- *Corresponding Author:
- Rajashree Hirlekar
Vivekanand Education Society’s College of Pharmacy, Chembur, Mumbai, Maharashtra 400074, India
E-mail: rajashree.hirlekar@ves.ac.in
| Date of Received | 28 May 2021 |
| Date of Revision | 12 July 2023 |
| Date of Acceptance | 19 January 2024 |
| Indian J Pharm Sci 2024;86(1):1-8 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Pulmonary drug delivery system is one of the most intriguing and beneficial route for drug delivery as it offers several advantages over the conventional oral route of administration. Many devices are being studied to achieve optimum drug delivery through pulmonary route. Nebulizers, dry powder inhalers, pressurized metered dose inhalers are developed and marketed to target delivery through the pulmonary system. Nebulizers are devices which convert liquid into aerosols of appropriate size for inhalation which can efficiently reach the pulmonary region. The different types of nebulizers include jet nebulizer, ultrasonic nebulizer, vibrating mesh nebulizer. They are further subdivided according to the modification in their structure. The advancement in science has led to the development of more ‘intelligent nebulizers’ which include I-neb nebulizer and AKITA nebulizer, they monitor the breathing pattern of the patient and release the drug accordingly resulting in minimal wastage of the drug. Nebulizers sales have shown growth in the market owing to an increase in the incidences of chronic obstructive pulmonary disorder, asthma in the young and elderly, thus, a combination of smart nebulizer and microparticle formulation will help in achieving the desired results.
Keywords
Pulmonary delivery, nebulizer, asthma, jet nebulizer, ultrasonic nebulizer, inhalers
Route of administration of drugs is a very important factor, and it significantly affects the pharmacokinetics and pharmacodynamics of the drug. Therefore, choosing an appropriate route for drug delivery is critical. The oral route is the most convenient but it also has many limitations like low bioavailability due to first-pass metabolism, higher risk of adverse effects and slower action of drugs. In order to overcome these limitations, many different routes are being studied, one such route which is being extensively explored and studied is the ‘Pulmonary Route of administration’. As the first-pass metabolism is eliminated in this mode of delivery, higher bioavailability can be achieved at lower doses. Pulmonary Drug Delivery Systems (PDDS) has an edge over other conventional routes owing to high permeability, large surface area of alveoli and faster onset of action[1]. PDD is also known as ‘oral inhalation administration’, and when compared to the intranasal administration only 20 % drug wastage takes place[2,3]. Another reason for PDD, being one of the most researched areas is that both local and systemic drugs can be administered through this route to treat various disorders; direct entry into lungs makes it a desirable route for local action and the large surface area due to the alveoli promises in a better systemic delivery through PDD. Various targets which are being studied for pulmonary delivery are explained briefly.
Local targets: Local delivery may be required in case of cystic fibrosis, lung cancer, tuberculosis, asthma and many drugs are being investigated for treating these diseases using oral inhalation technique. One such example is interferon which can be used in the treatment of tuberculosis and Idiopathic Pulmonary Fibrosis (IPF), phase 1 trial via i-Ban nebulizer using interferon showed that the drug was delivered safely and effectively with almost no side effects. The results showed that the diffusing capacity of the drug also increased using nebulizers which is beneficial in long term treatment[4,5].
Systemic targets: The large surface area, minimal physical barrier between air space and bloodstream, highly dispersed nature of aerosol[6], elimination of first pass metabolism makes PDDS an attractive area for delivery of systemic drugs. Drugs with particle size 1-5 µ reach the alveolar region quite easily and then they enter the capillaries. The mechanism by which the molecules travel across the endothelial lining is still to be fully explored. As a general rule, molecules with high lipophilicity move through the cell and the ones with low lipophilicity travel through transcellular mechanism, many molecules also move through active transport mechanisms[7-9].
Insulin is the best example which substantiates the above theory. Insulin delivered through pulmonary route has shown better results in terms of bioavailability out of all the other alternate routes like nasal, rectal, buccal[10]. The insulin administered by breath-actuated vibrating mesh nebulizer achieved lung deposition of >70 % of emitted dose[11].
