- *Corresponding Author:
- S. Kalvatala
Department of Pharmaceutical Sciences, Lovely Professional University, Phagwara, Punjab 144411, India
E-mail: ckbhaipharma@gmail.com
| Date of Received | 03 September 2023 |
| Date of Revision | 11 May 2024 |
| Date of Acceptance | 04 November 2024 |
| Indian J Pharm Sci 2024;86(6):1948-1957 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Nanostructured lipid carriers are lipid-based nanoparticles that consist of a solid-lipid matrix and a liquid lipid component, by enhancing drugs solubility and stability it improves bioavailability and efficacy. Nanostructured lipid carriers have the skill to act as carriers loaded with various drugs, including hydrophilic and hydrophobic molecules and can be functionalized with targeting ligands or imaging agents for the purpose of targeted drug delivery and imaging. Ovarian cancer, which begins in the ovaries, is one of the most lethal forms of gynecological cancer, it also shows a very less survival rate in women due to its asymptomatic nature, late diagnosis and high recurrence rate. Different genetic variations and pathways such as tumor protein p53 mutation in high-grade serous tumors, Kirsten rat sarcoma viral oncogene homolog mutation in mucinous tumors and clear cells are used to distinguish between various types of this disease. The term “Dual targeting” describes the therapeutic strategy in which two separate molecules or pathways are targeted at once to amplify the therapeutic effect, it can be useful in overcoming drug resistance as it concurrently targets two independent pathways and lowers the risk that cancer cells will develop resistance to individual therapies. This article reviews the uses of nanostructured lipid carriers used for the treatment of ovarian cancer.
Keywords
Ovarian cancer, cubosome, dual targeting, ligand
Ovarian carcinoma is a lethal cancer that affects the female ovary. In the past few years, there have been over 10 000 instances of a fatal disease affecting the female population, which has resulted in the death of many women in worldwide. Chemotherapy is a relatively new method of curing cancer initially; it was only effective for early-stage ovarian cancer. A significant number of malignancies affecting the ovaries are detected at an advanced stage, primarily due to the inconspicuous development of tumors, delayed manifestation of symptoms and insufficient screening techniques. Cisplatin and paclitaxel are combined for the treatment of ovarian cancer[1]. However traditional treatment approaches have limitations including disease relapse, decreased effectiveness and potential toxic side effects[2]. Chemotherapy for ovarian carcinoma is related to several side effects such as nausea, hair loss and decreased blood cell counts[3].
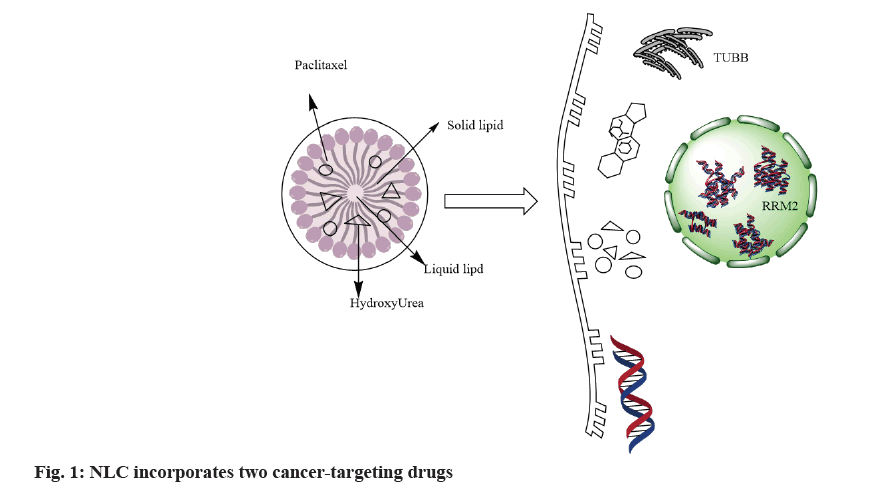
Scientists have developed various types of nanoparticles, including nanoparticles made of metals like silver and gold[4]. Nanoparticles are composed of organic materials such as lipids and polymers. Nanostructured Lipid Carriers (NLCs) consist of solid-lipid, liquid-lipids and surfactants or a combination of surfactant dispersion in water. Nano-lipid carriers have shown remarkable potential in drug loading and targeting[5]. NLCs possess several benefits to achieve higher drug loads and controlled release for both hydrophilic and hydrophobic therapeutic agents, leading to increased physical stability[6]. Attaching ligands allows passive and active targeting. Fig. 1 illustrates all NLCs materials and components in the marketed products and approved by regulatory agencies[7]
Ovarian Cancer Subtype
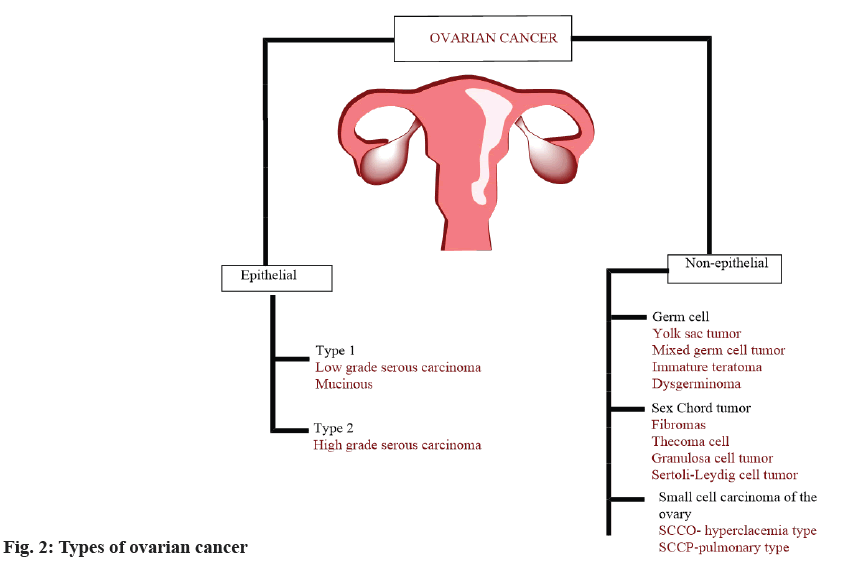
Cancer is the most common classification for epithelial tumours. Non-epithelial cancer includes germ cell cancer, stromal cell cancer and rare small cell carcinoma. Fig. 2 illustrates various ovarian cancer subtypes.
Epithelial Tissues Cancer that Occurs in Ovaries
Malignancies that originate from epithelial cells account for over 90 % of cases in the ovary. Postmenopausal epithelial ovarian cancer is caused by aging.
