- *Corresponding Author:
- H. R. Dehghan Manshadi
Department of Radiation Oncology, 7-Tir Hospital, Iran University of Medical Sciences, Tehran-1886718136,Iran
E-mail: dehghanhamidreza@gmail.com
| Date of Submission | 15 July 2016 |
| Date of Revision | 15 February 2017 |
| Date of Acceptance | 15 April 2017 |
| Indian J Pharm Sci 2017;79(3):335-344 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Doxorubicin is among the most powerful drugs used for the treatment of both adult and child cancers. Doxorubicin is a major cause of chemotherapy-induced cardiotoxicity that is a restricting factor for an optimum dose of the drug for treatment of the cancer patients. Many studies have explored pathophysiology and mechanisms of doxorubicin-induced cardiotoxicity. Cellular and animal experiments proposed that doxorubicin-induced cardiotoxicity mechanism is multifactorial. Oxidative stress has been considered as the primary cause of cardiotoxicity. Although there is no effective treatment for doxorubicin-induced cardiotoxicity currently but many investigations are being done to discover prevention treatments whereas no specific treatment has been approved. Studies have shown that reactive oxygen species and topoisomerase 2b are molecular targets for cardioprotection. Therapeutic imaging methods and cardio-biomarkers may be helpful in the improvement of rapid detection of cardiac damage. In this review, effects of doxorubicin on DNA damage, free radical generation, mitochondrial damage, cell death, and other parameters have been studied.
Keywords
Doxorubicin, cardiotoxicity, reactive oxygen species, mitochondrial damage, apoptosis
Doxorubicin is a secondary metabolite produced by Streptomyces peucetius var. caesius and belongs to anthracyclines (ANTs) family. It is an efficient antineoplastic agent used for the treatment of child and adult cancers such as solid tumours, leukaemia, lymphoma and breast cancer. Optimal administration of doxorubicin is hampered due to some toxicity such as hematopoietic suppression, nausea, vomiting, extravasation, alopecia, and cardiotoxicity [1]. Cytotoxic chemotherapy-induced cardiotoxicity has a high incidence [2]. Cardiotoxicity included short- and longterm toxic effects in the heart ranging from alterations in myocardial structure and function to severe cardiomyopathy and heart failure that may result in cardiac transplantation or death. Chronic cardiotoxicity occurred after prolonged administration of doxorubicin. Although the possibility of cardiotoxicity development is dose dependent, but it could occur even at lower dose due to individual variations [3].
Despite frequent attempts, the molecular mechanism of doxorubicin-induced cardiotoxicity has not been identified yet. Although different mechanisms of cardiotoxicity have been described in literature, including DNA damage, alteration of protein synthesis, formation of oxygen free radicals, cell membrane lesions and lipid peroxidation, mitochondrial damage, release of histamine and catecholamines, induction of immunogenic reactions, calcium homeostasis dysregulation whereas a combination of these factors trigger myocardial lesions [4,5]. In this report, some of the above-mentioned mechanisms have been reviewed.
