- *Corresponding Author:
- S. H. Kim
Cmri, Department of pharmacology, School of medicine, Kyungpook national university, Daegu-700 422
E-mail: shkim72@knu.ac.kr
| Date of Submission | 29 May 2013 |
| Date of Revision | 25 September 2013 |
| Date of Acceptance | 03 October 2013 |
| Indian J Pharm Sci 2013; 75(6): 664-671 |
Abstract
Allergic inflammatory diseases such as food allergy, asthma, sinusitis and atopic dermatitis are increasing worldwide. This study examined the effects of aqueous extract of Mosla punctulata on mast cell-mediated allergic inflammation and studied the possible mechanism of action. Aqueous extract of Mosla punctulata inhibited compound 48/80-induced systemic and immunoglobulin E-mediated local anaphylaxis and it also reduced intracellular calcium level and down-streamed histamine release from mast cells. In addition, aqueous extract of Mosla punctulata decreased gene expression and secretion of tumour necrosis factor alpha, an important proinflammatory cytokine, in mast cells. The inhibitory effect on tumour necrosis factor alpha expression was nuclear factor kappa B dependent. The results indicate that aqueous extract of Mosla punctulata inhibited mast cell-mediated allergic inflammatory reaction by suppressing histamine release and expression of tumour necrosis factor alpha, and involvement of calcium and nuclear factor kappa B in these effects. Hence it can be concluded that, the aqueous extract of Mosla punctulata might be a possible therapeutic candidate for allergic inflammatory disorders.
Keywords
Mosla punctulata, allergic inflammation, mast cells, histamine, tumor necrosis factor-a
Mast cells are effector cells in the initiation of inflammatory reactions associated with allergic disorders such as asthma, atopic dermatitis and sinusitis. In vertebrates, mast cells are widely distributed throughout vascularised tissues, particularly near surfaces exposed to the external environment, including the skin, airways, conjunctiva and gastrointestinal tract [1].
Upon exposure of an allergen, which is recognized by immunoglobulin E (IgE), antibodies bound to the high affinity IgE receptor (FcεRI) are expressed on mast cell surface, aggregation of FcεRI triggers a intracellular signalling process [2]. Phosphorylation of Src family kinases (Lyn, Syk and Fyn) and phospholipase C-γ are followed by calcium mobilization and activation of protein kinase C and nuclear factor kappa B (NF-κB) [3]. As a result, mast cells secrete both preformed and newly synthesised mediators including histamine, eicosanoids, proteases and several proinflammatory cytokines. Using these products, mast cells contribute not only immediate type hypersensitivity but also late reaction, like inflammatory responses.
Histamine is a major mediator leading to immediatetype hypersensitivity, which is released from activated mast cells. Histamine causes vasodilation and increases permeability of vessels near the allergic site. Thus, blood fluids including leukocytes, which participate in immune responses, enter the area causing swelling. By the release of chemotactic and proinflammatory mediators especially tumour necrosis factor alpha (TNF-α), mast cells affect on the late phase responses of an allergic inflammation [3]. The proinflammatory mediators released from mast cells change terminal microenvironment and attract neutrophils and basophils. Therefore reduction of these mediators is one of key indicators of relieved allergic inflammatory symptoms.
Mosla punctulata Nakai (Lamiaceae) is widely distributed in Korea, China, Taiwan and Japan. It was used in traditional medicine for treatment of skin itch and bronchitis. This crude drug contains essential oils, mainly, l-thujone and d-sabinene [4]. Thujone is known to inhibit tumour by increasing NK cells and cytotoxic T cell activity [5]. However, the antiallergic inflammatory effect of M. punctulata has not been studied yet. The objective of this study is to elucidate the effect of aqueous extract of M. punctulata (AEMP) on the allergic inflammation and to define the underlying mechanisms of these effects using in vivo and in vitro models.
Materials and Methods
The original stock of male Imprinting Control Region mice (6 weeks) was purchased from the Dae-Han Biolink (Daejeon, Korea). The animals were housed five per cage in a laminar air flow room maintained under a temperature of 22±2° and relative humidity of 55±5% throughout the study. The care and treatment of the mice were in accordance with the guidelines established by the Public Health Service Policy on the Humane Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee.
Compound 48/80, antidinitrophenyl (DNP) IgE, DNP-human serum albumin (HSA), phorbol 12-myristate 13-acetate (PMA) and calcium ionophore A23187 were purchased from Sigma, St. Louis, MO, USA. The human mast cell line (HMC-1) was grown in Iscove’s media (Life Technologies, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS) at 37° in 5% CO2. The passage ranging 4-8 of HMC-1 was used throughout the study.