High-Grade Serous Carcinoma (HGSC):
HGSC develops from cells that line the ovary, fallopian tube or peritoneum. Most epithelial ovarian tumors are lethal and aggressive[8]. Ovarian Surface Epithelium (OSE) is a cellular layer that lines the outer surface of the female reproductive organ. It develops from the embryonic tissue that lines the body cavity, called the coelomic epithelium. During ovulation, a mature embryo is released from a follicle in the ovary, the OSE may be compromised at the site of the rupture[9]. Some cases of OSE damage, invagination may occur during follicle recovery, leading to the formation of cysts within cortex of the affected tissues[10]. The Mullerian ducts, important to the maturation of the specific reproductive organs, arise from the invagination of the coelomic epithelium in the upper lateral portion of the gonadal ridge[11]. The Mullerian duct epithelium, ovarian mesothelium and peritoneal mesothelium originate from the same embryonic tissue layer[12]. OSE can undergo metaplasia, a process in which one tissue type is replaced by another[13]. When metaplastic potential coupled with genetic abnormalities, results in the formation of malignancy that resembles the typical epithelium of the fallopian tubes, uterus and endocervix[14,15].
Low-Grade Serous Carcinoma (LGSC):
LGSC is a less lethal form of the disease when compared to HGSC. It is a rare form of cancer which is mainly diagnosed in middle aged women[16]. A common approach to treat LGSC involves a combination of platinum and taxane-based chemotherapy, which is like treatment for HGSC. Hormonal therapy is another treatment option that could be investigated for those who have a disease that has not been eradicated by chemotherapy[17,18]. LGSC has symptoms with ovarian cancer and ultrasonography is a vital tool for the early evaluation of an adnexal tumor. The malignancy index is an important instrument that is utilized in ultrasound imaging[19]. Its primary purpose is to differentiate between benign and malignant adnexal (ovarian) lesions, this method considers the results of an ultrasound, the patient's menstrual status and the concentration of Cancer Antigen-125 (CA-125) in their blood[20,21]. Invasive LGSCs may show multicellular cysts with solid components or papillary projections, often accompanied by calcification[22], while noninvasive LGSCs often show multilocular cystic lesions with papillary projections. In contrast, high-grade serous ovarian LGSC is a less lethal form of the disease when compared to HGSC. It is a rare form of cancer which is mainly diagnosed in middle aged women[16]. A common approach to treat LGSC involves a combination of platinum and taxane-based chemotherapy, which is like treatment for HGSC. Hormonal therapy is another treatment option that could be investigated for those who have a disease that has not been eradicated by chemotherapy[17,18]. LGSC has symptoms with ovarian cancer and ultrasonography is a vital tool for the early evaluation of an adnexal tumor. The malignancy index is an important instrument that is utilized in ultrasound imaging[19]. Its primary purpose is to differentiate between benign and malignant adnexal (ovarian) lesions, this method considers the results of an ultrasound, the patient's menstrual status and the concentration of Cancer Antigen-125 (CA-125) in their blood[20,21]. Invasive LGSCs may show multicellular cysts with solid components or papillary projections, often accompanied by calcification[22], while noninvasive LGSCs often show multilocular cystic lesions with papillary projections. In contrast, high-grade serous ovarian tumors typically take the form of non-papillary solid masses that also feature scattered areas of cystic transformation, hemorrhage or necrosis[23,24].
Mucinous Ovarian Cancer (MOC):
MOC is a rare form of cancer in which the use of tobacco is considered a primary risk factor for developing MOC[25]. MOCs are characterized by the progression towards cancer that is indicated by their heterogeneous nature, which contains benign, borderline and carcinoma components[26]. Around 40 %-50 % of cases of Kirsten Rat Sarcoma viral oncogene homologue (KRAS) mutation are found in MOCs, while 25 or more than 25 cases are found in tumor protein p53 mutation. KRAS mutation is thought to be an early event detectable in the surrounding benign and borderline lesions[27,28].
Clear Cell Carcinoma (CCT):
CCT is depicted by the presence of hobnail-like cells with abundant cytoplasm containing glycogen[29]. CCT can manifest in several distinctive morphological forms, including papillary, tubulocystic, solid and mixed patterns[30]. In most cases, CCT will have a negative result for the progesterone receptor and Wilms tumor suppressor[31]. However, one study found that CCT tissues were more likely to have methylation of the Wilms' Tumor 1 (WTI) gene and WTI-antisense promoter than serous adenocarcinoma tissues[32]. It has been demonstrated that Hepatocyte Nuclear Factor-1 Beta (HNF-1β) has anti-apoptotic effects in clear cell carcinoma and this protein is regarded as a valid diagnostic for clear cell tumors[33].
Research into the molecular biology and genetics of clear cell carcinoma has shown that it often lacks mutations in P53, Breast Cancer gene 1 (BRCA1) and BRCA2 genes, but shows mutations in AT-Rich Interaction Domain 1A (ARID1A) and Phosphatidylinositol-4,5-bisphosphate 3-Kinase Catalytic subunit Alpha (PIK3CA) genes[34]. PIK3CA mutations are frequently found in CCT which leads to the activation of the Phosphatidylinositol 3-Kinase (P13K)-protein kinase B (AKT)-mammalian Target of Rapamycin (mTOR) pathways. It has been hypothesized that an increase in the activity of this pathway could be one of the factors that lead to the development of CCT. As a result, CCT has a unique molecular profile with common PIK3CA mutations and abnormal activation of the mTOR pathway[35,36].
Small Cell Carcinoma of the Ovary (SCCO):
SCCO is an exceedingly rare and aggressive subtype of ovarian cancer that mostly affects young women, often those under the age of 40 y[37]. It is characterized by small round blue cells that resemble those seen in small-cell lung carcinoma. It is classified into two types; Small cell Cancer of Ovary Hypercalcemia Type (SCOHT) and Small cell Cancer of Ovary Pulmonary Type (SCOPT). SCOHT tends to strike younger women (with an average onset age of 22), while SCOPT tends to strike older women (around 51). Both are extremely aggressive cancers, but they have different chemo and irradiation responses. Surgery and platinum-based chemotherapy are the main treatments for SCOHT; however, the optimum approach is unknown[20]. The combination of high-dose chemotherapy and stem cell transplantation has been proposed as a treatment for SCOHT. Many patients with SCOHT were found to have recurrent inactivating mutations in the SWI/SNF related, Matrix associated, Actin dependent Regulator of Chromatin, subfamily A, member 4 (SMARCA4) gene[38]. Researchers have identified the gene as the one that encodes the SWI/SNF chromatin remodelling complex's adenosine triphosphatase. A high frequency of mutations and a decrease of protein expression were detected in patients who were afflicted with the condition. These findings provide useful insights into the molecular pathogenesis of SCOHT and have the potential to have ramifications to examine the unusual subtype of ovarian cancer[39]. According to a recent study Enhancer of Zeste Homolog 2 (EZH2), which is a histone methyltransferase is found to have high levels of expression in SCOHT, as a result of the loss of SMARCA4. Based on research it appears that a therapeutic approach that targets EZH2 could be a promising technique for treating SCOHT, inhibition of EZH2 achieved through the use of pharmacological agents will be successful in treating this aggressive kind of ovarian cancer. However pulmonary type remains understudied due to its scarcity and further research is necessary to gain a better understanding of genetics and epigenetic alternations, this could potentially lead to the development of new targeted therapies[40].