Doxorubicin-induced DNA damage
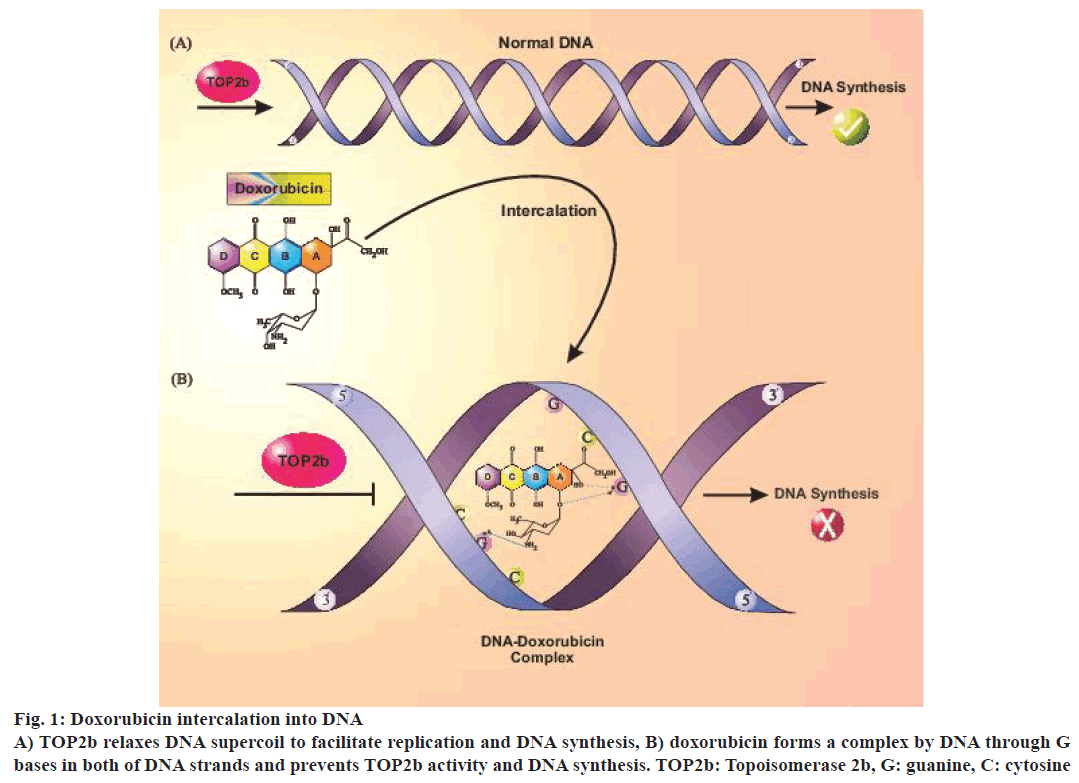
A major part of the anticancer effect of doxorubicin might be due to irreversible damage of tumour cell DNA. Proposed mechanisms for its antitumor effects included intercalation into DNA that caused prevention of micromolecule synthesis, reactive oxygen species (ROS) generation, DNA binding and cross-linkage and DNA damage by topoisomerase 2b (TOP2b) suppression and induction of apoptosis [6-8]. TOP2b was recently identified as doxorubicin-induced cardiotoxicity mediator in a rat model [9]. TOP2b unwinds DNA strands during replication, transcription or recombination and is present in all quiescent cells, including cardiomyocytes [10,11]. Doxorubicin has been known as a TOP2b poison that prevented DNA synthesis by intercalation into DNA strands. TOP2b changes DNA topology, which leads to transient breakage of double-strand DNA and DNA supercoil dysregulation that can result in cardiomyocyte death [12] (Figure 1).
P53 and apoptotic pathway activation have been shown in doxorubicin-induced cardiotoxicity [13]. TOP2b is required for P53 activation in response to doxorubicininduced DNA damage in cardiomyocytes whereas ROS generation from doxorubicin was due to a reduction in the expression of genes of antioxidant enzymes, which were also TOP2b dependent [14]. Alternatively, once the cell underwent DNA damage, DNA repair pathways get activated. Some of these enzymes cleave oxidized bases before replication, remove oxidized bases from the nucleotidic pool, or remove oxidized bases from DNA after replication [15,16]. These oxidized adducts were shown to be mutagenic compounds, which lead to prevention of DNA replication and increased DNA polymerase proofreading error [17].
Moreover, doxorubicin produced mitochondrial DNA (mtDNA) damage by the formation of adducts with its circular genome that led to mitochondrial machine disruption and mtDNA changes that included rearrangements, deletions and copy number reduction, which were observed in the heart, but not in the skeletal muscle of animal models and patients treated with doxorubicin. This suggested that mtDNA changes might accumulate with time, even in the absence of treatment, which resulted in a deficient respiratory chain that produced more ROS and triggered more mtDNA damage. Although doxorubicin has been toxic to both cancer and normal cells, mechanism of cell death in both cells might not be similar [18].
Doxorubicin-induced oxidative stress
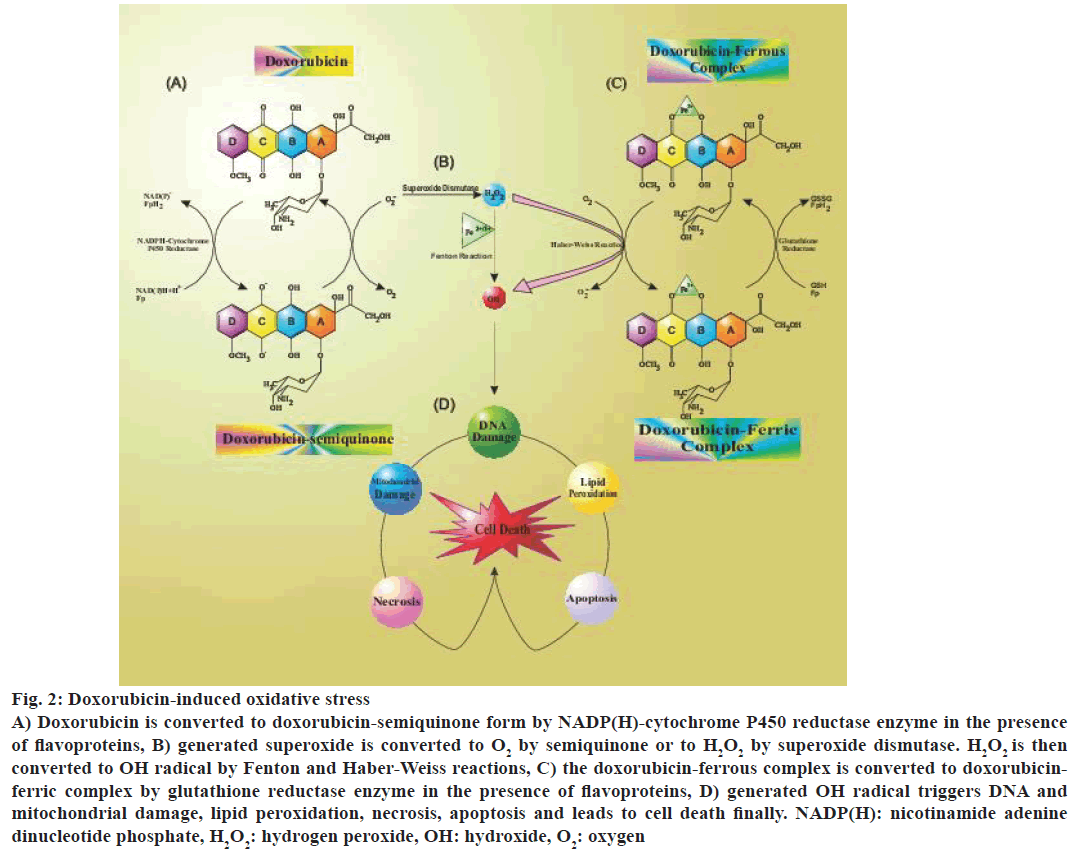