Preparation of AEMP
The plant of M. punctulata was collected in Wanju, South Korea, on the October 18, 2010. The voucher specimen (WSP-10-78) was deposited in the Herbarium of the College of Pharmacy, Woosuk University. M. punctulata was ground (400 g, 30 s) at room temperature using a Micro Hammer-Cutter mill (Culatti Co., Zurich, Switzerland). The particle size was 0.5-2 mm after grinding. The plant sample (100 g) was extracted twice with purified water (500 ml) at 70° for 5 h in a water bath. The extract was filtered through Whatman no. 1 filter paper and the filtrate was lyophilised using a 0.45 μm syringe filter. The yield of dried extract from crude materials was about 9%. The dried extract was dissolved in saline or Tyrode buffer A (HEPES 10 mM, NaCl 130 mM, KCl 5 mM, CaCl2 1.4 mM, MgCl2 1 mM, glucose 1.4 mM, 0.1% bovine serum albumin) before use.
Systemic anaphylaxis
Mice were given an intraperitoneal injection of 8 mg/kg of the mast cell degranulator compound 48/80. AEMP was administered intraperitoneally at doses of 1-1000 mg/kg 1 h before the injection of compound 48/80 (n=10/group). Mortality was monitored for 1 h after induction of anaphylactic shock [6]. After the mortality test, blood was obtained from the heart of each mouse to measure serum histamine content.
Passive cutaneous anaphylaxis
An IgE-dependent cutaneous reaction was carried out as described previously [7]. The passive cutaneous anaphylaxis (PCA) reaction was generated by sensitising skin with an intradermal injection of antiDNP IgE followed 48 h later with an injection of DNP–HSA into the mouse tail vein. Briefly, mice were injected intradermally with 0.5 μg of antiDNP IgE. After 48 h, each mouse (n=10/group) received an injection of 1 μg of DNP-HSA containing 4% Evans blue (1:4) via the tail vein. Thirty minutes after the challenge, the mice were killed and the dorsal skin (diameter, 1 cm) was removed for measurement of the pigmented area. The amount of dye was then determined colorimetrically after extraction with 1 ml of 1 M KOH and 9 ml of mixture of acetone and phosphoric acid (5:13). The absorbent intensity of the extraction was measured at 620 nm using spectrophotometer (Shimadzu, UV-1201, Japan).
Histamine level
Histamine contents in serum and HMC-1 cells were measured by o-phthaldialdehyde spectrofluorometric procedure as previously described [8]. HMC-1 cells (1×106 cells/ml) were preincubated with AEMP for 30 min, and then incubated for 30 min with PMA (20 nM) and calcium ionophore A23187 (1 μM) (PMACI) [7]. The cells were separated from the released histamine by centrifugation at 400 g for 5 min at 4°. The blood from the mice was centrifuged at 400 g for 10 min and the serum was withdrawn to measure histamine content.
Intracellular calcium
The intracellular calcium was measured with the use of the fluorescence indicator Fluo-3/AM (Molecular Probes, Eugene, OR, USA). HMC-1 cells were preincubated with Fluo-3/AM for 30 min at 37°. After washing the dye from the cell surface, the cells were pretreated with AEMP for 30 min prior to PMA (20 nM) and calcium ionophore A23187 (1 μM) treatment. The fluorescent intensity was recorded using a fluorescent plate reader (Molecular Devices, Sunnyvale, CA, USA) at an excitation of 488 nm and an emission of 515 nm.
Cell viability
In order to investigate the toxicity of AEMP, cell viability was evaluated by a colorimetric assay based on the conversion of 3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyltetrazolium bromide (MTT). MTT is transformed by mitochondrial dehydrogenases into formazan, enabling cell viability to be assessed. The cells were cultured on 1-1000 μg/ml AEMP, placed in single layers in 96-well plates, at an initial seeding density of 2×104 cells per well and cultured for 24 h. Then 20 μl of MTT solution prewarmed to 37° was added to each well and culture continued for 3 h under the same conditions. The reaction was then stopped by adding 100 μl of dimethylsulfoxide to each well. The solution was transferred to new wells and the absorbance measured at a wavelength of 570 nm using an enzyme-linked immunosorbent assay (ELISA) microplate reader.