Ovarian germ cell:
Eggs or ova are produced by specialised cells in the female body that are called ovarian germ cells. During the process of foetal development, these cells migrate to the developing ovary after having originated in primordial germ cells[25]. Different study is conducted on ovarian germ cell tumors, with dysgerminomas being the most common subtype, followed by immature teratomas, mature teratomas with malignant degeneration and mixed germ cell tumors. The KIT proto-oncogene, receptor tyrosine kinase gene, which is mutated in dysgerminomas, is involved in the activation of several intracellular signalling pathways[41]. In a study conducted by Fujiwara et al., a mutation in the KRAS gene was found in six cases of malignant ovarian germ cell tumors. These patients included two dysgerminomas and four immature teratomas[38]. Gain of 12P and isochromosome 12P are two examples of chromosome 12 abnormalities that have been linked to dysgerminomas. The KRAS gene has been linked to several different types of cancer due to its function in the Rat sarcoma protein (RAS)-Mitogen-activated protein kinase kinase kinase (RAF)- Mitogen-activated protein kinase kinase (MEK)-Extracellular signal regulated protein kinase (ERK)/c-Jun N-terminal kinase (JNK) pathway[42].
Yolk sac tumors are characterized by aneuploidy and frequently display gains in chromosome 12P, which are detected in approximately 60 %-70 % of ovarian cancer[43]. In contrast to other types of ovarian tumors, yolk sac tumors do not show any recurrent copy number variations. In addition, it has been discovered that yolk sac tumors typically demonstrate activation of the P13K/AKT/mTOR signalling pathway. This is a phenomenon that is also observed in certain types of cancer. Activation of the Transforming Growth Factor-beta (TGF-β)/ bone morphogenic proteins (BMP) and Wingless-Int (Wnt)/beta-catenin signalling pathways was not observed in dysgerminomas but was shown in tumours of the yolk sac.
Ovarian Sex Chord-Stromal Tumor (SCSTs):
Tumors originating in the specialized cells that sustain and regulate egg formation, known as ovarian SCSTs[44]. It originated from Granulosa Cell Tumors (GCT), theca cells, fibroma cells and Sertoli-Leydig cells[43]. The onset of ovarian SCSTs has been associated with a number of inherited conditions, including DICER1-pleuropulmonary blastoma familial tumor predisposition syndrome, Ollier disease and Maffucci syndrome. Peutz-Jeghers syndrome (PJS) is an autosomal dominant disease caused by mutations in the serine/threonine kinase 11 (STK11)/Liver Kinase B1 (LKB1) gene located on chromosome 19p13. Ovarian SCSTs linked with PJS have histological characteristics in-between those of Germ Cell Tumors (GCTs) and Sertoli cell tumors, Adenosine Monophosphate (AMP) kinase activating gene STK11/LKB1 is well-known as a tumor suppressor gene[45,46]. Enchondromas are benign cartilaginous tumors that are seen in patients with rare genetic illnesses including Ollier disease and Maffucci syndrome. Multiple enchondromas at different sites characterize Ollier disease, while multiple enchondromas and soft tissue hemangiomas characterize Maffucci syndrome. Mutations in the IDH gene, which produces isocitrate dehydrogenase are linked to enchondromas in Ollier disease and Maffucci syndrome. In this study, it is shown that hereditary syndrome such as PJS, Ollier disease and Maffucci syndrome and DICER1 syndrome are associated with an increased risk of ovarian SCSTs and may require specialized management and surveillance[47].
Granulosa Cell Carcinoma (GCC):
Granulosa cell tumors are rare and indolent neoplasms. Randomized clinical trials have never been used to identify the most effective treatment for these tumors. It is mainly diagnosed at a median age of around 50-60 y[48], with clinical symptoms that may include abdominal pain, distention, the presence of a palpable mass and mental disturbance. Based on clinical and histological characteristics it is of two types of juvenile and adult type[49]. Juvenile type granulosa cell tumors were found in prepubertal girls and younger women. Adult form of GCC is mostly found in older age women and distinguish by the presence of small, uniform cells arranged in cords or nests surrounded by a fibrous stroma[50,51]. The cells frequently possess small nuclei and indistinct nucleoli and they may take on the appearance of "coffee beans." For treatment of juvenile and adult GCC, surgery is the best option to remove the tumor while preserving fertility. Generally, progenesis of juvenile and adult GCC shows a high rate of cure in the early stage of the disease[52].
Sertoli-Leydig Cell (SLCTs):
SLCTs also known as androblastomas contain a very small percentage of ovarian tumors[53]. It is assigned into three types based on their degree of differentiation, which is directly linked to the patient’s prognosis they are differentiated, intermediately differentiated[54] and poorly differentiated. These tumors are mainly found in middle-aged women and manifest themselves in a unilateral location[55].
Cubosomal Loaded Drug
Cubosomes are a form of nanoparticle that has a unique structure consisting of a lipid bilayer enclosing an internal cubic lattice[56]. Because of their structure, they are perfect for the delivery of drugs, as they can encapsulate both pharmaceuticals that are hydrophilic and those that are hydrophobic. In order for cubosomes to serve their purpose as an efficient drug delivery system, they must be pre-loaded with enough quantity of bioactive, peptides, biologics or small-molecule drugs. Loading cargo onto cubosomes can be done three ways; within the lipid bilayer, on the lipid membrane and in the cubic phase's water channels[57]. Various methods are used for loading drugs into cubosomes such as the addition of a therapeutic agent to the molten lipid or co-lyophilizing it with the lipid film before dispersion[58]. Another method involves loading drug moieties onto preformed cubosomes using the incubation technique and it enables efficient loading of drugs onto the cubosomes, providing potential benefits for targeted drug delivery[59].
Cancer-Targeted Drug Delivery Using Cubosomes
The clinical application of anticancer drugs is limited due to non-specific distribution in living organisms which leads to adverse effects in normal tissue. Therefore, it is important to create drug delivery systems that can specifically target cancer cells while reducing collateral damage to healthy tissues, which led to the creation of highly efficient and precise drug delivery methods, such as cubosomes as it can offer biocompatibility, minimal toxicity, shows significant potential as drug delivery mechanism for cancer therapy[60]. To overcome different drug carriers have been developed to deliver agents at specific sites. Nanoparticles that concentrate in the affected tissue are used to deliver drugs or other therapeutic agents directly to the site of the disease, it offers several advantages such as enhanced efficacy, reduced toxicity and improved pharmacokinetics[61].
