- *Corresponding Author:
- N. Kurup
Department of Pharmaceutics, Principal K. M. Kundnani College of Pharmacy, Colaba Affiliated to University of Mumbai, Mumbai, Maharashtra 400005, India
E-mail: ns.kurup@kmkcp.edu.in
| Date of Received | 20 September 2021 |
| Date of Revision | 05 November 2022 |
| Date of Acceptance | 05 January 2023 |
| Indian J Pharm Sci 2023;85(1):13-22 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Phospholipids have managed to overcome several challenges, which hampered the therapeutic potential of conventional drug delivery systems. As formulation excipients, phospholipids have become progressively essential. This review intends to summarize the basic characteristics and usefulness of phospholipids in oral delivery systems (viz. nanocochleates and drug-phospholipid complex) to overcome problems relating to the solubility and permeability of anti-cancer agents. The first segment of the review will give insight into nanocochleates, which are cylindrical cigar-like structures that have the ability to deliver hydrophobic as well as hydrophilic drugs. In the second section, we have provided an overview of the phospholipid complex formed because of the interaction between drugs and phospholipids. In a nutshell, our review offers two strategies for boosting the use of phospholipids in the oral delivery of anti-cancer agents, which can help overcome the existing problems and open up new avenues and advances in developing oral drug delivery systems.
Keywords
Oral delivery, phospholipids, novel formulations, chemotherapeutic agents, nanocarrier system, cochleates
Cancer has remained a mystery that is still unresolved and is one of the leading causes of death worldwide. The current treatment approaches are based on surgery, radiation therapy, chemotherapy, stem cell therapy and many more, based on the nature of cancer and the stage of cancer. But this complex approach is far from being satisfactory[1]. Most of the anti-cancer drugs available in the market are parenteral formulations with serious side effects. Besides, several anti-cancer agents are also available in oral dosage form due to Patient comfort, ease of convenience over parenteral administration and willingness to establish chronic treatment regimens[2].
Having all the advantages described above, however, the effectiveness of the oral drug delivery systems is compromised due to the low oral bioavailability of chemotherapeutic agents. Widely known reasons for low bioavailability include low aqueous solubility, poor membrane permeation and early degradation by proteolytic enzymes. Thus, the optimal therapeutic dose might not reach the target, requiring a higher amount of drug for therapeutic efficacy. This can harm healthy cells and tissues, leading to severe side effects including nausea, acute vomiting, extensive hair loss and low blood cell count leading to anemia. Though the oral route is perhaps the most desired route of drug delivery, development of effective formulations to promote the delivery of drugs through oral route is the subject of great importance to the pharmaceutical industry. Most of the chemotherapeutic agents are hydrophobic and are challenging to formulate. However, the commercial availability of various oral chemotherapeutics can justify the preference for oral administration of anti-cancer drugs. Different anti-cancer drugs currently available in oral dosage forms are vinorelbine tartrate, etoposide, 5-fluorouracil, cyclophosphamide, capecitabine, etc[1].
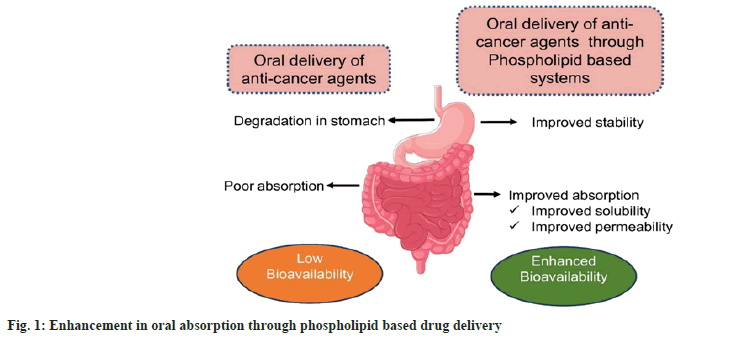
Conventional delivery systems such as tablets and capsules have been studied, but they encounter challenges, especially low solubility and bioavailability. Therefore, several strategies have been proposed to improve the solubility of chemotherapeutic agents by using pharmaceutical excipients, drug carriers or preparing prodrugs. Several biocompatible nanoparticulate systems based on polymers, lipids and oils are used successfully to increase the bioavailability of drugs when administered via oral route. The critical drawbacks of the encapsulation methods currently used for delivering anti-cancer drugs are low drug encapsulation, low retention efficiencies, instability and reticuloendothelial system absorption, drug leakage and poor accumulation at target sites. Therefore, there is a demand to formulate potential drug delivery systems for delivering anti-cancer agents, which will overcome the bioavailability problems. Furthermore, it is more suitable for patients who cannot withstand an intensive chemotherapy regimen on a long-term basis. Oral chemotherapy can significantly reduce the overall cost of treatment which can have an ultimate pharmacoeconomic advantage. Phospholipid-based formulations are currently at the forefront due to their excellent properties. They are investigated rigorously to achieve maximum drug delivery benefits (fig. 1). Anti-cancer drugs with poor solubility or bioavailability when given orally degrade and hence show low bioavailability. Therefore, drug delivery systems are important to attain the desired level in the systemic circulation. Various studies have shown improvement in drug delivery with phospholipid-based systems eg. Curcumin, Cyclosporin, Paclitaxel, etc. In this review, we explore the potential of nanocochleates and drug-phospholipid complex for oral delivery. However, the emphasis of this review is not on the comparison of these two strategies.