Brain as target: The physicochemical properties like low molecular weight and high lipophilicity that aids in the passage across the blood brain barrier (mechanism of many central nervous system drugs) are comparable to those that enable better uptake through the pulmonary route. Therefore, if compounds are formulated into proper aerosol size then they can be efficient in targeting the brain. Dihydroergotamine (DHE) a migraine medication which shows poor pharmacokinetic properties via the oral and rectal routes, when administered in inhaled form showed rapid absorption, high bioavailability and an improved t1/2 (15 h)[12]. Table 1 consists of examples of drugs along with their target which can be administered through PDDS[13-17].
| Drug | Disorder | Target | Device type | Company |
|---|---|---|---|---|
| Alpha 1- antitrypsin | Cystic fibrosis | Local (peripheral airway) | DPI | Kamada Ltd. |
| Flecainide | Paroxysmal atrial fibrillation | Heart | Nebulizer | Incarda therapeutics, Inc. |
| Levodopa | Parkinson | Brain | DPI | Pure IMS B.V. |
| Insulin | Diabetes mellitus | Systemic | Nebulizer | Dance biopharm Inc. |
| SB010 | Asthma and COPD | Local (central airway) | Nebulizer | Sternar AS. |
Table 1: PDDS-examples of drugs along with their targets.
Nebulizers
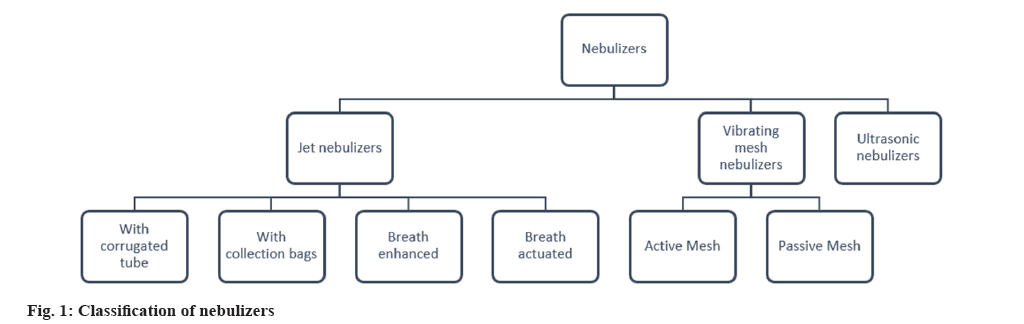
Many devices have been developed and marketed for PDD like nebulizers, Dry Powder Inhalers (DPIs), pressurized Metered Dose Inhalers (pMDIs). Not only they help in treating common respiration associated diseases like asthma, chronic obstructive pulmonary disorder (COPD) but also for Respiratory Syncytial Virus (RSV) infections, tuberculosis, influenza, schizophrenia and many other pulmonary disorders[18]. However, elderly and infants often do not find it easy to operate the pMDIs, also some patients with disorders like arthritis may find it tough to handle hand-held devices. According to a survey conducted in United States of America (USA) and Europe, nebulizers are the more preferred devices by children of 1 y to 5 y of age group as compared to pMDIs plus spacer[19]. Nebulizers are devices which convert liquid into aerosols of appropriate size for inhalation which can efficiently reach the pulmonary region. In the market various types of nebulizers are present. Fig. 1 depicts the various types of nebulizers.
Jet Nebulizers
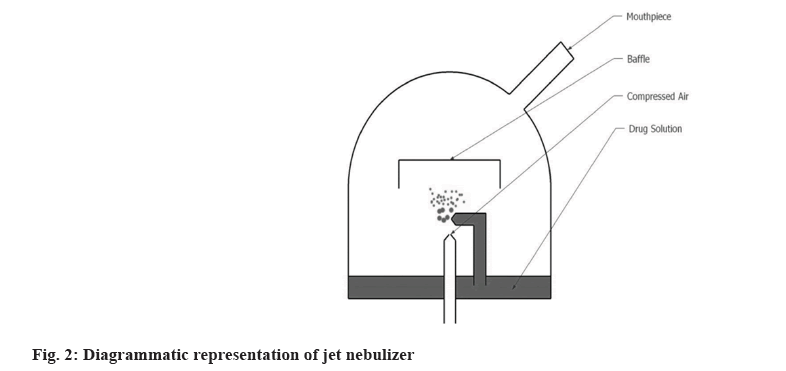
This type uses gas flow to produce aerosol. Either a compressed gas or gas generated by an electrical compressor is used. These devices have a flow rate of 6-10 l/min. The formulation enters the capillary tube from the nebulizer’s reservoir and is dispersed as aerosolized particles. Baffles present in the device help in reducing the size of the aerosol particles due to impaction as shown in fig. 2[20]. Jet nebulizer is also known as ‘Pneumatic’ nebulizer[21]. Antibiotics, mucolytics, liposomal formulations which cannot be delivered by DPI and pMDI can be efficiently delivered by Jet nebulizers. However, studies have shown poor drug delivery and huge wastage of drugs. In order to overcome these limitations different modifications are done in the nebulizer design. On the basis of these modifications, 4 types of Jet nebulizers are developed as follows. Jet nebulizer with corrugated tube; jet nebulizer with collection bag; breath-actuated and breath enhanced[22-25].