Doxorubicin appear to induce oxygen-derived free radical formation through two major pathways: a nonenzymatic pathway that used iron and an enzymatic pathway using mitochondrial respiratory chain [19,20]. The most commonly accepted theory of doxorubicin-induced cardiotoxicity implicated the formation of free radicals and superoxides. In the free radical theory, the reaction was initiated by loss of an electron from doxorubicin that triggered the formation of doxorubicin semiquinone radical aided by a reduced flavoenzyme such as NADPH-cytochrome P450 reductase. This radical appeared to be partly stable in the anoxic environment, but under the normoxic condition, its unpaired electron is given to oxygen leading to the formation of superoxide radicals. The semiquinone radical formed a complex with iron that resulted in the free radical complex doxorubicin-iron (Fe2+) [21-24] (Figure 2).
Figure 2: Doxorubicin-induced oxidative stress
A) Doxorubicin is converted to doxorubicin-semiquinone form by NADP(H)-cytochrome P450 reductase enzyme in the presence
of flavoproteins, B) generated superoxide is converted to O2 by semiquinone or to H2O2 by superoxide dismutase. H2O2 is then
converted to OH radical by Fenton and Haber-Weiss reactions, C) the doxorubicin-ferrous complex is converted to doxorubicinferric
complex by glutathione reductase enzyme in the presence of flavoproteins, D) generated OH radical triggers DNA and
mitochondrial damage, lipid peroxidation, necrosis, apoptosis and leads to cell death finally. NADP(H): nicotinamide adenine
dinucleotide phosphate, H2O2: hydrogen peroxide, OH: hydroxide, O2: oxygen
Appropriate flavoproteins such as complex Ι catalyzes reduced semiquinone radicals by accepting electrons from nicotinamide adenine dinucleotide (NADH) or nicotinamide adenine dinucleotide phosphate (NAD(P) H) and delivering them to doxorubicin. This sequence of reactions was known as the redox cycling, could be very deleterious since a low amount of doxorubicin has been found adequate for the formation of many superoxide radicals [25] (Figure 2). This radical damage triggered production of highly toxic aldehydes such as malondialdehyde (MAD). These aldehydes could diffuse easily into the cell through the cell membrane and get attached to micromolecular targets, thus acted as secondary cytotoxic messengers” [26].
NAD(P)H is a large polypeptide complex and researchers have suggested that the presence of definite single nucleotide polymorphism (SNP) in each subunit might make NAD(P)H complex susceptible to doxorubicin. Scientists were successful to show an association of doxorubicin-induced cardiotoxicity with NAD(P)H complex SNPs in non- Hodgkin lymphoma patients [27]. Doxorubicin-induced chronic cardiotoxicity was associated with an SNP in NCF4 subunit of NAD(P)H oxidase that was found responsible for NAD(P)H complex down-regulation, whereas acute cardiotoxicity was associated with SNPs in P22phox and Rac2 subunits [27]. Thus, these genetic polymorphisms in NAD(P)H oxidase might serve as a screening tool to diagnose patients with high risk for doxorubicin-induced cardiotoxicity in the future but polymorphisms of other genes could also be important. Some researchers have revealed that doxorubicininduced cardiotoxicity was associated with CBR3 gene variant V22M in carbonyl reductase domain, a doxorubicin metabolizing enzyme [28].