Cubosome-Loaded with Anticancer Drugs
Paclitaxel:
Paclitaxel is used in combination with platinum-based agents as a first-line chemotherapeutic drug[62]. PTX binds to β-tubulin, blocking the mitotic spindle and stopping the cell cycle at the metaphase-anaphase junction[62]. This process enhances the polymerization of microtubules, leading to the inhibition of the cell cycle. PTX conjugation to β-tubulins stabilizes microtubules, preventing their dynamic rearrangement to maintain interphase and mitotic functions that produce unusual bundles throughout the cell cycle[63], this slows cancer cell growth. PTX-based NSCLC and other cancer therapies are being studied to improve efficacy and safety[64].
In this study, paclitaxel showed extensive antitumor effectiveness in vivo mice tumor screening[65,66]. Although clinical trial shows the result of paclitaxel on different cancer mushroomed, as the source being extremely slow-growing Pacific yew many of them were not able to begin as planned[67]. Researchers soon released that more quantity of dried bark is required for the production of paclitaxel extract[68]. The discovery of platinum cytotoxic properties led to increased responsiveness to paclitaxel therapy in patients with ovarian cancers that were resistant to platinum. Another concern was that paclitaxel wasn't soluble in water, but this was fixed by making the drug soluble in ethanol and Cremophor E [69,70].
Cisplatin:
The platinum-based chemotherapy drug cisplatin is widely used to treat ovarian cancer[71]. It is an alkyl agent which produces an immensely reactive moiety that facilitates the cross-linking of Deoxyribonucleic Acid (DNA)[72], forming DNA adducts which in turn hindered the repair of DNA which subsequently leads to DNA damage. Ovarian cancer patients who undergo therapy with cisplatin frequently experience recurrence and develop resistance to chemotherapy[73,74].
Cisplatin can develop resistance in cancer cells through different changes[75]. Drug transfer decreases intracellular cisplatin accumulation, increased quantities of intracellular scavengers such as glutathione and/or metallothionein, leading to more efficient drug detoxification variations in the mechanisms leading to apoptosis[64,76].
Icariin (ICA):
Cubosomes with GMO and P407 as a stabilizer was used to transport icariin[77]. The use of cubosomes loaded with ICA for the cure of ovarian cancer was investigated[78]. The cubosome formulation was optimized using a Box-Behnken statistical approach[79]. Formulations' drug entrapment efficiencies varied with different particle sizes. ICA-Cubs, which were optimised ICA-loaded cubosomes, showed increased cytotoxicity and apoptotic potential in tests against ovarian cancer cell lines (SKOV-3 and Caov 3)[80]. Optimised ICA-Cubs were found to be ineffective against EA.hy926 endothelial cells. Analysis of cell cycle arrest using optimised cubs against ICA-raw showed a promising function for optimised cubs in the pre-G1 and G2/M phases. This higher production of reactive oxygen species may be responsible for the heightened apoptotic potential of the ICA. Therefore, it is hypothesised that ICA-Cubs treatment has a stronger capacity to lower tumor necrosis factor production within the cytosol of the SKOV-3 cells, which could restrict angiogenesis in the tumour microenvironment and cancer cell growth, proliferation, invasion and metastasis[81]. Caspase-3 production is greatly increased by the formulation technique in ICA-Cubs compared to ICA-raw[82]. This may be due to the increased internalization of ICA in the novel formulation, as the quantity of caspase-3 in ovarian cancer cells was unaffected by the placebo formulation[83]. This role of caspase-3 in the therapeutic group may be connected with p53 expression, which in turn may affect the development and spread of cancer cells. Ovarian cancer cell growth could be inhibited by ICA-loaded cubosomes due to their increased drug solubility and cellular permeability[84].
Dual Targeting Strategy for Cancer Treatment
The dual targeting strategy is an approach that tries to improve the selectivity and specificity of cancer treatment by focusing on various pathways and substances that are essential to the survival and proliferation of cancer cells[85,86]. It is a technique that can also be applied to drug delivery systems, may include delivering numerous drugs at once to the site of the tumor in an effort to improve the effectiveness of the treatment[87].
Bispecific antibodies have shown great promise as a dual-targeting strategy for cancer therapy. Based on the mechanism of action it is divided into two types. Direct targeting of particular components, such as cell surface receptors or soluble substances, is included in the first category of treatment approaches. This can be done by binding and neutralizing two ligands or receptors, neutralizing a receptor and a ligand, activating two receptors, activating one receptor and neutralizing another receptor, or activating a soluble factor.
Bispecific antibodies are used in the second type of dual targeting method, which involves delivering a therapeutically active component, such as effector molecules or effector cells, to the target region. In order to increase the effectiveness of a single molecule's treatment, this strategy incorporates both direct and indirect activities inside the same molecule. These dual targeting techniques have the possibilities to increase the specificity and efficacy of therapeutic interventions in a variety of illness conditions by focusing on the unique qualities of bispecific antibodies, such as their ability to simultaneously bind to several targets[88].
Dual receptor targeting as a potential cancer treatment:
Growth signals transmitted by receptors that are increased or amplified in tumor cells are essential for tumor formation and progression[89,90]. The Epidermal Growth Factor (EGF) receptor family (EGFR, Human Epidermal growth factor Receptor 2 (HER2), HER3 and HER4) and Insulin-like Growth Factor-1 Receptor (IGF-1R) exhibit this phenomenon. Cell proliferation, survival, differentiation and migration are all controlled by these receptors and their downstream signaling pathways, which include Ras/Raf/ERK/MAPK and PI3K/AKT[91]. However, pathway switching between two receptors can cause the reciprocal receptor to upregulate and activate, as seen in EGFR and IGF-1R[92].
This pathway facilitates tumor cell malignant phenotype maintenance and contributes to disease relapse[93]. In addition, several tumors have been discovered to have co-expression and crosstalk of many growth-promoting receptors, including EGFR and IGF-1R[94]. Therefore, it was hypothesized that targeting two distinct receptors on a tumor cell would increase the antiproliferative effect and preventing developing the resistance[95,96].
Dual-ligand cancer treatment:
Neovascularization, which is stimulated by vascular growth factors, is necessary for the progression of solid tumors[97]. These angiogenic agents stimulate endothelial cell proliferation, migration, extracellular matrix remodeling, vascular permeability and blood vessel survival. In addition to Vascular Endothelial Growth Factor A (VEGF-A), Angiopoietin-2 and osteopontin are two proteins that have been found to play a function in angiogenesis. Anti-VEGF antibodies like bevacizumab are used to treat metastatic colorectal cancer and a number of other solid tumors because of their ability to block the growth of new blood vessels. There may be a way to increase anti-angiogenic activity by combining the neutralization of multiple angiogenic compounds[98].Researchers created a novel bispecific Dual Variable Domain-Immunoglobulin (DVD-Ig) that combines the variable domain of bevacizumab (VEGF antibody) with those of an anti-osteopontin antibody (hu1A12). The two DVD-Igs, VEGF/OPN-BsAb were designed to have increased anti-angiogenic effects. The potential therapeutic benefits of the bispecific DVD-Ig were evaluated and found to be promising, as it inhibited primary tumor growth and spontaneous metastasis in the ovary. After comparing the binding behavior of both antibodies, VEGF/OPN-BsAb was selected for further analysis, in vitro, the bispecific antibody inhibited endothelial cell proliferation and reduced micro-vessel density in a hepatocellular carcinoma model (HCCLM3)VEGF/OPN-BsAb also inhibited primary tumor growth of the primary tumor, but also demonstrated effectiveness in preventing spontaneous ovarian metastasis, this finding indicates its potential as a therapeutic agent for surgery of metastatic cancer[99].