Ultrasonic Nebulizer
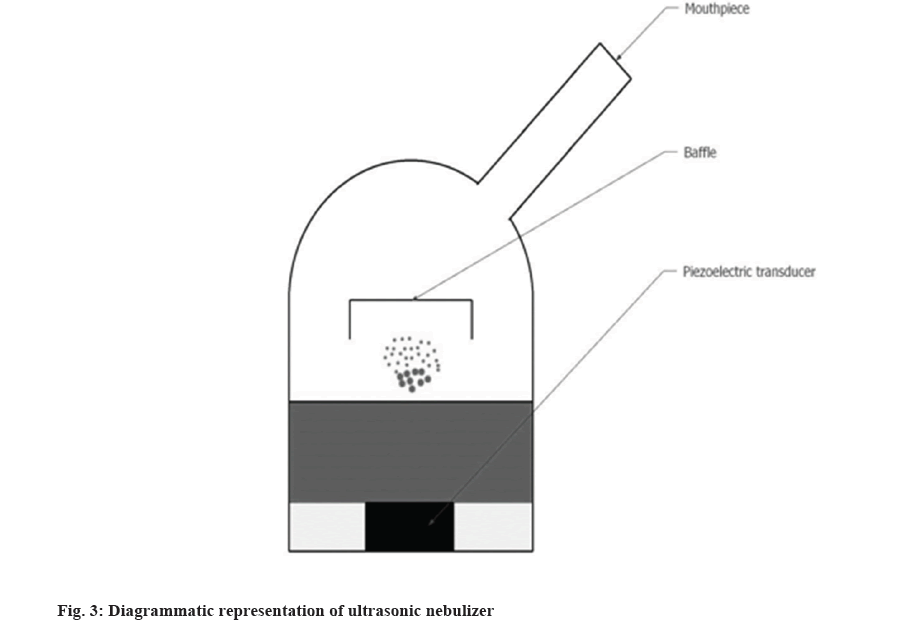
Liquid in the form of thin-film when flows on a vibrating surface of the frequency of more than 20 kHz break into fine droplets, this is the principle is used in ultrasonic atomization. Ultrasonic nebulizers were invented in the 1960s to overcome the shortcomings of jet nebulizers. They are more portable, silent in operation, and have greater output than jet nebulizers. Fig. 3 shows the components of the ultrasonic nebulizer. However, lung deposition is similar to jet nebulizers. A Piezoelectric disc acts as a vibrating surface which produces ultrasonic vibrations. These vibrations travel to the surface of the liquid where they form standing waves. These standing waves break on the surface which creates the mist on the surface. The particles produced are in the range of 2-4 µ[26].
Two mechanisms which are responsible for the generations of mist are capillary waves generated on the surface of the liquid create droplets at the crests of the waves; cavitations which occur in the liquid due to the ultrasonic frequency of the waves create a vacuum inside the cavity which implodes beneath the liquid surface. These imploding cavitations especially at the surface create hydraulic shocks that cause direct ejection of the droplets. It is important to note that highly viscous liquids or suspensions or glutinous liquids cannot be nebulized[27-29].
There are two types of ultrasonic nebulizers, one type where the drug and the piezoelectric material are in direct contact with each other and the second type where the water interface is present in between the piezoelectric transducer and the drug. In the former type of nebulizer, the piezoelectric disc vibrates at an ultrasonic frequency, heat is generated which can degrade thermo-labile drugs. The temperature at the disc is reported to be around 75° whereas in the latter type of nebulizer, a reservoir of water is present between the drug and the transducer, this arrangement prevents heating of the drug material.
Newer Approaches in Ultrasonic Nebulization
Using Surface Acoustic Waves (SAW)-ultrasonic waves produced by SAW induced on a piezoelectric device show more percentage of material converted into aerosol than standard inducers. Extrusion of liquid from micro and mesh-sized perforated can be achieved when the liquid is forced through the mesh with oscillations or when the mesh is vibrating, this modification led to the development of vibrating mesh nebulizers[30,31].