Sources and Properties of Phospholipids
Phospholipids are categorized as Generally Regarded As Safe (GRAS) by the United States Food and Drug Administration[3]. They have very low toxicity and can therefore are suitable for all the routes of administration. Phospholipids can be obtained from naturally occurring materials such as eggs, bovine brain, soybean, sunflower, rapeseed, cottonseed, etc. They can also be of synthetic origin (Hydrogenated phosphatidylcholine and synthetic phosphatidylcholine)[4]. However, for oral delivery, naturally occurring phospholipids are recommended. Phospholipids are an essential part of the cell membrane. They are amphiphilic molecules comprising of a hydrophilic head region formed of a negatively charged phosphate group, two hydrophobic tails formed of long-chain fatty acids and a glycerol or alcohol group that links the head and tail areas, enabling them to build a lipidic bilayer or vesicles in biological systems[5,6].
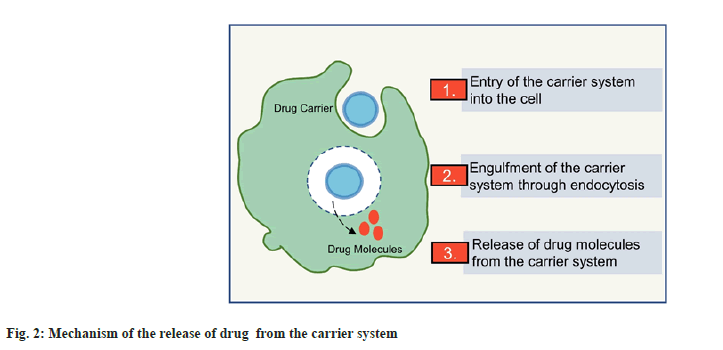
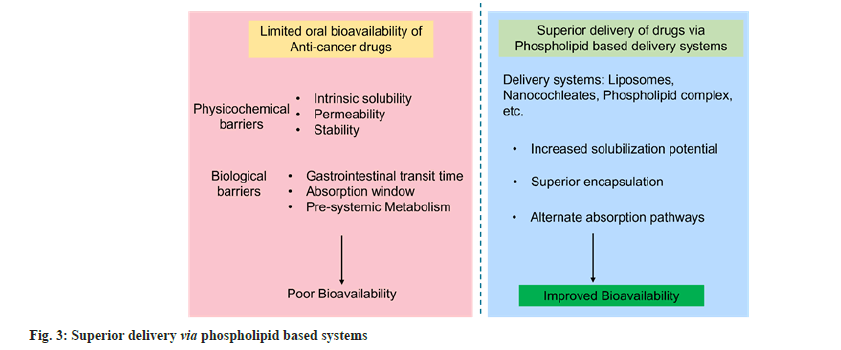
Owing to their amphiphilic nature, phospholipids are multipurpose excipients that can be used in oral formulations as emulsifiers, wetting agents, solubilizers and liposome former. Besides, they can be used as a matrix material for solid dispersions and slow-release tablet formulations[7]. Phospholipid-based delivery systems may provide a solution to the low bioavailability problems and therefore are widely studied for delivering anti-cancer agents. Fig. 2 shows the brief mechanism of delivery through phospholipid-based carriers. Many phospholipid-based liposomal formulations have reached the market, including Doxil®, Myocet®, Daunoxome®, etc[8]. However, the application and development of liposomes as oral drug delivery carriers is limited because of the chemical and enzymatic destabilization of the lipid vesicles in the intestine[9]. Therefore, it is beneficial to maximize the therapeutic potential of phospholipids using different strategies, thus giving the basis for successfully delivering anti-cancer agents via oral route. The poor biopharmaceutical and physicochemical properties associated with the various anticancer drugs limiting their oral deliverability can be effectively circumvented by the utilization of phospholipids and pharmaceutical approaches such as nanocochleates and phospholipid complexes. These novel drug delivery systems owing to their special properties can bypass various barriers to drug delivery across the gastrointestinal tract[1,3,6,9]. Fig. 3 shows a pictorial representation of superior delivery with phospholipid based systems.
Nanocochleates
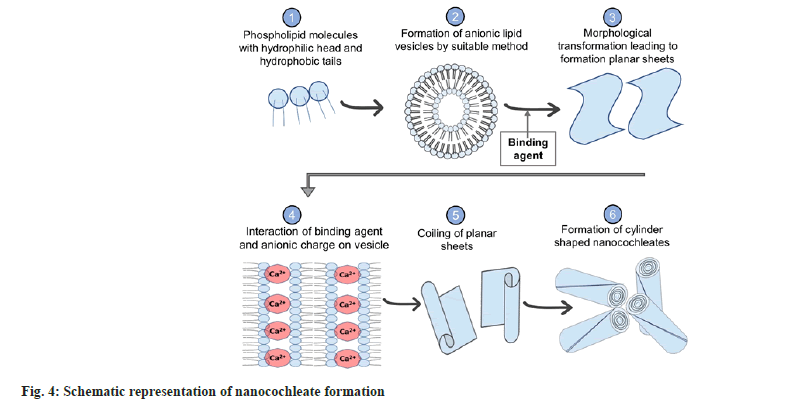
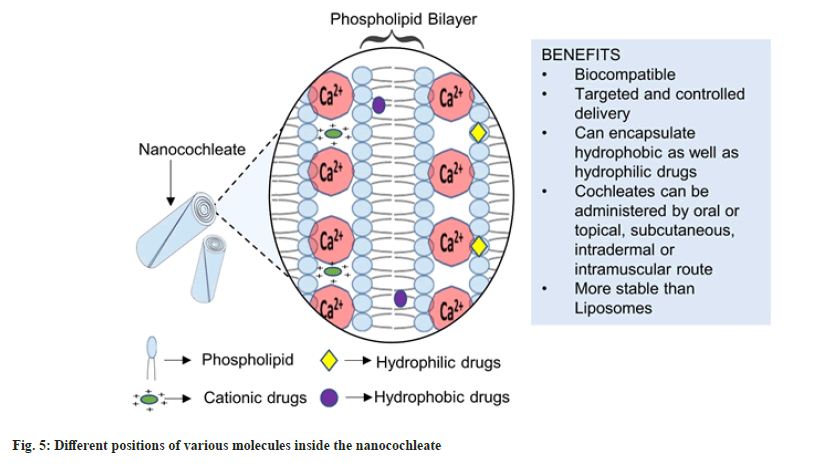
Dr. Dimitrious Papahadjoupoulos first discovered Cochleates in 1975. Cochleates or Nanocochleates are prepared from the pre-formed bilayer lipid vesicles (having anionic charge) or liposomes in the presence of binding agents. The fusion of bilayers produces morphological transformation to form large sheets that coil to form cochleate having a cylindrical cigar-like shape. Cochleate in Greek refers to “a snail with a spiral shell,” which resembles the folding pattern of the cylinder (fig. 4). These cochleates can incorporate hydrophilic, hydrophobic as well as charged molecules (fig. 5). Naturally occurring Lipids such as Phospholipids or lecithins are used extensively to prepare cochleates. Lipids obtained from natural origin employed for cochleate formation are phosphatidylserine, phosphatidylcholine, etc[10-12].