Conflict of interest:
The authors declared no conflict of interests.
References
- Malvezzi M, Carioli G, Rodriguez T, Negri E, La Vecchia C. Global trends and predictions in ovarian cancer mortality. Ann Oncol 2016;27(11):2017-25.
[Crossref] [Google Scholar] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68(6):394-424.
[Crossref] [Google Scholar] [PubMed]
- Momenimovahed Z, Tiznobaik A, Taheri S, Salehiniya H. Ovarian cancer in the world: Epidemiology and risk factors. Int J Womens Health 2019;11:287-99.
[Crossref] [Google Scholar] [PubMed]
- Khalifa AM, Elsheikh MA, Khalifa AM, Elnaggar YS. Current strategies for different paclitaxel-loaded nano-delivery Systems towards therapeutic applications for ovarian carcinoma: A review article. J Control Release 2019;311:125-37.
[Crossref] [Google Scholar] [PubMed]
- Iqbal MA, Md S, Sahni JK, Baboota S, Dang S, Ali J. Nanostructured lipid carriers system: Recent advances in drug delivery. J Drug Target 2012;20(10):813-30.
[Crossref] [Google Scholar] [PubMed]
- Cortez AJ, Tudrej P, Kujawa KA, Lisowska KM. Advances in ovarian cancer therapy. Cancer Chemother Pharmacol 2018;81:17-38.
[Crossref] [Google Scholar] [PubMed]
- Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Tangen CM, et al. Fluorouracil plus levamisole as effective adjuvant therapy after resection of stage III colon carcinoma: A final report. Ann Intern Med 1995;122(5):321-6.
[Crossref] [Google Scholar] [PubMed]
- Lv L, Zhuang YX, Zhang HW, Tian NN, Dang WZ, Wu SY. Capsaicin-loaded folic acid-conjugated lipid nanoparticles for enhanced therapeutic efficacy in ovarian cancers. Biomed Pharmacother 2017;91:999-1005.
[Crossref] [Google Scholar] [PubMed]
- Kanska J, Zakhour M, Taylor-Harding B, Karlan BY, Wiedemeyer WR. Cyclin E as a potential therapeutic target in high grade serous ovarian cancer. Gynecol Oncol 2016;143(1):152-8.
[Crossref] [Google Scholar] [PubMed]
- Kim J, Park EY, Kim O, Schilder JM, Coffey DM, Cho CH, et al. Cell origins of high-grade serous ovarian cancer. Cancers 2018;10(11):433.
[Crossref] [Google Scholar] [PubMed]
- Bowtell DD, Böhm S, Ahmed AA, Aspuria PJ, Bast Jr RC, Beral V, et al. Rethinking ovarian cancer II: reducing mortality from high-grade serous ovarian cancer. Nat Rev Cancer 2015;15(11):668-79.
[Crossref] [Google Scholar] [PubMed]
- Akhmedkhanov A, Toniolo P, Zeleniuch-Jacquotte A, Kato I, Koenig KL, Shore RE. Aspirin and epithelial ovarian cancer. Prev Med 2001;33(6):682-7.
[Crossref] [Google Scholar] [PubMed]
- Turcotte M, Spring K, Pommey S, Chouinard G, Cousineau I, George J, et al. CD73 is associated with poor prognosis in high-grade serous ovarian cancer. Cancer Res 2015;75(21):4494-503.
[Crossref] [Google Scholar] [PubMed]
- Bosetti C, Gallus S, La Vecchia C. Aspirin and cancer risk: An update to 2001. Eur J Cancer Prev 2002;11(6):535-42.
[Crossref] [Google Scholar] [PubMed]
- Mitra AK, Davis DA, Tomar S, Roy L, Gurler H, Xie J, et al. In vivo tumor growth of high-grade serous ovarian cancer cell lines. Gynecol Oncol 2015;138(2):372-7.
[Crossref] [Google Scholar] [PubMed]
- Crane EK, Sun CC, Ramirez PT, Schmeler KM, Malpica A, Gershenson DM. The role of secondary cytoreduction in low-grade serous ovarian cancer or peritoneal cancer. Gynecol Oncol 2015;136(1):25-9.
[Crossref] [Google Scholar] [PubMed]
- Grisham RN, Iyer G. Low-grade serous ovarian cancer: current treatment paradigms and future directions. Curr Treat Options Oncol 2018;19:1-6.
[Crossref] [Google Scholar] [PubMed]
- Grisham RN, Sylvester BE, Won H, McDermott G, DeLair D, Ramirez R, et al. Extreme outlier analysis identifies occult mitogen-activated protein kinase pathway mutations in patients with low-grade serous ovarian cancer. J Clin Oncol 2015;33(34):4099-105.
[Crossref] [Google Scholar] [PubMed]
- Gadducci A, Cosio S. Therapeutic approach to low-grade serous ovarian carcinoma: State of art and perspectives of clinical research. Cancers 2020;12(5):1336.
[Crossref] [Google Scholar] [PubMed]
- Oneda E, Zorzi F, Gorio A, Quaglia F, Abeni C, Rota L, et al. Differential diagnosis of small cell carcinoma of the ovary or ovarian metastases of small cell carcinoma of the lung: A case report and review of the literature. Case Rep Oncol 2020;13(2):822-8.
[Crossref] [Google Scholar] [PubMed]
- Etemadmoghadam D, Azar WJ, Lei Y, Moujaber T, Garsed DW, Kennedy CJ, et al. EIF1AX and NRAS mutations co-occur and cooperate in low-grade serous ovarian carcinomas. Cancer Res 2017;77(16):4268-78.
[Crossref] [Google Scholar] [PubMed]
- Della Pepa C, Tonini G, Santini D, Losito S, Pisano C, Di Napoli M, et al. Low grade serous ovarian carcinoma: From the molecular characterization to the best therapeutic strategy. Cancer Treat Rev 2015;41(2):136-43.
[Crossref] [Google Scholar] [PubMed]
- Horta M, Cunha TM. Sex cord-stromal tumors of the ovary: A comprehensive review and update for radiologists. Diagn Interv Radiol 2015;21(4):277.
[Crossref] [Google Scholar] [PubMed]
- Grabowski JP, Harter P, Heitz F, Pujade-Lauraine E, Reuss A, Kristensen G, et al. Operability and chemotherapy responsiveness in advanced low-grade serous ovarian cancer. An analysis of the AGO Study Group metadatabase. Gynecol Oncol 2016;140(3):457-62.