Compared to cardiac mitochondria, liver mitochondria lacked the NADH-associated pathway to have an equal reduction from cytosol to the respiratory chain. Therefore, liver mitochondria would not produce enough amount of doxorubicin semiquinone [29]. Doxorubicin entered the mitochondria and suppressed respiratory chain by binding to cardiolipin, a cardiac specific and polyunsaturated fatty acid-rich phospholipid found in the mitochondrial internal membrane. Cardiolipin has been found to have high affinity to doxorubicin [30]. Besides ROS, reactive nitrogen species (RNS) were also implicated in doxorubicin-induced cardiotoxicity and there appeared to be a crosstalk between doxorubicin and NO production. Studies have revealed that doxorubicin bound to reductase domain of endothelial nitric oxide synthase (eNOS, NOS3) led to increased superoxide and reduction of nitric oxide formation. Formation of peroxynitrite might also play a significant role in cardiotoxicity [31]. Continuous administration of doxorubicin in vivo has been shown to induce NO production inside of cardiomyocyte with inducible nitric oxide synthase (iNOS, NOS2) over-expression in both mRNA and protein level. In contrast, doxorubicin administration did not change the expression of other two isoforms of this enzyme. Although Investigations have demonstrated the involvement of eNOS isoform in doxorubicin-induced acute cardiotoxicity [32].
Doxorubicin also reduced the activity of cardiac enzymes superoxide dismutase (SOD), glutathione-Stransferase (GST), and catalase (CAT). Upregulation of manganese superoxide dismutase (MnSOD) has been shown to increase cell survival in the presence of doxorubicin as a free radical scavenger in mitochondria [43]. Calceolarioside protected against doxorubicin-induced apoptosis by upregulating many SOD, heme oxygenase (HO) and potential maintenance of mitochondrial membrane [43]. Furthermore, it attenuated reduced glutathione level significantly. Generally, antioxidant storage was low in heart tissue compared to other organs in the body that made the heart more susceptible to damage by doxorubicininduced free radicals [33].
Role of mitochondria in doxorubicin-induced cardiotoxicity
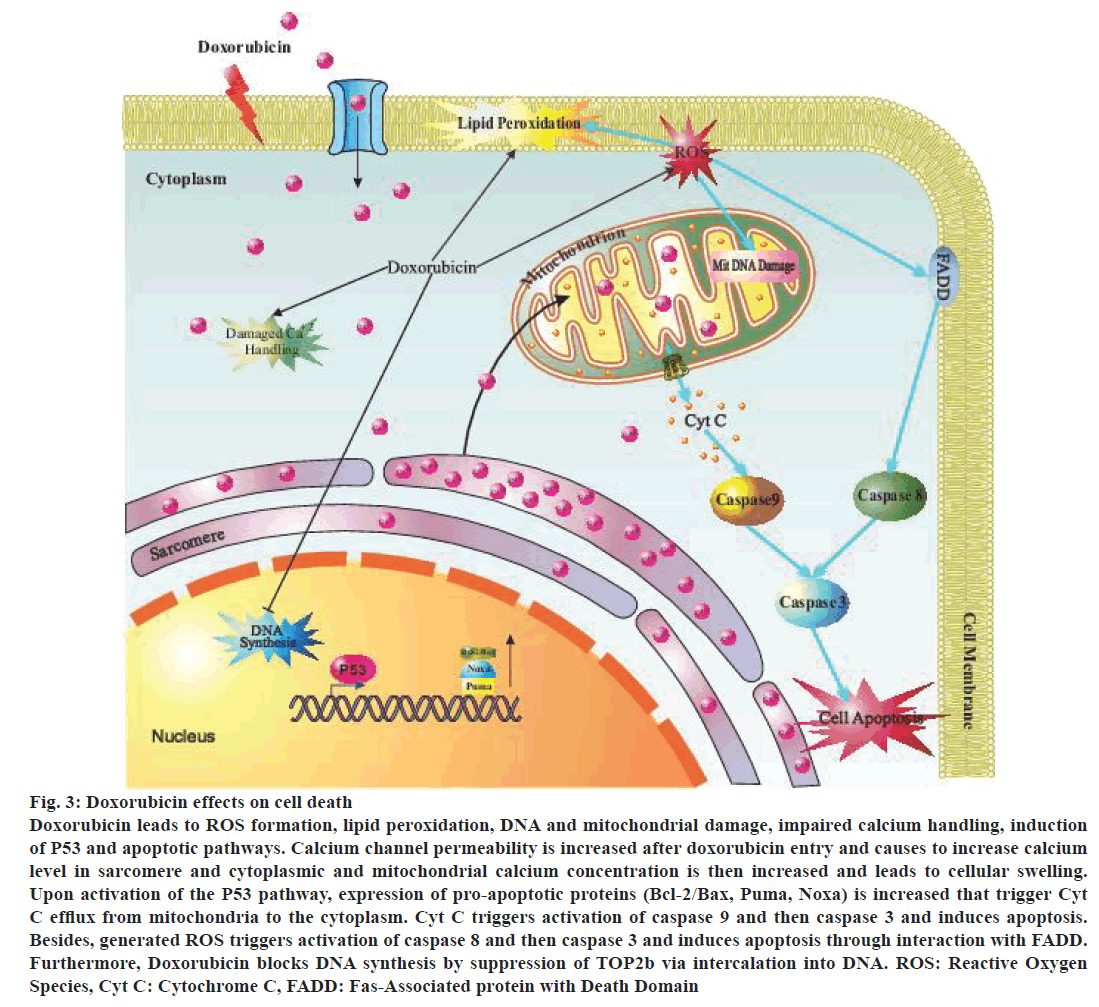
The enzymatic pathway for the formation of free radicals was mediated by mitochondria. Doxorubicin has a high affinity for cardiolipin, an abundant phospholipid in the internal membrane of mitochondria. This affinity permitted doxorubicin to penetrate into the cardiomyocytes [34]. The specific mechanism has not been identified, but the doxorubicin-induced damage to mitochondria likely triggered respiratory chain defect at first that permitted continuous production of free radicals and mitochondrial damage, which might have led to release of cytochrome C triggered apoptosis. P53 pathway activation led to protein translocation into the nucleus and induced changes in gene expression of the proteins to prevent cell division leading in apoptosis [35]. Doxorubicin triggers activation of various molecular signals from AMP-activated protein kinase that induces apoptosis to affect Bcl-2/Bax apoptosis pathway. By changing the Bcl-2/Bax ratio, downstream activation of different caspases can trigger apoptosis [36] (Figure 3).