For interaction with the negative charge on phospholipids, generally positively charged multivalent cations are used. These cations bind to the lipid non-covalently. Commonly used binding agents are divalent metal cations such as Ca2+, Ba2+, Mg2+ and Zn2+[11,13]. Monovalent cations such as Na+ and trivalent cations such as Al3+ also have been tested for their binding action[14]. Certain drugs with cationic charges, such as tobramycin and polylysine, have also been studied for their binding ability to form cochleate structures[15]. Recently Judeh et al.[16] investigated the properties of amikacin bridged cochleates[16]. Moreover, in particular, Ca2+ is used as a binding agent because it has the ability to improve membrane fusion and phagocytosis. However, the major obstacle in using Ca2+ as a binding agent is the aggregation of particles, which hampers the stability of nanocochleates. Citric acid may be used to overcome this difficulty, which acts as a dispersing and stabilizing agent[17]. Other aggregation inhibitors reported are casein, milk, albumin, hydroxy cellulose, methylcellulose, etc[18].
The enhanced anti-cancer activity of nanocochleates may be because of the increased number of small size molecules leading to membrane fusion and the Enhanced Permeability and Retention (EPR) effect. This enhanced effect of nanocochleates can be attributed to their cylindrical shape. The recommended particle size and zeta potential ranges are 100-200 nm and ±30 mV, respectively. This enhances the EPR effect and prevents the aggregation of particles. Other evaluation parameters include entrapment efficiency, drug loading, in vitro release studies, transmission electron microscopy, scanning electron microscopy, etc[19-21].
Mechanism of nanocochleates:
The theory behind the release and action of cochleate at the cellular level in in vivo conditions is unclear. It is known that the interior surface of cochleate having an encapsulated drug molecule remains protected from the harsh environment in the body. The outer layer may degrade, which will lead to gradual drug release and improved absorption. There are two proposed mechanisms; interaction of the cochleate’s calcium-rich layer with the cell membrane leads to disruption and morphological transformation of the cell membrane, causing the outer layer of cochleate to fuse with the cell membrane and through phagocytosis. A low concentration of calcium inside the cochleate triggers the opening of the cylindrical cochleate structure, causing the encapsulated drug to be released[11,22,23].
Methods of preparation:
Hydrogel isolation method: The hydrogel method involves the use of two polymer solutions for cochleate preparation. Briefly, the pre-formed liposomal dispersion loaded with the drug is prepared. It is then mixed with polymer A (such as dextran, polyethylene glycol and phosphatidylserine). The prepared mixture of liposomes and polymer A is added to polymer B (such as polyvinyl pyrrolidone), which is immiscible with polymer A, forming a two-phase aqueous system. The next step involves adding a cation salt solution to the biphasic system; the cation diffuses into polymer B and then into the mixture of liposome particles and polymer A. Small size cochleate are formed because of the cationic cross-linkage of the polymer[11,24].
Emulsification-lyophilization method: In this process, cochleates are formed after the formation of multiple emulsions. The lipid is dissolved in a solvent (viz. chloroform, cyclohexane); this is considered as the oil phase. The inner water phase comprises of the solution of a binding agent and a lyophilizing agent and the buffer constitutes the outer water phase. At the initial stage, a primary emulsion with submicron particle size is prepared with a probe sonicator’s aid using oil phase and inner water phases. This emulsion is further added as a dispersed phase to the outer water phase and gently emulsified to form a double emulsion, which is then subjected to lyophilization. Cochleates are formed on rehydration of lyophilized powder[10].
Trapping method: The trapping method includes the dropwise addition of calcium chloride solution to the pre-formed liposomes, which are formed by mixing water and phospholipids. It is the most common method for nanocochleate preparation[21,25].
Dialysis method: In this method, nanocochleates are prepared using a process involving dialysis. The first approach is called ‘Liposomes before cochleates dialysis method’. It involves a mixture of lipids and detergent. It is a two-step process that involves the removal of detergents from the mixture to form lipid vesicles. These vesicles are then again subjected to dialysis in the presence of a binding agent to form cochleates. The second approach is called the ‘Direct cochleates dialysis method’. This method involves removing detergent directly by dialysis against calcium chloride solution. The process probably does not include the formation of liposome intermediate[10].