[Crossref] [Google Scholar] [PubMed]
- Maoz A, Matsuo K, Ciccone MA, Matsuzaki S, Klar M, Roman LD, et al. Molecular pathways and targeted therapies for malignant ovarian germ cell tumors and sex cord–stromal tumors: A contemporary review. Cancers 2020;12(6):1398.
[Crossref] [Google Scholar] [PubMed]
- Rescorla FJ. Malignant germ cell tumors. The surgery of childhood tumors. 2016:333-44.
- Cheng SG. Recurrent somatic enzyme. Enzyme Res 2010;2(1):1-9.
- van Nieuwenhuysen E, Busschaert P, Neven P, Han SN, Moerman P, Liontos M, et al. The genetic landscape of 87 ovarian germ cell tumors. Gynecol Oncol 2018;151(1):61-8.
[Crossref] [Google Scholar] [PubMed]
- Wettersten HI, Aboud OA, Lara Jr PN, Weiss RH. Metabolic reprogramming in clear cell renal cell carcinoma. Nat Rev Nephrol 2017;13(7):410-9.
[Crossref] [Google Scholar] [PubMed]
- Makhov P, Joshi S, Ghatalia P, Kutikov A, Uzzo RG, Kolenko VM. Resistance to systemic therapies in clear cell renal cell carcinoma: Mechanisms and management strategies. Mol Cancer Ther 2018;17(7):1355-64.
[Crossref] [Google Scholar] [PubMed]
- Acién P, Acién M. Disorders of sex development: Classification, review and impact on fertility. J Clin Med 2020;9(11):3555.
[Crossref] [Google Scholar] [PubMed]
- Chen VW, Ruiz B, Killeen JL, Coté TR, Wu XC, Correa CN, et al. Pathology and classification of ovarian tumors. Cancer 2003;97(S10):2631-42.
[Crossref] [Google Scholar] [PubMed]
- DeLair DF, Burke KA, Selenica P, Lim RS, Scott SN, Middha S, et al. The genetic landscape of endometrial clear cell carcinomas. J Pathol 2017;243(2):230-41.
[Crossref] [Google Scholar] [PubMed]
- Wolf MM, Kimryn Rathmell W, Beckermann KE. Modeling clear cell renal cell carcinoma and therapeutic implications. Oncogene 2020;39(17):3413-26.
[Crossref] [Google Scholar] [PubMed]
- Jung SE, Lee JM, Rha SE, Byun JY, Jung JI, Hahn ST. CT and MR imaging of ovarian tumors with emphasis on differential diagnosis. Radiographics 2002;22(6):1305-25.
[Crossref] [Google Scholar] [PubMed]
- Atkins MB, Tannir NM. Current and emerging therapies for first-line treatment of metastatic clear cell renal cell carcinoma. Cancer Treat Rev 2018;70:127-37.
[Crossref] [Google Scholar] [PubMed]
- Simões MF, da Costa AA, Silva TN, Fernandes L, Bovolim G, Torrezan GT, et al. Case report of small cell carcinoma of the ovary, hypercalcemic type (ovarian rhabdoid tumor) with SMARCB1 mutation: A literature review of a rare and aggressive condition. Curr Oncol 2022;29(2):411-22.
[Crossref] [Google Scholar] [PubMed]
- Fujiwara K, Shintani D, Nishikawa T. Clear cell carcinoma of the ovary. Ann Oncol 2016:27 Suppl 1:i50-2
- Elsherif S, Javadi S, Viswanathan C, Faria S, Bhosale P. Low-grade epithelial ovarian cancer: What a radiologist should know. Br J Radiol 2019;92(1095):20180571.
[Crossref] [Google Scholar] [PubMed]
- Nasioudis D, Chapman-Davis E, Frey MK, Caputo TA, Witkin SS, Holcomb K. Small cell carcinoma of the ovary: A rare tumor with a poor prognosis. Int J Gynecol Cancer 2018;28(5).
[Crossref] [Google Scholar] [PubMed]
- Kraggerud SM, Hoei-Hansen CE, Alagaratnam S, Skotheim RI, Abeler VM, Rajpert-De Meyts E, et al. Molecular characteristics of malignant ovarian germ cell tumors and comparison with testicular counterparts: Implications for pathogenesis. Endocr Rev 2013;34(3):339-76.
[Crossref] [Google Scholar] [PubMed]
- van Nieuwenhuysen E, Lambrechts S, Lambrechts D, Leunen K, Amant F, Vergote I. Genetic changes in nonepithelial ovarian cancer. Expert Rev Anticancer Ther 2013;13(7):871-82.
[Crossref] [Google Scholar] [PubMed]
- Edefonti V, Decarli A, Vecchia CL, Bosetti C, Randi G, Franceschi S, et al. Nutrient dietary patterns and the risk of breast and ovarian cancers. Int J Cancer 2008;122(3):609-13.
[Crossref] [Google Scholar] [PubMed]
- Gloor E. Ovarian sex cord tumor with annular tubules: Clinicopathologic report of two benign and one malignant cases with long follow-ups. Virchows Arch A Path Anat and Histol 1979;384:185-93.
[Crossref] [Google Scholar] [PubMed]
- Marrucci O, Nicoletti P, Mauriello A, Facchetti S, Pietropolli A, Patrizi L, et al. Erratum to “Uterine Tumor Resembling Ovarian Sex Cord Tumors Type II with Vaginal Vault Recurrence”. Case Rep Obstet Gynecol 2020;2020:5205723.
- Sternberg WH, Dhurandhar HN. Functional ovarian tumors of stromal and sex cord origin. Hum Pathol 1977;8(5):565-82.
[Crossref] [Google Scholar] [PubMed]
- Benagiano G, Bigotti G, D'Alessandro P, Napolitano C. Endocrine and morphological study of a case of ovarian sex-cord tumor with annular tubules in a woman with Peutz-Jeghers syndrome. Int J Gynecol Obstet 1988;26(3):441-52.
[Crossref] [Google Scholar] [PubMed]
- Kanthan R, Senger JL, Kanthan S. The multifaceted granulosa cell tumours—myths and realities: A review. ISRN Obstet Gynecol 2012;2012(1):878635.
[Crossref] [Google Scholar] [PubMed]
- Peluso JJ, Pru JK. Non-canonical progesterone signaling in granulosa cell function. Reproduction 2014;147(5):R169-78.
[Crossref] [Google Scholar] [PubMed]
- Young RH, Dickersin GR, Scully RE. Juvenile granulosa cell tumor of the ovary: A clinicopathological analysis of 125 cases. Am J Surg Pathol 1984;8(8):575-96.
[Crossref] [Google Scholar] [PubMed]
- McFee RM, Romereim SM, Snider AP, Summers AF, Pohlmeier WE, Kurz SG, et al. A high-androgen microenvironment inhibits granulosa cell proliferation and alters cell identity. Mol Cell Endocrinol 2021;531:111288.