Real-time polymerase chain reaction
The total cellular RNA was isolated from the cells (1×106/well in a 24-well plate) after stimulation with PMA (20 nM) and A23187 (1 μM) with or without AEMP for 2 h using TRI reagent (Molecular Research Center, Cincinnati, OH, USA) according to the manufacturer’s protocol. A quantitative real-time polymerase chain reaction (PCR) assay was carried out using the Thermal Cycler Dice TP850 (Takarabio Inc., Shiga, Japan). Briefly, 2 μl of cDNA (100 ng), 1 μl of sense and antisense primer solution (0.4 μM), 12.5 μl of SYBR Premix Ex Taq (Takarabio) and 9.5 μl of dH2O were mixed together to obtain a final 25 μl reaction mixture in each reaction tube. The conditions for the PCR were similar to our previous report[9]. The normalization and quantification of mRNA expression was performed using the TP850 software supplied by the manufacturer.
Enzyme-linked immunosorbent assay
The secretion of TNF-α was measured by the modification of an ELISA as described previously [10]. HMC-1 cells were cultured with media and resuspended in Tyrode buffer A. The cells were stimulated with PMACI for 8 h in the absence or presence of AEMP. The ELISA was performed by coating 96-well plates with 6.25 ng/well of monoclonal antibody with specificity for TNF-α.
Western blot
HMC-1 were washed 3 times with phosphate buffered saline (PBS) and resuspended in lysis buffer. Samples were electrophoresed using 12% sodium dodecyl sulphate-polyacrylamide gel electrophoresis, as described elsewhere [11] and then transferred to a nitrocellulose membrane. The nucleus p65 NF-κB was assayed using antiNF-κB (p65) antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Immunodetection was done by using super signal west pico chemiluminescent substrate (Thermo scientific, Waltham, MA, USA).
Transient transfection and luciferase activity assay
For transient transfection, HMC-1 cells were seeded at 2×106 in 6-well plates for a day before transient transfection. The expression vectors containing the NF-κB luciferase reporter construct (pNF-κB-LUC, plasmid containing NF-κB binding site; Stratagene, Grand Island, NY, USA) were transfected with serum and antibiotics free Iscove’s medium containing 8 μl lipofectamine 2000 reagent (Invitrogen). After 5 h of incubation, medium was replaced with Iscove’s medium containing 10% FBS and antibiotics. Cells were allowed to recover at 37° for 30 h and subsequently were stimulated as indicated. Cell lysates were prepared and assay for luciferase activity using Luciferase Assay System (Promega, Madison, WI, USA), according to the manufacturer’s instructions.
Statistical analysis
Statistical analyses were performed using SAS statistical software (SAS Institute, Cary, NC, USA). Treatment effects were analysed using analysis of variance, followed by Duncan’s multiple range tests. P<0.05 was to indicate significance.
Results
Effect of AEMP on systemic and local anaphylaxis
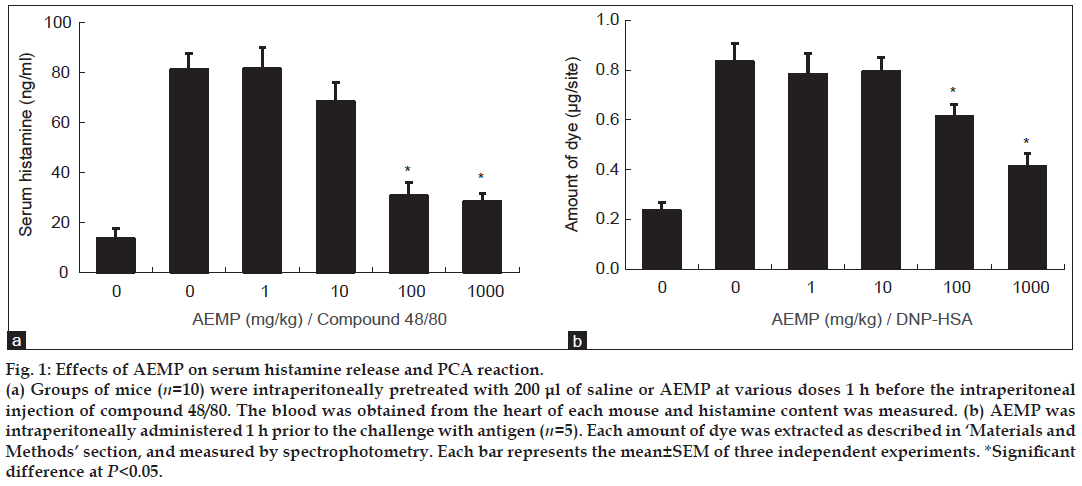