Electron transfer chain proteins are required to bind to cardiolipin for complete function and since doxorubicin disrupted protein-cardiolipin bond, therefore, generated more superoxide (O2-). Other mitochondrial membrane proteins such as those are responsible for carnitine transport may be affected by doxorubicin that results in attenuation of mitochondrial function [37]. These events likely disrupt mitochondrial and cellular metabolism eventually because they produced more than 90% of ATP that is utilised by cardiomyocytes [38]. This functional damage triggered pathological alterations in the ultrastructure, such as mitochondrial swelling and formation of myelin-like fingers inside mitochondria [39] (Figure 3).
Figure 3: Doxorubicin effects on cell death
Doxorubicin leads to ROS formation, lipid peroxidation, DNA and mitochondrial damage, impaired calcium handling, induction
of P53 and apoptotic pathways. Calcium channel permeability is increased after doxorubicin entry and causes to increase calcium
level in sarcomere and cytoplasmic and mitochondrial calcium concentration is then increased and leads to cellular swelling.
Upon activation of the P53 pathway, expression of pro-apoptotic proteins (Bcl-2/Bax, Puma, Noxa) is increased that trigger Cyt
C efflux from mitochondria to the cytoplasm. Cyt C triggers activation of caspase 9 and then caspase 3 and induces apoptosis.
Besides, generated ROS triggers activation of caspase 8 and then caspase 3 and induces apoptosis through interaction with FADD.
Furthermore, Doxorubicin blocks DNA synthesis by suppression of TOP2b via intercalation into DNA. ROS: Reactive Oxygen
Species, Cyt C: Cytochrome C, FADD: Fas-Associated protein with Death Domain
Other researchers have proposed that targeting of the myocardial energy network to be a part of doxorubicininduced cardiotoxicity because a significant reduction in phosphate energy pool occurred in cardiomyocytes [40]. Doxorubicin also reduced respiratory chain complex activity and attenuated function of adenine nucleotide translocator (ANT) or voltage-dependent anion channel (VDAC) or both (responsible proteins for the formation and transportation of ATP from mitochondria to cytosol). Researchers showed that treatment with doxorubicin affected mitochondrial gene expression [41]. It is likely that doxorubicin-induced alterations might affect gene expression of metabolic enzymes, including oxidative and glycolytic enzymes. They used transgenic mitochondrial reporter mouse to show that doxorubicin suppressed cardiac mitochondrial metabolism and biogenesis and triggered apoptosis. The mouse was permitted to inhale carbon monoxide (CO) to upregulate the required genes for mitochondrial biosynthesis and nuclear-encoded HO. This revealed that doxorubicin interfered with transcription regulation in both nucleus and mitochondria [42,43]. Loss of cardiomyocyte following activation of apoptotic and necrotic pathways were an interesting description for doxorubicin-induced cardiotoxicity [44-46] (Figure 3).
