F, Griffin N, Faulkner S, Rowe CW, Williams L, Roselli S, et al. The neurotrophic tyrosine kinase receptor TrkA and its ligand NGF are increased in squamous cell carcinomas of the lung. Sci Rep 2018;8(1):8135.
[Crossref] [Google Scholar] [PubMed]
- Rets A, Clayton AL, Christensen RD, Agarwal AM. Molecular diagnostic update in hereditary hemolytic anemia and neonatal hyperbilirubinemia. Int J Lab Hematol 2019;41(S1):95-101.
[Crossref] [Google Scholar] [PubMed]
- Moattari M, Kouchesfehani HM, Kaka G, Sadraie SH, Naghdi M. Evaluation of nerve growth factor (NGF) treated mesenchymal stem cells for recovery in neurotmesis model of peripheral nerve injury. J Craniomaxillofac Surg 2018;46(6):898-904.
[Crossref] [Google Scholar] [PubMed]
- Martorana F, Gaglio D, Bianco MR, Aprea F, Virtuoso A, Bonanomi M, et al. Differentiation by nerve growth factor (NGF) involves mechanisms of crosstalk between energy homeostasis and mitochondrial remodeling. Cell Death Dis 2018;9(3):391.
[Crossref] [Google Scholar] [PubMed]
- Akhter R, Saleem S, Saha A, Biswas SC. The pro-apoptotic protein Bmf co-operates with Bim and Puma in neuron death induced by ß-amyloid or NGF deprivation. Mol Cell Neurosci 2018;88:249-57.
[Crossref] [Google Scholar] [PubMed]
- Kaur A, Riaz MS, Murugaiah V, Varghese PM, Singh SK, Kishore U. A recombinant fragment of human surfactant protein D induces apoptosis in pancreatic cancer cell lines via fas-mediated pathway. Front Immunol 2018;9:1126-31.
[Crossref] [Google Scholar] [PubMed]
- Dahourou LD, Gbati OB, Millogo A, Dicko A, Roamba CR, Pangui LJ. Analysis of the knowledge, attitudes and practices of populations in four villages of the Boucle du Mouhoun Region (Burkina Faso) regarding Taenia solium life cycle. Health 2018;10(1):95-106.
- Pissetti CW, Tanaka SC, Hortolani AC, Marqui AB. Gene polymorphisms in FAS (Rs3740286 and Rs4064) are involved in endometriosis development in Brazilian women, but not those in CASP8 (rs13416436 and rs2037815). Rev Bras Ginecol Obstet 2018;40(8):450-7.
[Crossref] [Google Scholar] [PubMed]