Delivery of drugs:
Several drugs have been investigated for their use in Nanocochleate-based therapy, which are summarised in Table 1.
| S No. | Name of drug encapsulated | Biologic utility | Comments | Reference |
|---|---|---|---|---|
| 1 | Amikacin | Antibiotic | Better in vitro drug release profile along with increased stability | [16] |
| 2 | Amphotericin B and Miltefosine | Anti-leishmania | Oral cochleates demonstrated potent efficacy | [29] |
| 3 | Andrographolide | Hepatoprotective | Improved drug release with higher encapsulation efficiency | [22] |
| 4 | Andrographolide | Anti-cancer | 1.18-fold increase in oral bioavailability along with the reduction in accumulation in other organs | [14] |
| 5 | Artemisinin | Malaria | Higher encapsulation efficiency and controlled release action | [30] |
| 6 | Artemisinin | Malaria | Sustained in vitro release with better permeability and in vitro bioavailability | [31] |
| 7 | Curcumin | Breast cancer | Improved cytotoxicity | [32] |
| 8 | Cyclosporine | Immuno-suppressant | Enhanced oral bioavailability by 3-folds | [33] |
| 9 | Erlotinib and Dexketoprofen | Non-small-cell lung cancer | Higher entrapment efficiency | [34] |
| 10 | Glipizide | Diabetes | Increased oral bioavailability | [35] |
| 11 | Hydroxy-camptothecin | Hepatic cancer | Enhanced oral bioavailability | [36] |
| 12 | Imatinib and Dexketoprofen | Fibrosarcoma | Enhanced drug release and higher efficacy | [37] |
| 13 | Nelfinavir Mesylate | Anti-viral | Oral bioavailability increased by 3.8 fold compared with dispersion form | [38] |
| 14 | Paclitaxel | Colon cancer | oral route showed a 25-fold reduction in the tumor growth | [39] |
| 15 | Quercetin | Breast cancer | Enhanced encapsulation efficiency | [40] |
| 16 | Raloxifene | Breast cancer | Increased anti-tumor action along with enzyme inhibition | [19] |
| 17 | Resveratrol | Diabetes mellitus | Significant decrease in glucose levels | [41] |
| 18 | Rifampicin | Anti-Tubercular | Increased permeability compared with pure drug | [42] |
| 19 | Sorafenib Tosylate | Hepatocellular carcinoma | 2.18-fold increase in the oral bioavailability | [43] |
| 20 | Trans-Resveratrol | Hepatocellular carcinoma | Enhanced oral bioavailability and anti-cancer activity | [44] |
| 21 | Thyme oil | Increased antioxidant activity | [45] | |
| 22 | Vitamin D3 | Osteoporosis | Increased bioavailability and osteoprotective effect | [46] |
Table 1: List of Drugs benefitted by Nanocochleates.
Drug-Phospholipid Complex
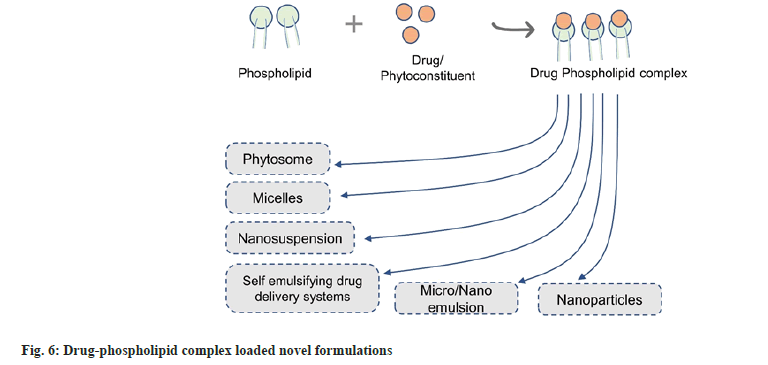
The drug-phospholipid complex is a colloidal dispersion in which the active agent is covalently bound to phospholipid. Phosphatidylcholine is primarily employed to form the drug-phospholipid complex. Choline phospholipids are present in abundance in eukaryotic cells. Initially, phospholipid complexes were studied to enhance the bioavailability of phytoconstituents. Later they were found to be effective in increasing the bioavailability of biopharmaceutical classification system class II and IV drugs[6,26]. Phospholipid-complexation can be used as an effective strategy for delivering both naturally occurring agents as well as synthetic drugs, frequently referred to as phytosome and pharmacosome, respectively[27]. The complexes formed can further be incorporated into various delivery systems such as microemulsions, nanosuspensions, etc (fig. 5). In this type of nano-drug release system, the dual characteristics of the drug-phospholipid complex and nano-preparation are present, which could not only enhance the solubility, stability and bioavailability of the drug but also reduce the dose and toxic side effects. It may also be beneficial in achieving targeted drug delivery (fig. 6).
If the absorption of drugs is having limited dissolution or permeation, their bioavailability can be increased by forming phospholipid complexes. Various characterization techniques include differential calorimetric studies, transmission electron microscopy, fourier transform infrared spectroscopy, nuclear magnetic resonance spectroscopy and scanning electron microscopy.
Mechanism of complex formation:
The polyphenolic compounds or drug molecule forms chemical bonds with the phospholipid molecules. Free carboxylic or active hydrogen atom-like amino, hydroxyl groups is esterified with the hydroxyl group of the phospholipid, which results in the formation of a drug phospholipid complex. A spacer chain may be used to promote complex formation. The complex possesses both hydrophilic and lipophilic properties. They enhance the bioavailability of drugs by facilitating the migration of drugs across the cell membrane and tissues[28].
Methods of preparation:
Solvent evaporation: One of the most well-known methods used for the preparation of drug-phospholipid complex is the solvent evaporation method. In this method, the compound of interest and phospholipids are dissolved in the solvent or solvent mixture, refluxed for a certain period of time and then evaporated using the rota evaporator. The rota evaporator solvent evaporation operates on the principle of reducing the boiling point by vacuum application, followed by rotation in order to increase the solution’s heating surface area[6].