Animal studies have revealed that apoptotic cell death occurred in vivo after exposing to doxorubicin. Cell culture studies have also demonstrated doxorubicininduced apoptotic and necrotic cell death [46] (Figure 3). Mitochondrial damage and apoptosis evidence have been found in endomyocardial biopsies of the patients treated with doxorubicin [47,48]. Biochemical pathways of apoptosis and necrosis have been present in cardiomyocytic death following doxorubicin administration. In doxorubicin-induced apoptosis in the heart, a mitochondrial pathway might have been involved that required Bax, cytochrome C and caspase-3 [49] (Figure 3).
Generally, treatment with doxorubicin increased mitochondrial oxidative stress and disrupted intracellular calcium levels [50]. Intracellular calcium was raised leading to increased mitochondrial calcium levels finally [51]. This increased calcium level led to increase in the permeability of mitochondrial membrane that triggered transmembrane potential disruption, mitochondrial swelling and increased the permeability of its outer membrane to apoptotic factors such as cytochrome C (Figure 3). Caspase activity could be affected by doxorubicin. In the cytosol, cytochrome C formed a complex with adaptor protein apoptosis protease activating factor-1 (APAF-1), dATP and caspase-9, the apoptosome. It has been demonstrated that caspase-3 activation was associated with apoptosis induced by in vivo and in vitro administration of doxorubicin [52].
The current hypothesis of necrosis and some forms of apoptotic cell death included the prolonged opening of a conductive pore in mitochondria, the mitochondrial permeability transition pore (mPTP) [53]. Formation of mPTP has been proposed through conformational changes associated with the binding site of ANT with VDAC between inner and outer membranes of mitochondria [54]. Recent studies revealed that genetic deletion of ANT and VDAC isoforms did not lead to loss of mPTP, suggesting that none of these proteins were essential components [55]. In contrast, deletion of cyclophilin D reduced ischemia-reperfusion-induced cell death, suggesting a role for cyclophilin D in mPTP [56].
The evidence demonstrated that phosphate transporter was an essential component of mPTP and phosphate transporter interaction with ANT regulated mPTP opening. For a long time, it was speculated that mPTP had a role in apoptotic cell death as the protagonist of mitochondrial permeability. Recent data suggested that increased permeability in the mitochondrial membrane has been one of the major factors in necrotic and apoptotic cell death. These data were important since necrosis occured in many lesions of adult hearts, including cardiotoxic effects of anticancer drugs. In fact, recent experiments have revealed that mPTP did not start apoptosis but played a significant role in necrosis, especially in the heart. Other studies on cyclophilin D deficient mouse have shown that mPTP played an important role in cell necrosis [57,58].
Overall, these data revealed that opening of mPTP involved in cardiac cell necrosis compared to cytochrome C released in apoptosis. It is well understood that mitochondria played a significant role in the pathogenesis of doxorubicin-induced cardiotoxicity. Prevention of mitochondrial disruption would prevent myocardial alterations and result in better function of the heart. In addition, more experiments are required to understand the specific function of mitochondria in this pathogenesis.
Doxorubicin-induced cardiotoxicity correlation with apoptosis, necrosis and autophagy
It has been accepted that doxorubicin-induced oxidative stress activated apoptotic signalling leading to cardiomyocyte apoptosis and both intrinsic and extrinsic apoptotic pathways were involved [59,60]. It appeared that doxorubicin could induce apoptosis through a mechanism that would not include ROS formation and oxidative stress directly, although apoptosis generated free radicals by itself [61]. In a doxorubicin model, oxidative stress activated heatshock factor 1 (HSF1) that produced more heat shock protein 25 (HSP25), which stabilized P53 and increased production of pro-apoptotic proteins (Figure 3).
Heat-shock protein family played a significant role in these processes. These proteins were acted as molecular chaperones that stabilized their target proteins involved in antiapoptotic signalling and prevented cardiac dephosphorylation, ubiquitination, and degradation [62]. Scientists showed that HSP27 overexpression that had a cardiac protective role against ischemia/reperfusion damage also prevented apoptosis and doxorubicininduced myocardial dysfunction due to the protective role of HSP27 in the regulation of oxidative stress response and maintenance of mitochondrial function [63]. HSP10 and HSP60 overexpression also led to an increase in post-translational modification of Bcl-2 proteins that amplify antiapoptotic signalling likely through their effects as molecular chaperones. HSP27 increased AKT phosphorylation (one of the major survival pathways) [64].