To determine the effect of AEMP in allergic reaction, an in vivo model of a systemic anaphylaxis was used. After the intraperitoneal injection of compound 48/80, the mice were monitored for 1 h, after which the mortality rate was determined. Injection of compound 48/80 (8 mg/kg) into mice induced fatal shock in 100% of animals. When AEMP was intraperitoneally pretreated at doses ranging from 1 to 1000 mg/kg for 1 h, the mortality was dose dependently reduced. AEMP at dose of 1000 mg/kg completely inhibited compound 48/80-induced fatal shock (Table 1). In addition, the mortality of mice administered with AEMP (1000 mg/kg) 5, 10, 15 and 20 min after compound 48/80 injection increased time dependently (Table 2). The effect of AEMP on the compound 48/80-induced serum histamine release was investigated as well. AEMP was intraperitoneally administered 1 h prior to the injection of compound 48/80. Injection of compound 48/80 markedly caused an increase in serum histamine release, which was inhibited by AEMP treatment in a dose-dependent manner (fig. 1a). To confirm the antiallergic effects of AEMP, we used a PCA model induced by antiDNP-IgE and DNP-HSA. To compare the amount of dye with control, the left dorsal skin of these mice was injected with saline alone. AEMP (1-1000 mg/kg) was intraperitoneally administered 1 h prior to the challenge with antigen. AEMP dosedependently inhibited PCA reaction (fig. 1b).
| AEMP treatment | Compound 48/80 | Mortality |
|---|---|---|
| (mg/kg, BW) | (mg/kg, BW) | (%) |
| 0 | 8 | 100 |
| 1 | 8 | 100 |
| 10 | 8 | 80 |
| 100 | 8 | 20 |
| 1000 | 8 | 0 |
| 1000 | 0 | 0 |
Groups of mice (n=10/group) were intraperitoneally pretreated with 200 µl of saline or AEMP at various doses 1 h before the intraperitoneal injection of compound 48/80. Mortality (%) within 1 h following compound 48/80 injection is represented as the number of dead mice×100/total number of experimental mice. AEMP=Aqueous extract of M. punctulata.
Table 1: Effect of aemp on compound 48/80 induced systemic anaphylaxis
| AEMP treatment | Compound 48/80 | Time | Mortality |
|---|---|---|---|
| (mg/kg, BW) | (mg/kg, BW) | (min) | (%) |
| 0 | 8 | 100 | |
| 1000 | 8 | 0 | 0 |
| 1000 | 8 | 5 | 20 |
| 1000 | 8 | 10 | 60 |
| 1000 | 8 | 15 | 90 |
| 1000 | 8 | 20 | 100 |
Mice (n=10/group) were intraperitoneally treated with 200 µl of saline or AEMP.
AEMP (1000 mg/kg) was given 5, 10, 15 and 20 min after the intraperitoneal injection of compound 48/80. Mortality (%) within 1 h following compound 48/80 injection is represented as the number of dead mice×100/total number of experimental mice. AEMP=Aqueous extract of M. punctulata.
Table 2: Time-dependent effects of aemp on compound 48/80-induced systemic anaphylaxis
Figure 1: Effects of AEMP on serum histamine release and PCA reaction.
(a) Groups of mice (n=10) were intraperitoneally pretreated with 200 μl of saline or AEMP at various doses 1 h before the intraperitoneal
injection of compound 48/80. The blood was obtained from the heart of each mouse and histamine content was measured. (b) AEMP was
intraperitoneally administered 1 h prior to the challenge with antigen (n=5). Each amount of dye was extracted as described in ‘Materials and
Methods’ section, and measured by spectrophotometry. Each bar represents the mean±SEM of three independent experiments. *Significant
difference at P<0.05.