Vibrating Mesh Nebulizer
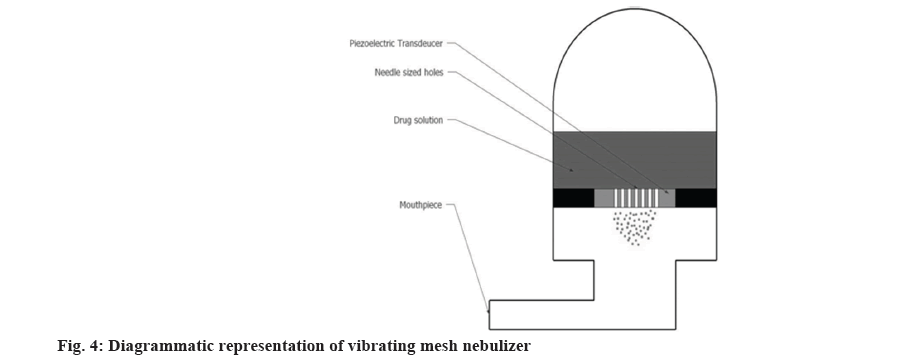
Advancement in the nebulizer technologies resulted into the idea of mesh nebulizers. In vibrating mesh nebulizer, micro pump technology is used for the production of aerosol, liquid medication is forced through the mesh to produce aerosol as shown in fig. 4. This system is either battery or electrically operated depending on the manufacturer. This kind of technology helps in achieving a desired particle size which can reach the preferred airway region. The factors affecting the performance of the device include mode of vibration, mesh shape and material of the mesh[32]. Vibrating mesh nebulizers formulate small and more deliverable aerosol particles as compared to the above two types of nebulizers thus resulting into better efficiency[33]. A study demonstrated that mesh nebulizers showed higher anatomical selectivity as compared to jet nebulizer. Although the particle size generated by both nasal jet and nasal mesh nebulizer was comparable it was observed that the later showed significant peripheral aerosol deposition in the nasal cavity without deposition in the lungs[34]. Vibrating mesh nebulizers are divided into 2 categories.
Active mesh nebulizer:
In this a piezo element is used which with the help of electric current vibrates the mesh which is in contact with the liquid medication resulting into production of aerosol. Aeroneb and eFlow are some of the examples of this category[35,36].
Passive mesh nebulizer:
It consists of a transducer which generates passive vibrations in the perforated plate with 6000 tapered holes to produce aerosol. Microair NE-U22 is an example of passive mesh nebulizer[37]. The pros and cons of all the above discussed nebulizers are mentioned in Table 2[38,39].
| Types | Advantages | Disadvantages |
|---|---|---|
| Jet nebulizers | Easy to use | Long administration time |
| Minimum cognitive ability required | Less portability | |
| Ultrasonic nebulizers | Large volumes of liquid can be nebulized | Lower efficiency |
| Shorter delivery time than jet | May breakdown complex molecules | |
| Vibrating mesh nebulizers | Short administration time | Frequent cleaning is required |
| More portable | Limited medications can be administered |
Table 2: Advantages and disadvantages of different types of nebulizer.
Intelligent Nebulizers
The conventional nebulizers failed to deliver appropriate amounts of drug as their constant drug output makes the amount of drug inhaled directly dependent on the breathing pattern of the patient. This led to the development of more advanced nebulizers termed as ‘smart’ or ‘intelligent’ nebulizers. These advanced nebulizers consist of Adaptive Aerosol Technology (AAD), they analyse the breathing pattern of the patient and thus helps in exact drug delivery minimizing wastage. The dosimetric function of the AAD system enables the delivery of drugs having narrow therapeutic index[40]. Owing to the AAD technology, variation in drug delivery is nearly eliminated which aids the patient in adhering to the treatment. The AAD system detects the change in the airflow pressure of the first three breaths, to confirm the correct starting point for aerosol delivery. This process continues throughout the treatment, and the AAD system continuously adapts to the breathing patterns of the patient. Different Nebulizers using this technology have been discussed in brief further.
I-Neb Nebulizer
HaloLite and Prodose AAD systems were the first and second generation AAD technology respectively. HaloLite mechanism was based on the active venturi jet nebulizer technology, it was mainly used to aerosolized asthma medications, a paediatric version was also developed for children of 6 mo to 5 y of age. The Prodose system had one step advance algorithms as compared to the HaloLite which resulted into more accurate dosing than the latter one. I-Neb is the third generation AAD technology. It is an efficient combination of vibrating mesh nebulizer and the AAD disc which aids in precise and reproducible dose delivery. It is a small, lightweight and virtually silent nebulizer. I-neb delivers the aerosol in two different breathing pattern algorithms: The Tidal Breathing Mode (TBM) and the Target Inhalation Mode (TIM)[41]. Feedback is recorded after the drug is delivered in the form of an electronic diary which helps clinicians to check patient compliance to the therapy.