[Crossref] [Google Scholar] [PubMed]
- Schumer ST, Cannistra SA. Granulosa cell tumor of the ovary. J Clin Oncol 2003;21(6):1180-9.
[Crossref] [Google Scholar] [PubMed]
- Oliva E, Alvarez T, Young RH. Sertoli cell tumors of the ovary: A clinicopathologic and immunohistochemical study of 54 cases. Am J Surg Pathol 2005;29(2):143-56.
[Crossref] [Google Scholar] [PubMed]
- Young RH, Dudley AG, Scully RE. Granulosa cell, Sertoli-Leydig cell and unclassified sex cord-stromal tumors associated with pregnancy: A clinicopathological analysis of thirty-six cases. Gynecol Oncol 1984;18(2):181-205.
[Crossref] [Google Scholar] [PubMed]
- Weng CS, Chen MY, Wang TY, Tsai HW, Hung YC, Yu KJ, et al. Sertoli–Leydig cell tumors of the ovary: A Taiwanese gynecologic oncology group study. Taiwan J Obstet Gynecol 2013;52(1):66-70.
[Crossref] [Google Scholar] [PubMed]
- Shen HH, Lake V, Le Brun AP, James M, Duff AP, Peng Y, et al. Targeted detection of phosphatidylserine in biomimetic membranes and in vitro cell systems using annexin V-containing cubosomes. Biomaterials 2013;34(33):8361-9.
[Crossref] [Google Scholar] [PubMed]
- Elnaggar YS, Etman SM, Abdelmonsif DA, Abdallah OY. Novel piperine-loaded Tween-integrated monoolein cubosomes as brain-targeted oral nanomedicine in Alzheimer’s disease: Pharmaceutical, biological and toxicological studies. Int J Nanomed 2015;10:5459-73.
[Crossref] [Google Scholar] [PubMed]
- Aleandri S, Bandera D, Mezzenga R, Landau EM. Biotinylated cubosomes: A versatile tool for active targeting and codelivery of paclitaxel and a fluorescein-based lipid dye. Langmuir 2015;31(46):12770-6.
[Crossref] [Google Scholar] [PubMed]
- Caltagirone C, Falchi AM, Lampis S, Lippolis V, Meli V, Monduzzi M, et al. Cancer-cell-targeted theranostic cubosomes. Langmuir 2014;30(21):6228-36.
[Crossref] [Google Scholar] [PubMed]
- Fan C, Gao W, Chen Z, Fan H, Li M, Deng F, et al. Tumor selectivity of stealth multi-functionalized superparamagnetic iron oxide nanoparticles. Int J Pharm 2011;404(1-2):180-90.
[Crossref] [Google Scholar] [PubMed]
- Leaf C. Why we're losing the war on cancer (and how to win it). Fortune-European Ed. 2004;149(5):42-55.
[Google Scholar] [PubMed]
- Zhai J, Luwor RB, Ahmed N, Escalona R, Tan FH, Fong C, et al. Paclitaxel-loaded self-assembled lipid nanoparticles as targeted drug delivery systems for the treatment of aggressive ovarian cancer. ACS Appl Mater Interfaces 2018;10(30):25174-85.
[Crossref] [Google Scholar] [PubMed]
- Zhang L, Li J, Tian D, Sun L, Wang X, Tian M. Theranostic combinatorial drug-loaded coated cubosomes for enhanced targeting and efficacy against cancer cells. Cell Death Dis 2020;11(1):1.
[Crossref] [Google Scholar] [PubMed]
- Huang CY, Ju DT, Chang CF, Reddy PM, Velmurugan BK. A review on the effects of current chemotherapy drugs and natural agents in treating non–small cell lung cancer. Biomedicine 2017;7(4):12-23.
[Crossref] [Google Scholar] [PubMed]
- Kampan NC, Madondo MT, McNally OM, Quinn M, Plebanski M. Paclitaxel and its evolving role in the management of ovarian cancer. Biomed Res Int 2015;2015(1):413076.
[Crossref] [Google Scholar] [PubMed]
- Gajda E, Godlewska M, Mariak Z, Nazaruk E, Gawel D. Combinatory treatment with miR-7-5p and drug-loaded cubosomes effectively impairs cancer cells. Int J Mol Sci 2020;21(14):5039.
[Crossref] [Google Scholar] [PubMed]
- Flak DK, Adamski V, Nowaczyk G, Szutkowski K, Synowitz M, Jurga S, et al. AT101-loaded cubosomes as an alternative for improved glioblastoma therapy. Int J Nanomed 2020:7415-31.
[Crossref] [Google Scholar] [PubMed]
- Murgia S, Biffi S, Mezzenga R. Recent advances of non-lamellar lyotropic liquid crystalline nanoparticles in nanomedicine. Curr Opin Colloid Interface Sci 2020;48:28-39.
- Parness J, Horwitz SB. Taxol binds to polymerized tubulin in vitro. J Cell Biol 1981;91(2):479-87.
[Crossref] [Google Scholar] [PubMed]
- Alavi M, Webster TJ. Nano liposomal and cubosomal formulations with platinum-based anticancer agents: Therapeutic advances and challenges. Nanomed 2020;15(24):2399-410.
[Crossref] [Google Scholar] [PubMed]
- Grabosch S, Bulatovic M, Zeng F, Ma T, Zhang L, Ross M, et al. Cisplatin-induced immune modulation in ovarian cancer mouse models with distinct inflammation profiles. Oncogene 2019;38(13):2380-93.
[Crossref] [Google Scholar] [PubMed]
- Yan XY, Qu XZ, Xu L, Yu SH, Tian R, Zhong XR, et al. Insight into the role of p62 in the cisplatin resistant mechanisms of ovarian cancer. Cancer Cell Int 2020;20:1-1.
[Crossref] [Google Scholar] [PubMed]
- Kleih M, Böpple K, Dong M, Gaißler A, Heine S, Olayioye MA, et al. Direct impact of cisplatin on mitochondria induces ROS production that dictates cell fate of ovarian cancer cells. Cell Death Dis 2019;10(11):851.
[Crossref] [Google Scholar] [PubMed]
- Han Y, Kim B, Cho U, Park IS, Kim SI, Dhanasekaran DN, et al. Mitochondrial fission causes cisplatin resistance under hypoxic conditions via ROS in ovarian cancer cells. Oncogene 2019;38(45):7089-105.
- Cohen SM, Lippard SJ. Cisplatin: From DNA damage to cancer chemotherapy. Prog Nucleic Acid Res Mol Biol 2001:67:93-130.
[Crossref] [Google Scholar] [PubMed]
- Zhao Z, Ji M, Wang Q, He N, Li Y. Circular RNA Cdr1as upregulates SCAI to suppress cisplatin resistance in ovarian cancer via miR-1270 suppression. Mol Ther Nucleic Acids 2019;18:24-33.