Salting out anti-solvent precipitation method: In this method for complex preparation, both the phospholipid and drug are added in a flask containing a common organic solvent. The mixture is refluxed at desired temperature using a magnetic stirrer. The solution is later concentrated and an anti-solvent like n-hexane is added. Phospholipid complex is obtained as a precipitate which is further filtered under vacuum[6,27].
Mechanical dispersion method: In this method, the lipids dissolved in an organic solvent are brought in contact with the aqueous phase containing the drug. Initially, the phospholipid is dissolved in diethyl ether which is later slowly injected into an aqueous solution of the phytoconstituents to be encapsulated. The subsequent removal of the organic solvent under reduced pressure leads to the formation of phyto-phospholipid complex.
Others: Novel methods for the phospholipid complex preparation include supercritical fluids, which include gas anti-solvent technique, compressed anti-solvent process, supercritical anti-solvent method.
Delivery of drugs:
Drug-phospholipid complex based formulations are studied extensively in order to get the best possible benefits in drug delivery, as shown in Table 2.
| S No. | Name of drug | Delivery type | Comments | Reference |
|---|---|---|---|---|
| 1 | Aspirin | Phospholipid complex | Improved solubility and bioavailability | [47] |
| 2 | Biochanin | Nanosized phospholipid complex | Significant increase in intestinal permeability and oral bioavailability | [48] |
| 3 | Catechin | Phospholipid complex | Improved lipid solubility with sustained-release action | [49] |
| 4 | Curcumin | Phospholipid complex | Improved therapeutic efficacy and safety | [50] |
| 5 | Docetaxel | Self-micro emulsifying drug delivery system | Improved Permeability and dissolution profile | [51] |
| 6 | Embelin | Phospholipid complex | Improved solubility and dissolution profile compared with free embelin | [52] |
| 7 | Emodin | Phospholipid complex | Enhanced solubility in water and octanol | [53] |
| 8 | Erlotinib | Phospholipid complex | Enhanced bioavailability and higher in vitro cytotoxicity | [54] |
| 9 | Etoposide | Self-emulsifying drug delivery system | Enhanced drug release and oral bioavailability | [55] |
| 10 | Evodiamine | Phospholipid complex | Enhanced oral bioavailability | [56] |
| 11 | Fexofenadine | Phospholipid complex | Increased lipophilicity and intestinal permeation | [57] |
| 12 | Ibuprofen | Phospholipid complex | Increased solubility in phosphate buffer | [58] |
| 13 | Methotrexate | Self-assembled nanoparticles | Sustained release and significant cytotoxic effect than free drug | [59] |
| 14 | Mangiferin | Soft nanoparticles | Improved aqueous solubility, in vitro release and bioavailability | [60] |
| 15 | Naringenin | Phospholipid complex | Better solubility along with improved dissolution profile | [61] |
| 16 | Paclitaxel | Self-nano emulsifying drug delivery system | 2.13-fold higher bioavailability | [62] |
| 17 | Puerarin | Microemulsion | Improved bioavailability | [63] |
| 18 | Quercetin | Phospholipid complex | Increased oral bioavailability compared with pure drug | [64] |
| 19 | Rifampicin | Phospholipid complex | Enhanced oral bioavailability and solubility | [65] |
| 20 | Rutin | Nano lipid complex | Improved oral bioavailability and better hepatoprotective action | [66] |
| 21 | Silybin | Nanosuspension | Potent hepatoprotective effect | [67] |
| 22 | Sinigrin | Phytosome-complex | Reduced toxicity and enhanced activity | [68] |
| 23 | Tamoxifen | Phospholipid complex | Enhanced oral bioavailability and aqueous solubility | [69] |
Table 2: Investigations on Drug-Phospholipid Complex for Enhanced Drug Delivery.
Conclusion
An increase in the number of cancer cases has driven researchers to find novel therapies for fighting cancer over the last few decades. Among the new modes of treatments, nanosystems, especially phospholipid-based systems, have gained attention because of their unique advantages such as biocompatibility, small size and high surface area. Phospholipid-based formulations have emerged as a boon for improving the bioavailability of anti-cancer agents. Many of the anti-cancer agents suffer from low oral bioavailability, therefore to overcome the limitations of such drug molecules, promising outcomes of these strategies give additional research potential. Furthermore, these strategies can have the perks of being cost-effective as they are easy to formulate compared with other delivery systems. These can serve as platform technologies to enhance the clinical effectiveness of drugs that are potent but difficult to deliver orally. These strategies could be seen as an alternative to existing delivery systems with improved efficiency and bioavailability. Nonetheless, given the growing interest in oral drug delivery, these approaches could see a significant rise in commercial formulations in the near future.
Conflict of interest:
The authors have no conflicts of interest regarding this investigation.
References
- Tariq M, T Singh A, Iqbal Z, J Ahmad F, Talegaonkar S. Investigative approaches for oral delivery of anticancer drugs: A patent review. Recent Pat Drug Deliv Formul 2016;10(1):24-43.
[Crossref] [Google Scholar] [PubMed]
- Gupta M, Sharma V, Chauhan NS. Nanotechnology for oral delivery of anticancer drugs: An insight potential. Nanostruct Oral Med 2017:467-510.
- van Hoogevest P, Wendel A. The use of natural and synthetic phospholipids as pharmaceutical excipients. Eur J Lipid Sci Technol 2014;116(9):1088-107.
[Crossref] [Google Scholar] [PubMed]
- Singh RP, Gangadharappa HV, Mruthunjaya K. Phospholipids: Unique carriers for drug delivery systems. J Drug Deliv Sci Technol 2017;39:166-79.