In the TBM, the patient inhales voluntarily during tidal breathing while a pressure sensor monitors the inspiratory flow rate and length of the inhalation. Based on the volume of breath in breathing pattern, aerosol is released in a pulse form during the first 50 %-80 % of the inhalation time. In TIM pattern, a patient for 8 s has to practice slow and deep inhalation and the aerosol pulse is released for 7 s leaving 1 s for aerosol deposition in the lungs, 30 breaths are monitored and the information is recorded for future treatment. If the patient finds it difficult to lengthen his breath for 8 s the I-neb adapts to the short breathing pattern and releases the aerosol accordingly.
In a study conducted by Philips Respironics, when I-neb in both modes of breathing (TIM and TBM) was evaluated it was concluded that lung deposition achieved with the slow and deep inhalation as in TIM was superior to the lung deposition achieved during tidal breathing in TBM i.e. I-Neb with TIM pattern will be more beneficial for patients who require multiple dosing daily. However, TIM had a shorter nebulization time compared to TBM[42].
Akita
Individualized Control Inhalation (ICI) technology allows specific targeting of the drug to a particular lung region, e.g., α1-antitrypsin showed higher alveolar deposition when administered for α1-antitrypsin deficiency. ICI can be achieved through AKITA. It is a reusable, breath-actuated nebulizer. The inspiratory flow of the aerosol from AKITA is around 12-15 l/min due to this even the slightly large particles reach the lungs evading the physiological obstructions. It can either be combined with a mesh or jet for delivering the drug. A computer algorithm is present in the form of a smart card in the nebulizer which helps in achieving ICI. No aerosolization takes place during exhalation. Due to the controlled breathing delivery, less patient variability is achieved which results in adherence to the treatment[43,44]. The system is user-friendly and can also be used at hospitals[45].
These smart nebulizers help in achieving the desired dosage more efficiently. However, recent advances in the PDDS consist of particle engineering. Particle size is a fundamental factor which affects both pharmacokinetic and pharmacodynamic properties of the drug. Scientists are exploring various particle sizes for optimum delivery which includes microparticles and nanoparticles, the small size of the particle helps in achieving better bioavailability. Various techniques like micronization, spray drying, spray freezing, and supercritical fluid, and are used for developing inhalable microparticles. The aerosolization and dissolution rate of inhalable microparticles of budesonide showed better results than the other formulations. Particles of size 3 µ can be achieved by supercritical fluid technique.
Popular Commercial Nebulizers
Nebulizer sales have shown a constant growth with increased incidence of COPD in elderly and children[46]. Table 3 summarizes the popular nebulizers available in the market along with their salient features[47-50]. Apart from these known technologies the other patented technologies include Technosphere and Pulmosphere. Faster onset of action is seen for drugs inhaled as technosphere formulation. These drugs also bypass hepatic first pass metabolism thus aiding in better bioavailability. Technosphere insulin administered through the inhalation route showed greater efficiency in maintaining the Postprandial Glucose (PPG) levels as it had around 20 % higher bioavailability and faster rate of elimination. Pulmosphere is another novel technology which includes emulsion-based spray drying technique. Since the drug is delivered irrespective of its physicochemical properties or dose administered a significant improvement is observed in both the drug targeting and dose consistency. Dry powder Tobramycin was prepared by Pulmosphere technology which showed rapid delivery, better pulmonary deposition and more convenient administration compared to other nebulized formulations. Other technologies like liposomes, solid lipid nanoparticles are also being explored for controlled pulmonary delivery[51,52]. The SARS-CoV-2 pandemic has forced researchers all over the world to discover different PDDS technologies which will help in combating the infection. Antiviral formulations when administered through these nebulizers may help in achieving desired results at an increased pace. Combination of an intelligent nebulizer along with a micro or nano antiviral formulation may emerge as a saviour in these tough times. However, a lot of research needs to be done in this field but nebulizers can be a ray of hope for elderly and children suffering from COPD, asthma and other respiratory disorders which are at higher risk during the pandemic.