Effect of AEMP on the histamine and intracellular calcium from HMC-1
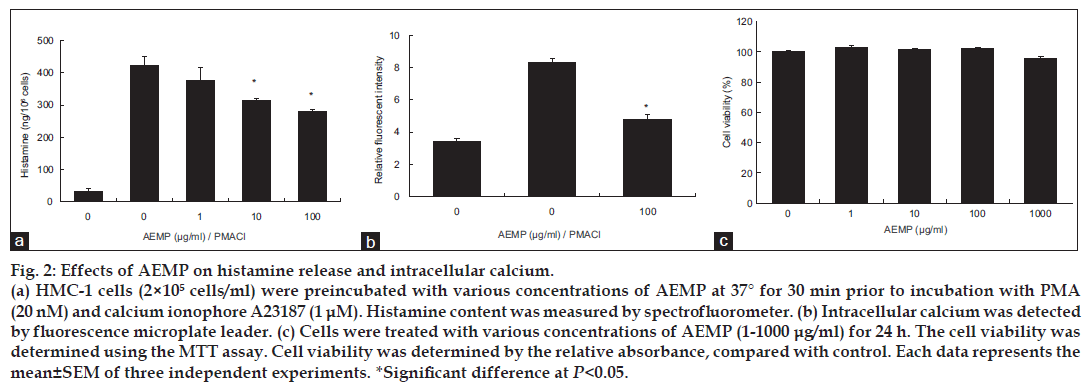
We estimated the effects of AEMP on degranulation of mast cells. Release of histamine from activated mast cells is a hallmark degranulation. HMC-1 cells released a high level of histamine when simulated with PMACI (fig. 2a). When AEMP (1-100 μg/ ml) was pretreated for 30 min, histamine level was dose-dependently inhibited in PMACI-stimulated HMC-1. Calcium movements across membranes of mast cells are critical to histamine release [12]. To investigate the mechanism responsible for the reduction of histamine after AEMP treatment, we assayed the level of intracellular calcium. When HMC-1 cells were stimulated with PMACI, the intracellular calcium level was significantly elevated (fig. 2b). Preincubation of AEMP (100 μg/ml) with HMC-1 decreased the intracellular calcium level induced by PMACI. The level of intracellular calcium was shown by the relative fluorescent intensity. The concentration and duration of AEMP treatment used in these studies had no significant effect on the cell viability of HMC-1 cells. Up to 1000 μg/ml of AEMP did not show cytotoxicity (fig. 2c).
Figure 2: Effects of AEMP on histamine release and intracellular calcium.
(a) HMC-1 cells (2×105 cells/ml) were preincubated with various concentrations of AEMP at 37° for 30 min prior to incubation with PMA (20 nM) and calcium ionophore A23187 (1 μM). Histamine content was measured by spectrofluorometer. (b) Intracellular calcium was detected by fluorescence microplate leader. (c) Cells were treated with various concentrations of AEMP (1-1000 μg/ml) for 24 h. The cell viability was determined using the MTT assay. Cell viability was determined by the relative absorbance, compared with control. Each data represents the mean±SEM of three independent experiments. *Significant difference at P<0.05.
Effect of AEMP on the expression and secretion of TNF-α
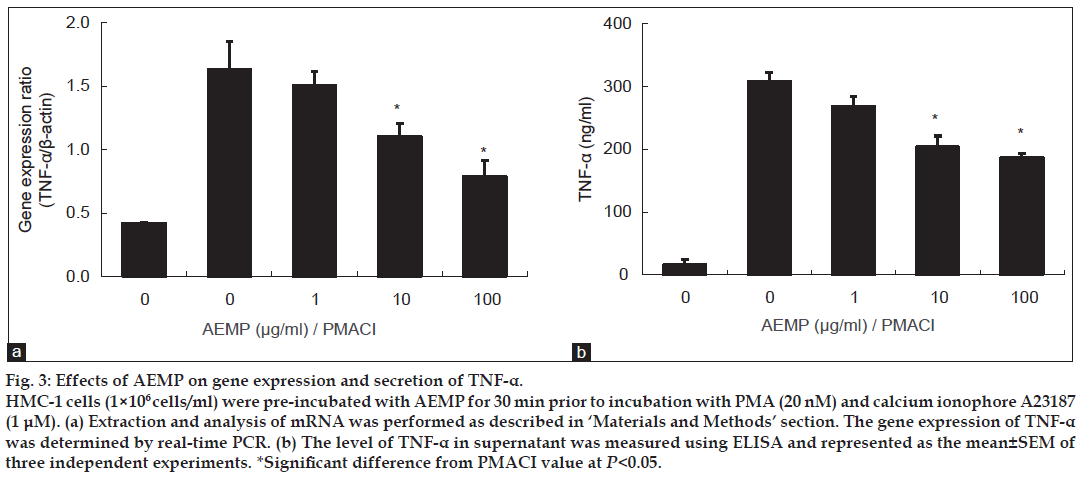
HMC-1 cell line is a useful model for researching cytokine activation pathway [13,14]. We investigated the inhibitory effect of AEMP on the expression of proinflammatory cytokine, TNF-α. Previously, we reported that gene expression of TNF-α was peaked at 4 h after treatment of PMACI [15]. HMC-1 cells were stimulated by PMACI during 4 h, and the cells were preincubated with AEMP for 30 min. As shown in fig. 3a, AEMP dose-dependently inhibited PMACI-induced gene expression of TNF-α. To confirm the correlation of mRNA expression with protein secretion, we measured the level of TNF-α by ELISA. When the HMC-1 cells were stimulated with PMACI for 8 h, the secretion of cytokines was remarkably induced. Secretion of TNF-α was significantly inhibited by AEMP in PMACI-stimulated HMC-1 (fig. 3b).