Moreover, some of the HSP are found to be secreted to extracellular matrix, which entered the blood stream and acted as a ligand for toll-like receptors (TLRs). It has been demonstrated that HSP60 signalling could be blocked by antibodies as well as by TLR-2 antagonists against these peptides, whereas HSP70 interacted with TLR-4 [65]. Thus, TLR-2 and TLR-4 roles in doxorubicin-induced cardiotoxicity have been identified. TLR-2 mediated signalling through proinflammatory nuclear factor-κB (NF-κB) pathway is involved in cytokine production, apoptosis, and cardiac dysfunction after treatment with doxorubicin and deletion of this receptor in knockout mouse maintained cardiac function and prevented apoptosis. TLR-4 also acted via NF-κB pathway and knockout mouse showed better cardiac function after treatment with doxorubicin than wild types [66]. This pathway is not only in cardiomyocyte although it is thought to be specific in the most of the cells. NF-κB activation by doxorubicin has been demonstrated in endothelial and kidney cells. Furthermore, NF-κB signalling is not specific for TLRs and initiates regulation of transcription and translation route of many other proteins, including inflammatory cytokines that are likely involved in apoptosis [67].
Utilization of an NO donor S-nitrosyl-N-acetylpenicillamine (SNAP) produced an antiapoptotic effect by caspase activity suppression through S-nitrosylation in treated cardiomyocytes with doxorubicin. Blocking of volume-sensitive chloride channel has been shown that prevents caspase-3 dependent apoptosis in doxorubicin toxicity [68].
Cellular necrosis in cardiomyocytes typically has been associated with mitochondrial and cytoplasm swelling, coagulated sarcomer and plasma membrane rupture (Figure 3). This type of cell death could be well controlled by mPTP-dependent mechanisms. ROS rise led to increased mitochondrial calcium level and promoted mPTP opening that caused mitochondrial swelling and reduction of used ATP and thus necrotic cell death is induced [69].
Autophagy has been considered as a process, which was global degradation and recycling of cytoplasmic components such as aged proteins and organelles [70]. Autophagy is important in the heart due to turnover of organelles in basal low levels on their normal conditions and upregulation in response to stress, such as ischemia-reperfusion and in cardiovascular diseases such as heart failure. Recent studies have proposed that autophagic cell death might play an important role in doxorubicin-induced myocardial dysfunction [71]. Overall, mitochondria can be considered as a junction for apoptosis, necrosis, and autophagy processes.
Doxorubicin is an effective chemotherapeutic agent that increased survival of cancer patients, but its administration is hampered due to cardiotoxicity. Cardiotoxicity, an apparent side effect of doxorubicin, might emerge twenty years after treatment as an acute or chronic side effect. This side effect is especially significant for children that have been treated with doxorubicin. Since this side effect is dose restricting and can lead to increased disease severity or even death. Understanding of the mechanisms of action is critical. It is thought that doxorubicin-induced cardiotoxicity was mediated by oxidative stress.
Until now, the most accepted hypothesis was the free radical theory. Since treatment of cardiac diseases is very expensive, physicians should consider the best cardioprotective option while treating patients with doxorubicin. Administration of cardioprotective agents in an effective option, but development of these effective strategies requires a sound understanding of the molecular mechanisms of doxorubicin-induced cardiotoxicity. Dexrazoxane reduced prolonged cardiotoxicity in cancer patients without leading to secondary malignancies [72]. Multiple factors interfered with doxorubicin-induced cardiotoxicity including gender, age, drug dose and radiation. Multiple techniques can facilitate monitoring of doxorubicininduced cardiotoxicity, such as echocardiography, cardiac magnetic resonance imaging and serum biomarkers and all these have been found to be important in the survival of cancer patients.
Many have studied the association of cardiotoxicity with doxorubicin-related metabolism gene polymorphisms. Jensen et al. demonstrated that cardiotoxicity could occur in association with demographic characteristics, suggesting a correlation between individual genetics and toxicity development and that many gene polymorphisms are associated with increased cardiotoxicity [73]. Furthermore, Aminken et al. observed that RARG gene variant in the coding region increased the susceptibility of anthracycline-induced cardiotoxicity in children affected with cancer [74]. Reichwagen et al. found that NADPH oxidase gene polymorphisms were associated with anthracyclineinduced cardiotoxicity in patients affected with B-cell lymphoma [75]. Krajinovic et al. revealed that ABCC5 and NOS3 gene polymorphisms have been associated with the development of doxorubicin-induced cardiotoxicity in children with acute lymphoblastic leukemia [76].