[Crossref] [Google Scholar] [PubMed]
- Khan J, Alexander A, Saraf S, Saraf S. Recent advances and future prospects of phyto-phospholipid complexation technique for improving pharmacokinetic profile of plant actives. J Control Release 2013;168(1):50-60.
[Crossref] [Google Scholar] [PubMed]
- Kuche K, Bhargavi N, Dora CP, Jain S. Drug-phospholipid complex-a go through strategy for enhanced oral bioavailability. AAPS PharmSciTech 2019;20(2):43.
[Crossref] [Google Scholar] [PubMed]
- van Hoogevest P. Review: An update on the use of oral phospholipid excipients. Eur J Pharm Sci 2017;108:1-2.
[Crossref] [Google Scholar] [PubMed]
- Teixeira MC, Carbone C, Souto EB. Beyond liposomes: Recent advances on lipid based nanostructures for poorly soluble/poorly permeable drug delivery. Prog Lipid Res 2017;68:1-1.
[Crossref] [Google Scholar] [PubMed]
- Zhang L, Wang S, Zhang M, Sun J. Nanocarriers for oral drug delivery. J Drug Target 2013;21(6):515-27.
[Crossref] [Google Scholar] [PubMed]
- Nagarsekar K. Cochleates: New insights into drug delivery system (Doctoral dissertation); 2016.
- Pawar A, Bothiraja C, Shaikh K, Mali A. An insight into cochleates, a potential drug delivery system. RSC Adv 2015;5(99):81188-202.
- Nagarsekar K, Zma J. Recent advances and developments in cochleate technology. Nanomed Nanotechnol 2017;2(2):000119.
- Shanmugam T, Banerjee R. Nanostructured self-assembled lipid materials for drug delivery and tissue engineering. Ther Deliv 2011;2(11):1485-516.
[Crossref] [Google Scholar] [PubMed]
- Ahiwale RJ, Chellampillai B, Pawar AP. Investigation of 1, 2-Dimyristoyl-Sn-Glycero-3-Phosphoglycerol-Sodium (DMPG-Na) Lipid with various metal cations in nanocochleate preformulation: Application for Andrographolide oral delivery in cancer therapy. AAPS PharmSciTech 2020;21(7):279.
[Crossref] [Google Scholar] [PubMed]
- Syed UM, Woo AF, Plakogiannis F, Jin T, Zhu H. Cochleates bridged by drug molecules. Int J Pharm 2008;363(1-2):118-25.
[Crossref] [Google Scholar] [PubMed]
- Judeh Z. Insights into the mechanism of formation of non-conventional cochleates and its impact on their functional properties. J Mol Liq 2021;335:116249.
- Bozó T, Wacha A, Mihály J, Bóta A, Kellermayer MS. Dispersion and stabilization of cochleate nanoparticles. Eur J Pharm Biopharm 2017;117:270-5.
- Mannino R, Gould-Fogerite S, Krause-Elsmore S, Delmarre D, Lu R, inventors; University of Medicine, Dentistry of New Jersey, Biodelivery Sciences Inc, assignee. Novel encochleation methods, cochleates and methods of use. United States patent application US 10/822,230; 2005.
- Ağardan N, Değim Z, Yılmaz Ş, Altıntaş L, Topal T. The effectiveness of raloxifene-loaded liposomes and cochleates in breast cancer therapy. AAPS PharmSciTech 2016;17(4):968-77.
[Crossref] [Google Scholar] [PubMed]
- Poudel I, Ahiwale R, Pawar A, Mahadik K, Bothiraja C. Development of novel biotinylated chitosan-decorated docetaxel-loaded nanocochleates for breast cancer targeting. Artif Cells Nanomed Biotechnol 2018;46(2):229-40.
[Crossref] [Google Scholar] [PubMed]
- Bhosale RR, Gangadharappa HV, Gowda DV, Osmani RA, Vaghela R. A review on nanocochleates: The inimitable nanoparticulate drug carriers. Adv Sci Eng Med 2017;9(5):359-69.
- Asprea M, Tatini F, Piazzini V, Rossi F, Bergonzi MC, Bilia AR. Stable, monodisperse and highly cell-permeating nanocochleates from natural soy lecithin liposomes. Pharmaceutics 2019;11(1):34.
[Crossref] [Google Scholar] [PubMed]
- Shende P, Khair R, Gaud RS. Nanostructured cochleates: A multi-layered platform for cellular transportation of therapeutics. Drug Dev Ind Pharm 2019;45(6):869-81.
[Crossref] [Google Scholar] [PubMed]
- Yücel Ç, Altintaş Y, Değim Z, Yılmaz Ş, Arsoy T, Altıntaş L, et al. Novel approach to the treatment of diabetes: Embryonic stem cell and insulin-loaded liposomes and nanocochleates. J Nanosci Nanotechnol 2019;19(7):3706-19.
[Crossref] [Google Scholar] [PubMed]
- Tilawat M, Bonde S. Nanocochleates: A potential drug delivery system. J Mol Liq 2021;334:116115.
- Semalty A. Cyclodextrin and phospholipid complexation in solubility and dissolution enhancement: A critical and meta-analysis. Exp Opin Drug Deliv 2014;11(8):1255-72.
[Crossref] [Google Scholar] [PubMed]
- Gnananath K, Nataraj KS, Rao BG. Phospholipid complex technique for superior bioavailability of phytoconstituents. Adv Pharm Bull 2017;7(1):35-42.
[Crossref] [Google Scholar] [PubMed]
- Pandita A, Sharma P. Pharmacosomes: An emerging novel vesicular drug delivery system for poorly soluble synthetic and herbal drugs. ISRN Pharm 2013;2013:e348186.