Figure 3: Effects of AEMP on gene expression and secretion of TNF-α.
HMC-1 cells (1×106 cells/ml) were pre-incubated with AEMP for 30 min prior to incubation with PMA (20 nM) and calcium ionophore A23187 (1 μM). (a) Extraction and analysis of mRNA was performed as described in ‘Materials and Methods’ section. The gene expression of TNF-α was determined by real-time PCR. (b) The level of TNF-α in supernatant was measured using ELISA and represented as the mean±SEM of three independent experiments. *Significant difference from PMACI value at P<0.05.
Effect of AEMP on the NF-κB
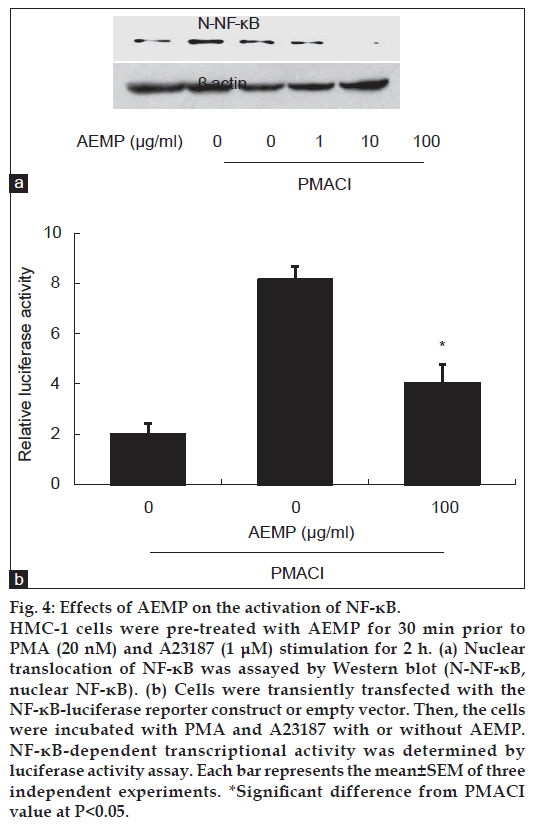
To investigate the intracellular mechanism responsible for the inhibitory effect of AEMP on the expression of TNF-α, we examined the effect of AEMP on the activation of transcription factors, NF-κB. NF-κB is an important transcriptional regulator of TNF-α and plays a crucial role in immune and inflammatory responses [16]. Stimulation of HMC-1 with PMACI induced the nuclear translocation of p65 NF-κB after 2 h of incubation. AEMP inhibited the PMACIinduced nuclear translocation of NF-κB (fig. 4a). To confirm the inhibitory effect of AEMP on the NF-κB activation, we examined the effect of AEMP on NF-κB-dependent gene reporter assay. HMC-1 cells were transiently transfected with a NF-κB-luciferase reporter construct or an empty vector. Exposure of cells to PMACI increased the luciferase activity in the cells transfected with NF-κB-luciferase reporter construct (fig. 4b). AEMP significantly reduced PMACI-induced luciferase activity.
Figure 4: Effects of AEMP on the activation of NF-κB.
HMC-1 cells were pre-treated with AEMP for 30 min prior to PMA (20 nM) and A23187 (1 μM) stimulation for 2 h. (a) Nuclear translocation of NF-κB was assayed by Western blot (N-NF-κB, nuclear NF-κB). (b) Cells were transiently transfected with the NF-κB-luciferase reporter construct or empty vector. Then, the cells were incubated with PMA and A23187 with or without AEMP. NF-κB-dependent transcriptional activity was determined by luciferase activity assay. Each bar represents the mean±SEM of three independent experiments. *Significant difference from PMACI value at P<0.05.