| S No. | Name of the nebulizer | Principle for aerosol production | Features |
|---|---|---|---|
| 1 | Dr. Trust portable ultrasonic mesh nebulizer | Uses a vibrating mesh at ultrasonic frequency | Compact |
| Can be used by holding in one hand | |||
| Comparatively expensive | |||
| 2 | OMRON compression nebulizer | Uses compressed air | Popular brand |
| Consist of internal air compressor | |||
| Comparatively cheap | |||
| Can be used for infants | |||
| 3 | Bruitcare intelligent mesh nebulizer | Uses a vibrating mesh at ultrasonic frequency | Smart nebulizer |
| Operated using smartphone | |||
| Drug is released during active inhalation | |||
| 4 | Fly portable ultrasonic nebulizer | Uses a vibrating mesh at ultrasonic frequency | Highly compact |
| No mask or accessory required | |||
| Easy to use | |||
| High patient compliance | |||
| 5 | OMRON micro air 360° | Uses a vibrating mesh at ultrasonic frequency | Compact |
| New mesh technology principle | |||
| Better medication delivery to lungs |
Table 3: Popular nebulizers available in the market.
Conclusion
PDDS offers many advantages over the conventional systems. PDDS can be achieved using nebulizers, DPI and other inhalers. However, nebulizers are more efficient in COPD and asthma as well as for infants and older patients. Nebulizers have evolved from the basic jet to completely automated smart nebulizers. This development has helped in achieving more accurate dose and minimizing drug wastage. Even with these advancements in nebulization technology popular nebulizers in the market include the basic and inexpensive devices, owing to the higher cost of the advanced nebulizers. Thus, a combination of an efficient smart and economical nebulizer along with a micro particle drug formulation may augment the drug action and a desirable outcome can be achieved.
Acknowledgements:
The authors wish to thank all researchers for providing an eminent literature source for devising this manuscript.
Conflict of interest:
The authors declared no conflict of interests.
References
- Liang Z, Ni R, Zhou J, Mao S. Recent advances in controlled pulmonary drug delivery. Drug Discov Today 2015;20(3):380-9.
[Crossref] [Google Scholar] [PubMed]
- Berger WE. Paediatric pulmonary drug delivery: Considerations in asthma treatment. Expert Opin Drug Deliv 2005;2(6):965-80.
[Crossref] [Google Scholar] [PubMed]
- Groneberg DA, Witt C, Wagner U, Chung KF, Fischer A. Fundamentals of pulmonary drug delivery. Respir Med 2003;97(4):382-7.
[Google Scholar] [PubMed]
- de Kruijf W, Ehrhardt C. Inhalation delivery of complex drugs—the next steps. Curr Opin Pharmacol 2017;36:52-7.
[Crossref] [Google Scholar] [PubMed]
- Diaz KT, Skaria S, Harris K, Solomita M, Lau S, Bauer K, et al. Delivery and safety of inhaled interferon-γ in idiopathic pulmonary fibrosis. J Aerosol Med Pulm Drug Deliv 2012;25(2):79-87.
[Crossref] [Google Scholar] [PubMed]
- Patton JS, Byron PR. Inhaling medicines: Delivering drugs to the body through the lungs. Nat Rev Drug Discov 2007;6(1):67-74.
[Crossref] [Google Scholar] [PubMed]
- Siekmeier R, Scheuch G. Systemic treatment by inhalation of macromolecules–principles, problems, and examples. J Physiol Pharmacol 2008;59(6):53-79.
[Google Scholar] [PubMed]
- Patton JS, Brain JD, Davies LA, Fiegel J, Gumbleton M, Kim KJ, et al. The particle has landed—characterizing the fate of inhaled pharmaceuticals. J Aerosol Med Pulm Drug Deliv 2010;23(S2):S-71.
[Crossref] [Google Scholar] [PubMed]
- Siekmeier R, Scheuch G. Treatment of systemic diseases by inhalation of biomolecule aerosols. J Physiol Pharmacol 2009;60(5):15-26.
[Google Scholar] [PubMed]
- Cavaiola TS, Edelman S. Inhaled insulin: A breath of fresh air? A review of inhaled insulin. Clin Ther 2014;36(8):1275-89.
[Crossref] [Google Scholar] [PubMed]
- Fink JB, Molloy L, Patton JS, Galindo-Filho VC, de Melo Barcelar J, Alcoforado L, et al. Good things in small packages: An innovative delivery approach for inhaled insulin. Pharm Res 2017;34:2568-78.