[Crossref] [Google Scholar] [PubMed]
- Pham TT, Barratt G, Michel JP, Loiseau PM, Saint-Pierre-Chazalet M. Interactions of antileishmanial drugs with monolayers of lipids used in the development of amphotericin B–miltefosine-loaded nanocochleates. Colloids and Surf B Biointerfaces 2013;106:224-33.
[Crossref] [Google Scholar] [PubMed]
- Judeh Z. Alginate-coating of artemisinin-loaded cochleates results in better control over gastro-intestinal release for effective oral delivery. J Drug Deliv Sci Technol 2019;52:27-36.
- Wong PW, Judeh Z. Continuous, high-throughput production of artemisinin-loaded supramolecular cochleates using simple off-the-shelf flow focusing device. Mater Sci Eng C 2020;108:110410.
[Crossref] [Google Scholar] [PubMed]
- Nadaf SJ, Killedar SG. Curcumin nanocochleates: Use of design of experiments, solid state characterization, in vitro apoptosis and cytotoxicity against breast cancer MCF-7 cells. J Drug Deliv Sci Technol 2018;47:337-50.
- Liu M, Zhong X, Yang Z. Chitosan functionalized nanocochleates for enhanced oral absorption of cyclosporine A. Sci Rep 2017;7(1):41322.
[Crossref] [Google Scholar] [PubMed]
- Çoban Ö, Değim Z. Development of nanocochleates containing erlotinib HCl and dexketoprofen trometamol and evaluation of in vitro characteristic properties. Turkish J Pharm Sci 2018;15(1):16-21.
[Google Scholar] [PubMed]
- Jain A, Kamble R, Patil S. Electrospray technology as a probe for single step fabrication of glipizide loaded nanocochleates with enhanced bioavailability. J Dispers Sci Technol 2021:1-9.
- Zhong X, Chen B, Yang Z. Nanocochleates as the potential delivery systems for oral antitumor of hydroxycamptothecin. J Biomed Nanotechnol 2018;14(7):1339-46.
[Crossref] [Google Scholar] [PubMed]
- Çoban Ö, Değim Z, Yılmaz Ş, Altıntaş L, Arsoy T, Sözmen M. Efficacy of targeted liposomes and nanocochleates containing imatinib plus dexketoprofen against fibrosarcoma. Drug Dev Res 2019;80(5):556-65.
[Crossref] [Google Scholar] [PubMed]
- Belubbi T, Shevade S, Dhawan V, Sridhar V, Majumdar A, Nunes R, et al. Lipid architectonics for superior oral bioavailability of nelfinavir mesylate: Comparative in vitro and in vivo assessment. AAPS PharmSciTech 2018;19(8):3584-98.
[Crossref] [Google Scholar] [PubMed]
- Shanmugam T, Joshi N, Ahamad N, Deshmukh A, Banerjee R. Enhanced absorption, and efficacy of oral self-assembled paclitaxel nanocochleates in multi-drug resistant colon cancer. Int J Pharm 2020;586:119482.
[Crossref] [Google Scholar] [PubMed]
- Sonwane SA, Chavan MJ, Hase DP, Chumbhale DS, Ambare AS, Bodakhe YT. Preparation, characterization and in vitro anticancer testing of quercetin-loaded nanocochleates. Pharm Res 2017:1-7.
- Yücel Ç, Karatoprak GŞ, Atmar A. Novel resveratrol-loaded nanocochleates and effectiveness in the treatment of diabetes. Fabad J Pharm Sci 2018;43(2):35-44.
- Yadav A, Chaudhary S. Formulation development and characterization of nanocochleates for the improvement of permeability of drug. J Adv Pharm Edu Res 2016;6(3).
- Ahiwale RJ, Chellampillai B, Pawar AP. Investigation of novel sorafenib tosylate loaded biomaterial based nano-cochleates dispersion system for treatment of hepatocellular carcinoma. J Dispersion Sci Technol 2021;43(10):1-9.
- El-Melegy MG, Eltaher HM, Gaballah A, El-Kamel AH. Enhanced oral permeability of Trans-Resveratrol using nanocochleates for boosting anticancer efficacy; in vitro and ex vivo appraisal. Eur J Pharm Biopharm 2021;168:166-83.
[Crossref] [Google Scholar] [PubMed]
- Asprea M, Leto I, Bergonzi MC, Bilia AR. Thyme essential oil loaded in nanocochleates: Encapsulation efficiency, in vitro release study and antioxidant activity. LWT 2017;77:497-502.
- Eskandarynasab M, Etemad-Moghadam S, Alaeddini M, Doustimotlagh AH, Nazeri A, Dehpour AR, et al. Novel osteoprotective nanocochleate formulation: A dual combination therapy-codelivery system against glucocorticoid induced osteoporosis. Nanomed 2020;29:102273.
[Crossref] [Google Scholar] [PubMed]
- Semalty A, Semalty M, Singh D, Rawat MS. Development and characterization of aspirin-phospholipid complex for improved drug delivery. Int J Pharm Sci Nanotechnol 2010;3(2):940-7.
- Singh SK, Rashid M, Bhalala K, Malik Y, Chaturvedi S, Raju KS, et al. A novel nanosized phospholipid complex of Biochanin A for improving oral bioavailability: Preparation and in vitro/in vivo characterizations. J Drug Deliv Sci Technol 2021;61:102254.
- Semalty A, Semalty M, Singh D, Rawat MS. Phyto-phospholipid complex of catechin in value added herbal drug delivery. J Incl Phenom Macrocycl Chem 2012;73(1):377-86.