Discussion
Anaphylaxis is a life-threatening syndrome induced by a sudden systemic release of inflammatory mediators such as histamine, various cytokines and lipidderived mediators [17]. We showed that AEMP reduces mast cell-mediated allergic inflammatory reactions using in vivo and in vitro models. AEMP inhibited compound 48/80-induced systemic allergic reaction. In addition, AEMP administered mice were protected from IgE-mediated PCA, which is one of the most important in vivo models of anaphylaxis in a local allergic reaction. These results suggest that AEMP might be useful in the treatment of allergic disease particularly skin reactions.
Mast cells function as a key mediator of allergic inflammation. After allergen exposure, mast cells are activated by cross-linking of adjacent IgE, aggregation of FcεRI triggers intracellular signalling process that results in the degranulation and secretion of histamine, protease, cytokine and chemokine. These events cause vasodilation, vascular permeability and contraction of bronchial smooth muscle. After several hours from allergen exposure various inflammatory cells, such as T cells, eosinophils, macrophages and basophils, are activated and migrate to the allergic region. These responses are mediated by mast cell products [3]. Mast cell degranulation is preceded by the calcium influx. Calcium leads the granule membrane fusion via the SNAP receptor protein and secretory carrier membrane protein, after which granules are broken up [18]. Calcium is a critical factor for the degranulation of mast cells. Calcium movements across membranes of mast cells represent a major target for effective antiallergic drugs, as these are essential events linking stimulation to secretion [19]. Our results showed that AEMP decreased histamine and intracellular calcium in mast cells. These results suggest that AEMP inhibits the degranulation of mast cells by the decreasing of intracellular calcium level.
The HMC-1 cell line is one of the useful cells for studying cytokine activation pathways [16]. The various types of cytokines produced by HMC-1 with PMACI stimulation supports the well recognised role of mast cells in immediate type hypersensitivity. TNF-α is a major cytokine stored and released by mast cells. It promotes inflammation, leukocyte infiltration, chemotaxis of neutrophils and T cells [20]. TNF-α is also involved in eosinophil survival, and thereby contributes to chronic inflammation [21]. Therefore, the inhibition of TNF-α is one of the main points of reduced allergic inflammatory symptom. In the present study, AEMP inhibited the gene expression and secretion of TNF-α in PMACI-stimulated mast cells. This result suggests that the antiallergic inflammatory effect of AEMP results from its inhibition of TNF-α from mast cells.
Expression of TNF-α is regulated by the transcription factor, NF-κB. NF-κB regulates the expression of multiple inflammatory and immune genes and plays a critical role in chronic inflammatory diseases. The role of NF-κB and its regulation of cytokine production in allergic inflammation has been characterised [22]. In PMACI-stimulated mast cells, AEMP decreased the nuclear translocation of NF-κB and NF-κBdependent gene expression. These results demonstrated that AEMP attenuates activation of NF-κB and downstream TNF-α expression.
Intracellular calcium is crucially involved in gene transcription of inflammatory cytokines as well. Several reports have demonstrated associations between intracellular calcium and inflammatory cytokine production from mast cells [23,24]. Depletion of intracellular calcium with various calcium blockers inhibits IgE-induced TNF-α and IL-6 production in mast cells. Furthermore, NF-κB pathway mediates these effects. Because of the reducing effect of AEMP on the intracellular calcium, we suggest that one possible pathway of the inhibitory effect of AEMP on the TNF-α is mediated by the reduction of intracellular calcium in mast cells.
In summary, the present study demonstrates that AEMP significantly reduced mast cell-mediated allergic inflammation in in vivo and in vitro models. We suggest that AEMP reduces histamine release by the modulation of intracellular calcium. AEMP inhibits expression of inflammatory cytokines by the suppression of NF-κB activation. Because we used the whole AEMP, not a purified single compound, the biological effects of the individual active components are not clear at this time. The effort to identify the active components from AEMP in allergic inflammatory symptom is ongoing in our laboratory. However, the results presented in this report give an insight into the mechanism responsible for antiallergic inflammatory activity of AEMP and evidence that AEMP could contribute to the prevention or treatment of mast cell-mediated allergic inflammatory diseases.
Acknowledgements
This study was supported by a grant of the Korea Healthcare technology R and D Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea (A111375), NRF funded by the Ministry of Science, ICT and Future Planning (2012M3A9B6055416), and NRF- 2013R1A1A4A01006557
References
- Grimbaldeston MA, Metz M, Yu M, Tsai M, Galli SJ. Effector and potential immunoregulatory roles of mast cells in IgE-associated acquired immune responses. CurrOpinImmunol 2006;18:751-60.
- Rivera J, Fierro NA, Olivera A, Suzuki R. New insights on mast cell activation via the high affinity receptor for IgE. AdvImmunol 2008;98:85-120.
- Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature 2008;454:445-54.
- Yook CS. Medicinal Plants of Korea. Seoul: Jinmyeong Publishing Co.; 1981. p. 345.
- Siveen KS, Kuttan G. Augmentation of humoral and cell mediated immune responses by Thujone. IntImmunopharmacol 2011;11:1967-75.
- Bae Y, Lee S, Kim SH. Chrysin suppresses mast cell-mediated allergic inflammation: Involvement of calcium, caspase-1 and nuclear factor-kB.ToxicolApplPharmacol 2011;254:56-64.
- Kim HH, Yoo JS, Lee HS, Kwon TK, Shin TY, Kim SH. Elsholtziaciliata inhibits mast cell-mediated allergic inflammation: Role of calcium, p38 mitogen-activated protein kinase and nuclear factor-kB.
- ExpBiol Med (Maywood) 2011;236:1070-7. Kim SH, Lee S, Kim IK, Kwon TK, Moon JY, Park WH et al. Suppression of mast cell-mediated allergic reaction by Amomumxanthiodes. Food ChemToxicol 2007;45:2138-44.
- Choi EJ, Lee S, Kim HH, Singh TS, Choi JK, Choi HG, et al. Suppression of dust mite extract and 2,4-dinitrochlorobenzene-induced atopic dermatitis by the water extract of Linderaobtusiloba. J Ethnopharmacol 2011;137:802-7.
- Kim SH, Park SB, Kang SM, Jeon H, Lim JP, Kwon TK, et al. Antiallergic effects of Teucriumjaponicum on mast cell-mediated allergy model. Food ChemToxicol 2009;47:398-403.
- Lee S, Yun HS, Kim SH. The comparative effects of mesoporous silica nanoparticles and colloidal silica on inflammation and apoptosis. Biomaterials 2011;32:9434-43.
- Eisenhut M, Wallace H. Ion channels in inflammation. Pflugers Arch 2011;461:401-21.
- Kim GY, Lee JW, Ryu HC, Wei JD, Seong CM, Kim JH. Proinflammatory cytokine IL-1beta stimulates IL-8 synthesis in mast cells via a leukotriene B4 receptor 2-linked pathway, contributing to angiogenesis. J Immunol 2010;184:3946-54.
- Shin TY, Kim SH, Suk K, Ha JH, Kim I, Lee MG, et al.Antiallergic effects of Lycopuslucidus on mast cell-mediated allergy model. Toxicol ApplPharmacol 2005;209:255-62.
- 15. Park HH, Lee S, Oh JM, Lee MS, Yoon KH, Park BH, et al.Antiinflammatory activity of fisetin in human mast cells (HMC-1). Pharmacol Res 2007;55:31-7.
- Kim SH, Jun CD, Suk K, Choi BJ, Lim H, Park S, et al. Gallic acid inhibits histamine release and proinflammatory cytokine production in mast cells. ToxicolSci 2006;91:123-31.
- Boden SR, Wesley Burks A. Anaphylaxis: A history with emphasis on food allergy. Immunol Rev 2011;242:247-57.
- Castle JD, Guo Z, Liu L. Function of the t-SNARE SNAP-23 and secretory carrier membrane proteins (SCAMPs) in exocytosis in mast cells. MolImmunol 2002;38:1337-40.
- Beaven MA, Rogers J, Moore JP, Hesketh TR, Smith GA, Metcalfe JC.
- The mechanism of the calcium signal and correlation with histamine release in 2H3 cells. J BiolChem 1984;259:7129-36.
- Thomas PS. Tumour necrosis factor-alpha: The role of this multifunctional cytokine in asthma. Immunol Cell Biol 2001;79:132-40.
- Levi-Schaffer F, Temkin V, Malamud V, Feld S, Zilberman Y. Mast cells enhance eosinophil survival in vitro: Role of TNF-alpha and granulocyte-macrophage colony-stimulating factor. J Immunol 1998;160:5554-62.
- Blackwell TS, Blackwell TR, Christman JW. Impaired activation of nuclear factor-kappaB in endotoxin-tolerant rats is associated with down-regulation of chemokine gene expression and inhibition of neutrophilic lung inflammation. J Immunol 1997;158:5934-40.
- Tanaka S, Mikura S, Hashimoto E, Sugimoto Y, Ichikawa A. Ca2+ influx-mediated histamine synthesis and IL-6 release in mast cells activated by monomeric IgE. Eur J Immunol 2005;35:460-8.