[Crossref] [Google Scholar] [PubMed]
- Hampson NB, Kieburtz KD, LeWitt PA, Leinonen M, Freed MI. Prospective evaluation of pulmonary function in Parkinson's disease patients with motor fluctuations. Int J Neurosci 2017;127(3):276-84.
[Crossref] [Google Scholar] [PubMed]
- Kerem E, Bauer S, Strauss P, Jaffe N, Armoni S, Pugatsch T, et al. Safety and efficacy of inhaled human Alpha-1 Antitrypsin (AAT) in Cystic Fibrosis (CF): A report of a phase ii clinical study. InA26. New Data Regard Cystic Fibrosis 2009;179:A1185.
- Flecainide inhalation-InCarda Therapeutics. Adis Insight. 2023.
- Chua AL, Silberstein S. Inhaled drug therapy development for the treatment of migraine. Expert Opin Pharmacother 2016;17(13):1733-43.
[Crossref] [Google Scholar] [PubMed]
- Luinstra M, Grasmeijer F, Hagedoorn P, Moes JR, Frijlink HW, De Boer AH. A levodopa dry powder inhaler for the treatment of Parkinson’s disease patients in off periods. Eur J Pharm Biopharm 2015;97:22-9.
[Crossref] [Google Scholar] [PubMed]
- Krug N, Hohlfeld JM, Kirsten AM, Kornmann O, Beeh KM, Kappeler D, et al. Allergen-induced asthmatic responses modified by a GATA3-specific DNAzyme. New Engl J Med 2015;372(21):1987-95.
[Crossref] [Google Scholar] [PubMed]
- Hertel SP, Winter G, Friess W. Protein stability in pulmonary drug delivery via nebulization. Adv Drug Deliv Rev 2015;93:79-94.
- Nicolini G, Cremonesi G, Melani AS. Inhaled corticosteroid therapy with nebulized beclometasone dipropionate. Pulm Pharmacol Ther 2010;23(3):145-55.
[Crossref] [Google Scholar] [PubMed]
- Dolovich MB, Dhand R. Aerosol drug delivery: Developments in device design and clinical use. Lancet 2011;377(9770):1032-45.
[Crossref] [Google Scholar] [PubMed]
- Hess DR. Aerosol delivery devices in the treatment of asthma. Respir Care 2008;53(6):699-725.
[Google Scholar] [PubMed]
- Arı A. Jet, Ultrasonic, and mesh nebulizers: An evaluation of nebulizers for better clinical outcomes. Eurasian J Pulmonol 2014;16(1).
- Ho SL, Kwong WJ, O'Drowsky L, Coates AL. Evaluation of four breath-enhanced nebulizers for home use. J Aerosol Med 2001;14(4):467-75.
[Crossref] [Google Scholar] [PubMed]
- De Boer AH, Hagedoorn P, Frijlink HW. The choice of a compressor for the aerosolisation of tobramycin (TOBI®) with the PARI LC PLUS® reusable nebuliser. Int J Pharm 2003;268(1-2):59-69.
[Crossref] [Google Scholar] [PubMed]
- Ari A, Fink JB. Breath-actuated nebulizer vs. small-volume nebulizer: Efficacy, safety, and satisfaction. Respir Care 2012;57(8):1351-3.
[Crossref] [Google Scholar] [PubMed]
- Sabato K, Ward P, Hawk W, Gildengorin V, Asselin JM. Randomized controlled trial of a breath-actuated nebulizer in pediatric asthma patients in the emergency department. Respir Care 2011;56(6):761-70.
[Crossref] [Google Scholar] [PubMed]
- O'Callaghan C, Barry PW. The science of nebulised drug delivery. Thorax 1997;52(2):S31-44.
- Chandel A, Goyal AK, Ghosh G, Rath G. Recent advances in aerosolised drug delivery. Biomed Pharmacother 2019;112:108601.
[Crossref] [Google Scholar] [PubMed]
- Clark AR. Medical aerosol inhalers: Past, present, and future. Aerosol Sci Technol 1995;22(4):374-91.
- Yeo LY, Friend JR, McIntosh MP, Meeusen EN, Morton DA. Ultrasonic nebulization platforms for pulmonary drug delivery. Expert Opin Drug Deliv 2010;7(6):663-79.
[Crossref] [Google Scholar] [PubMed]
- Ari A, Restrepo RD. Aerosol delivery device selection for spontaneously breathing patients: 2012. Respir Care 2012;57(4):613-26.