- Khatik R, Dwivedi P, Shukla A, Srivastava P, Rath SK, Paliwal SK, et al. Development, characterization and toxicological evaluations of phospholipids complexes of curcumin for effective drug delivery in cancer chemotherapy. Drug Deliv 2016;23(3):1057-68.
[Crossref] [Google Scholar] [PubMed]
- Wang M, You SK, Lee HK, Han MG, Lee HM, Pham TM, et al. Development and evaluation of docetaxel-phospholipid complex loaded self-microemulsifying drug delivery system: Optimization and in vitro/ex vivo studies. Pharmaceutics 2020;12(6):544.
[Crossref] [Google Scholar] [PubMed]
- Pathan RA, Bhandari U. Preparation and characterization of embelin–phospholipid complex as effective drug delivery tool. J Incl Phenom Macrocycl Chem 2011;69(1):139-47.
- Singh D, Rawat MS, Semalty A, Semalty M. Emodin–phospholipid complex: A potential of herbal drug in the novel drug delivery system. J Therm Anal Calorim 2012;108(1):289-98.
- Dora CP, Kushwah V, Katiyar SS, Kumar P, Pillay V, Suresh S, et al. Improved oral bioavailability and therapeutic efficacy of erlotinib through molecular complexation with phospholipid. Int J Pharm 2017;534(1-2):1-3.
[Crossref] [Google Scholar] [PubMed]
- Wu Z, Guo D, Deng L, Zhang Y, Yang Q, Chen J. Preparation and evaluation of a self-emulsifying drug delivery system of etoposide–phospholipid complex. Drug Dev Ind Pharm 2011;37(1):103-12.
[Crossref] [Google Scholar] [PubMed]
- Tan Q, Liu S, Chen X, Wu M, Wang H, Yin H, et al. Design and evaluation of a novel evodiamine-phospholipid complex for improved oral bioavailability. AAPS PharmSciTech 2012;13(2):534-47.
[Crossref] [Google Scholar] [PubMed]
- Kaur S, Samal SK, Roy S, Sangamwar AT. Successful oral delivery of fexofenadine hydrochloride by improving permeability via phospholipid complexation. Eur J Pharm Sci 2020;149:105338.
- Amirinejad M, Davoodi J, Abbaspour MR, Akhgari A, Hadizadeh F, Badiee A. Preparation, characterization and improved release profile of ibuprofen-phospholipid association. J Drug Deliv Sci Technol 2020;60:101951.
- Li Y, Lin J, Liu G, Li Y, Song L, Fan Z, et al. Self-assembly of multifunctional integrated nanoparticles loaded with a methotrexate–phospholipid complex: Combining simplicity and efficacy in both targeting and anticancer effects. RSC Adv 2016;6(89):86717-27.
- Telange DR, Sohail NK, Hemke AT, Kharkar PS, Pethe AM. Phospholipid complex-loaded self-assembled phytosomal soft nanoparticles: Evidence of enhanced solubility, dissolution rate, ex vivo permeability, oral bioavailability, and antioxidant potential of mangiferin. Drug Deliv Transl Res 2021;11(3):1056-83.
[Crossref] [Google Scholar] [PubMed]
- Semalty A, Semalty M, Singh D, Rawat MS. Preparation and characterization of phospholipid complexes of naringenin for effective drug delivery. J Incl Phenom Macrocycl Chem 2010;67(3):253-60.
- Ding D, Sun B, Cui W, Chen Q, Zhang X, Zhang H, et al. Integration of phospholipid-drug complex into self-nanoemulsifying drug delivery system to facilitate oral delivery of paclitaxel. Asian J Pharm Sci 2019;14(5):552-8.
[Crossref] [Google Scholar] [PubMed]
- Wu JY, Li YJ, Han M, Hu XB, Yang L, Wang JM, et al. A microemulsion of puerarin–phospholipid complex for improving bioavailability: Preparation, in vitro and in vivo evaluations. Drug Dev Indu Pharm 2018;44(8):1336-41.
[Crossref] [Google Scholar] [PubMed]
- Singh D, SM Rawat M, Semalty A, Semalty M. Quercetin-phospholipid complex: An amorphous pharmaceutical system in herbal drug delivery. Curr Drug Discov Technol 2012;9(1):17-24.
[Crossref] [Google Scholar] [PubMed]
- Singh C, Bhatt TD, Gill MS, Suresh S. Novel rifampicin–phospholipid complex for tubercular therapy: Synthesis, physicochemical characterization and in vivo evaluation. Int J Pharm 2014;460(1-2):220-7.
[Crossref] [Google Scholar] [PubMed]
- Ravi GS, Charyulu RN, Dubey A, Prabhu P, Hebbar S, Mathias AC. Nano-lipid complex of rutin: Development, characterisation and in vivo investigation of hepatoprotective, antioxidant activity and bioavailability study in rats. AAPS PharmSciTech 2018;19(8):3631-49.
[Crossref] [Google Scholar] [PubMed]
- Chi C, Zhang C, Liu Y, Nie H, Zhou J, Ding Y. Phytosome-nanosuspensions for silybin-phospholipid complex with increased bioavailability and hepatoprotection efficacy. Eur J Pharm Sci 2020;144:105212.
[Crossref] [Google Scholar] [PubMed]
- Mazumder A, Dwivedi A, Fox LT, Brümmer A, Du Preez JL, Gerber M, et al. In vitro skin permeation of sinigrin from its phytosome complex. J Pharm Pharmacol 2016;68(12):1577-83.
[Crossref] [Google Scholar] [PubMed]
- Jena SK, Singh C, Dora CP, Suresh S. Development of tamoxifen-phospholipid complex: Novel approach for improving solubility and bioavailability. Int J Pharm 2014;473(1-2):1-9.
[Crossref] [Google Scholar] [PubMed]