[Crossref] [Google Scholar] [PubMed]
- Yetisgin AA, Cetinel S, Zuvin M, Kosar A, Kutlu O. Therapeutic nanoparticles and their targeted delivery applications. Molecules 2020;25(9):2193.
[Crossref] [Google Scholar] [PubMed]
- Cheng X, Tan S, Duan F, Yuan Q, Li Q, Deng G. Icariin induces apoptosis by suppressing autophagy in tamoxifen-resistant breast cancer cell line MCF-7/TAM. Breast Cancer 2019;26:766-75.
[Crossref] [Google Scholar] [PubMed]
- Jiang S, Chang H, Deng S, Fan D. Icariin enhances the chemosensitivity of cisplatin‑resistant ovarian cancer cells by suppressing autophagy via activation of the AKT/mTOR/ATG5 pathway. Int J Oncol 2019;54(6):1933-42.
[Crossref] [Google Scholar] [PubMed]
- Wang P, Zhang J, Xiong X, Yuan W, Qin S, Cao W, et al. Icariin suppresses cell cycle transition and cell migration in ovarian cancer cells. Oncol Rep 2019;41(4):2321-8.
[Crossref] [Google Scholar] [PubMed]
- Li N, Wang J, Wang X, Sun J, Li Z. Icariin exerts a protective effect against d-galactose induced premature ovarian failure via promoting DNA damage repair. Biomed Pharmacother 2019;118:109218.
[Crossref] [Google Scholar] [PubMed]
- Alhakamy NA, Fahmy UA, Badr-Eldin SM, Ahmed OA, Asfour HZ, Aldawsari HM, et al. Optimized icariin phytosomes exhibit enhanced cytotoxicity and apoptosis-inducing activities in ovarian cancer cells. Pharmaceutics 2020;12(4):346.
[Crossref] [Google Scholar] [PubMed]
- Cao LH, Qiao JY, Huang HY, Fang XY, Zhang R, Miao MS, et al. PI3K–AKT signaling activation and icariin: The potential effects on the perimenopausal depression-like rat model. Molecules 2019;24(20):3700.
[Crossref] [Google Scholar] [PubMed]
- Fahmy UA, Fahmy O, Alhakamy NA. Optimized icariin cubosomes exhibit augmented cytotoxicity against SKOV-3 ovarian cancer cells. Pharmaceutics 2020;13(1):20.
[Crossref] [Google Scholar] [PubMed]
- Zhong L, Wang R, Wang Y, Peng S, Ma Y, Ding S, et al. Dual inhibition of VEGF and PARP suppresses KRAS-mutant colorectal cancer. Neoplasia 2020;22(9):365-75.
[Crossref] [Google Scholar] [PubMed]
- Amer Ridha A, Kashanian S, Rafipour R, Hemati Azandaryani A, Zhaleh H, Mahdavian E. A promising dual-drug targeted delivery system in cancer therapy: Nanocomplexes of folate–apoferritin-conjugated cationic solid lipid nanoparticles. Pharm Dev Technol 2021;26(6):673-81.
[Crossref] [Google Scholar] [PubMed]
- Samaddar S, Mazur J, Boehm D, Thompson DH. Development and in vitro characterization of bladder tumor cell targeted lipid-coated polyplex for dual delivery of plasmids and small molecules. Int J Nanomed 2019;14:9547-61.
[Crossref] [Google Scholar] [PubMed]
- Levit SL, Tang C. Polymeric nanoparticle delivery of combination therapy with synergistic effects in ovarian cancer. Nanomaterials 2021;11(4):1048.
[Crossref] [Google Scholar] [PubMed]
- Ludovini V, Bellezza G, Pistola L, Bianconi F, Di Carlo L, Sidoni A, et al. High coexpression of both insulin-like growth factor receptor-1 (IGFR-1) and epidermal growth factor receptor (EGFR) is associated with shorter disease-free survival in resected non-small-cell lung cancer patients. Ann Oncol 2009;20(5):842-9.
[Crossref] [Google Scholar] [PubMed]
- Choi HJ, Heo JH, Park JY, Jeong JY, Cho HJ, Park KS, et al. A novel PI3K/mTOR dual inhibitor, CMG002, overcomes the chemoresistance in ovarian cancer. Gynecol Oncol 2019;153(1):135-48.
[Crossref] [Google Scholar] [PubMed]
- Gartung A, Yang J, Sukhatme VP, Bielenberg DR, Fernandes D, Chang J, et al. Suppression of chemotherapy-induced cytokine/lipid mediator surge and ovarian cancer by a dual COX-2/sEH inhibitor. Proc Natl Acad Sci 2019;116(5):1698-703.
[Crossref] [Google Scholar] [PubMed]
- Tao Y, Pinzi V, Bourhis J, Deutsch E. Mechanisms of disease: Signaling of the insulin-like growth factor 1 receptor pathway—therapeutic perspectives in cancer. Nat Clin Pract Oncol 2007;4(10):591-602.
[Crossref] [Google Scholar] [PubMed]
- Ueda S, Hatsuse K, Tsuda H, Ogata S, Kawarabayashi N, Takigawa T, et al. Potential crosstalk between insulin-like growth factor receptor type 1 and epidermal growth factor receptor in progression and metastasis of pancreatic cancer. Mod Pathol 2006;19(6):788-96.
[Crossref] [Google Scholar] [PubMed]
- Liu Y, Chen S, Sun J, Zhu S, Chen C, Xie W, et al. Folate-targeted and oxygen/indocyanine green-loaded lipid nanoparticles for dual-mode imaging and photo-sonodynamic/photothermal therapy of ovarian cancer in vitro and in vivo. Mol Pharm 2019;16(10):4104-20.
[Crossref] [Google Scholar] [PubMed]
- Wieduwilt MJ, Moasser M. The epidermal growth factor receptor family: Biology driving targeted therapeutics. Cell Mol Life Sci 2008;65:1566-84.
[Crossref] [Google Scholar] [PubMed]
- Wang K, Zhu C, He Y, Zhang Z, Zhou W, Muhammad N, et al. Restraining cancer cells by dual metabolic inhibition with a mitochondrion‐targeted platinum (II) complex. Angew Chem Int Engl 2019;58(14):4638-43.
[Crossref] [Google Scholar] [PubMed]
- Ferrara N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr Rev 2004;25(4):581-611.
[Crossref] [Google Scholar] [PubMed]
- Kou G, Shi J, Chen L, Zhang D, Hou S, Zhao L, et al. A bispecific antibody effectively inhibits tumor growth and metastasis by simultaneous blocking vascular endothelial growth factor A and osteopontin. Cancer Lett 2010;299(2):130-6.
[Crossref] [Google Scholar] [PubMed]
- Madheswaran T, Kandasamy M, Bose RJ, Karuppagounder V. Current potential and challenges in the advances of liquid crystalline nanoparticles as drug delivery systems. Drug Discov Today 2019;24(7):1405-12.
[Crossref] [Google Scholar] [PubMed]