[Crossref] [Google Scholar] [PubMed]
- Pritchard JN, Hatley RH, Denyer J, Hollen DV. Mesh nebulizers have become the first choice for new nebulized pharmaceutical drug developments. Ther Deliv 2018;9(2):121-36.
[Crossref] [Google Scholar] [PubMed]
- Dhand R. Nebulizers that use a vibrating mesh or plate with multiple apertures to generate aerosol. Respir Care 2002;47(12):1406-6.
[Google Scholar] [PubMed]
- Vecellio L, De Gersem R, Le Guellec S, Reychler G, Pitance L, Le Pennec D, et al. Deposition of aerosols delivered by nasal route with jet and mesh nebulizers. Int J Pharm 2011;407(1-2):87-94.
[Crossref] [Google Scholar] [PubMed]
- Lenney W, Edenborough F, Kho P, Kovarik JM. Lung deposition of inhaled tobramycin with eFlow rapid/LC Plus jet nebuliser in healthy and cystic fibrosis subjects. J Cyst Fibros 2011;10(1):9-14.
[Crossref] [Google Scholar] [PubMed]
- Bakuridze L, Andrieu V, Dupont C, Dubus JC. Does repeated disinfection of the e-Flow rapid® nebulizer affect in vitro performance? J Cyst Fibros 2007;6(4):309-10.
[Crossref] [Google Scholar] [PubMed]
- Skaria S, Smaldone GC. Omron NE U22: Comparison between vibrating mesh and jet nebulizer. J Aerosol Med Pulm Drug Deliv 2010;23(3):173-80.
[Crossref] [Google Scholar] [PubMed]
- Tashkin DP. A review of nebulized drug delivery in COPD. Int J Chron Obstruct Pulm Dis 2016;11:2585-96.
[Crossref] [Google Scholar] [PubMed]
- Ari A, Fink JB. Guidelines for aerosol devices in infants, children and adults: Which to choose, why and how to achieve effective aerosol therapy. Expert Rev Respir Med 2011;5(4):561-72.
[Crossref] [Google Scholar] [PubMed]
- Denyer J, Nikander K, Smith NJ. Adaptive aerosol delivery (AAD®) technology. Expert Opin Drug Deliv 2004;1(1):165-76.
- Geller DE, Kesser KC. The I-neb adaptive aerosol delivery system enhances delivery of α1-antitrypsin with controlled inhalation. J Aerosol Med Pulm Drug Deliv 2010;23(S1):S-55.
[Crossref] [Google Scholar] [PubMed]
- Nikander K, Prince I, Coughlin S, Warren S, Taylor G. Mode of breathing—tidal or slow and deep—through the I-neb Adaptive Aerosol Delivery (AAD) system affects lung deposition of 99mTc-DTPA. J Aerosol Med Pulm Drug Deliv 2010;23(S1):S-37.
[Crossref] [Google Scholar] [PubMed]
- Rubin BK. Pediatric aerosol therapy: New devices and new drugs. Respir Care 2011;56(9):1411-23.
[Crossref] [Google Scholar] [PubMed]
- Luisetti M, Kroneberg P, Suzuki T, Kadija Z, Muellinger B, Campo I, et al. Physical properties, lung deposition modeling, and bioactivity of recombinant GM-CSF aerosolised with a highly efficient nebulizer. Pulm Pharmacol Ther 2011;24(1):123-7.
[Crossref] [Google Scholar] [PubMed]
- Our E, Century N, Caring O. Patient Care Services 2010. 2010;12(2):71–7.
- The best nebulizer machines: Review and buying guide. Reviews.in; 2023.
- Dr. Trust Portable Ultrasonic Mesh Nebulizer Machine. The best nebulizer machines: Review and buying guide. Reviews.in; 2023.
- Portable smart mesh nebulizer. Briutcare; 2023.
- Review on fly mesh nebulizer. Oxygen Concentrator Supplies; 2023.
- Rogueda PG, Traini D. The nanoscale in pulmonary delivery. Part 2: Formulation platforms. Expert Opin Drug Deliv 2007;4(6):607-20.
[Crossref] [Google Scholar] [PubMed]
- Chan HK, Chew NY. Novel alternative methods for the delivery of drugs for the treatment of asthma. Adv Drug Deliv Rev 2003;55(7):793-805.
[Crossref] [Google Scholar] [PubMed]
- Jain H, Bairagi A, Srivastava S, Singh SB, Mehra NK. Recent advances in the development of microparticles for pulmonary administration. Drug Discov Today 2020;25(10):1865-72.
[Crossref] [Google Scholar] [PubMed]