Recent data demonstrated that the primary damage of DNA induced by doxorubicin was different from oxidative stress-induced DNA damage and this might be a novel therapeutic target to reduce cardiotoxicity for better therapeutic outcomes in cancer patients. Currently, there is no standard guideline for the treatment of doxorubicin-induced cardiotoxicity. Thus, early detection of doxorubicin-induced cardiotoxicity is essential to prevent subsequent damage. Comprehensive studies are required to investigate biologic parameters, genetic variations and efficient imaging techniques for prognosis, prevention, and treatment of doxorubicininduced cardiotoxicity.
Conflict of interest
The authors report no declarations of interest.
Financial support and sponsorship
Nil.
References
- Jain D. Cardiotoxicity of doxorubicin and other anthracycline derivatives. J Nucl Cardiol 2000;7:53-62.
- Grenier MA, Lipshultz SE. Epidemiology of anthracycline cardiotoxicity in children and adults. Semin Oncol 1998;25:72-85.
- Lipshultz SE, Lipsitz SR, Mone SM, Goorin AM, Sallan SE, Sanders SP, et al. Female sex and drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N Engl J Med 1995;332:1738-43.
- Arola OJ, Saraste A, Pulkki K, Kallajoki M, Parvinen M, Voipio-Pulkki LM. Acute doxorubicin cardiotoxicity involves cardiomyocyte apoptosis. Cancer Res 2000;60:1789-92.
- Hatch GM, McClarty G. Regulation of cardiolipin biosynthesis in H9C2 cardiac myoblasts by Cytidine 5-triphosphate. J Biol Chem 1996;271:25810-6.
- Takemura G, Fujiwara H. Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog Cardiovasc Dis 2007;49:330-52.
- Gewirtz DA. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics Adriamycin and daunorubicin. Biochem Pharmacol 1999;57:727-41.
- Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev 2004;56:185-229.
- Cirillo R, Sacco G, Venturella S, Brightwell J, Giachetti A, Manzini S. Comparison of doxorubicin- and MEN 10755-induced long-term progressive cardiotoxicity in the rat. J Cardiovasc Pharmacol 2000;35:100-8.
- de Graaf H, Dolsma WV, Willemse PH, van der Graaf WT, Sleijfer DT, de Vries EG, et al. Cardiotoxicity from intensive chemotherapy combined with radiotherapy in breast cancer. Br J Cancer 1997;76:943-5.
- Suzuki T, Hayashi D, Yamazaki T, Mizuno T, Kanda Y, Komuro I, et al. Elevated B-type natriuretic peptide levels after anthracycline administration. Am Heart J 1998;136:362-3.
- Lipshultz SE, Rifai N, Sallan SE, Lipsitz SR, Dalton V, Sacks DB, et al. Predictive value of cardiac troponin-T in pediatric patients at risk for myocardial injury. Circulation 1997;96:2641-8.
- Nousiainen T, Jantunen E, Vanninen E, Remes J, Vuolteenaho O, Hartikainen J. Natriuretic peptides as markers of cardiotoxicity during doxorubicin treatment for non-Hodgkin’s Lymphoma. Eur J Haematol 1999;62:135-41.
- Minotti G, Mancuso C, Frustaci A, Mordente A, Santini SA, Calafiore AM, et al. Paradoxical inhibition of cardiac lipid peroxidation in cancer patients treated with doxorubicin. J Clin Invest 1996;98:650-61.
- Fujikawa K, Kamiya H, Yakushiji H, Fujii Y, Nakabeppu Y, Kasai H. The oxidized forms of dATP are substrates for the human MutT homologue, the hMTH1 protein. J Biol Chem 1999;274:18201-5.
- Takao M, Zhang QM, Yonei S, Yasui A. Differential subcellular localization of human MutY homolog (hMYH) and the functional activity of adenine:8-oxoguanine DNA glycosylase. Nucleic Acids Res 1999;27:3638-44.
- Parker MA, King V, Howard KP. Nuclear magnetic resonance study of doxorubicin binding to cardiolipin containing magnetically oriented phospholipid bilayers. Biochem Biophys Acta 2001;15:206-16.
- Pereira GC, Silva AM, Diogo CV, Carvalho FS, Monteiro P, Oliveira PJ. Drug-induced cardiac mitochondrial toxicity and protection: From doxorubicin to carvedilol. Curr Pharm Des 2011;17:2113-29.
- Gianni L, Zweier JL, Levy A, Myers CE. Characterization of the cycle of iron-mediated electron transfer from doxorubicin to molecular oxygen. J Biol Chem 1985;259:6056-8.
- Olson RD, Mushlin PS. Doxorubicin cardiotoxicity: analysis of prevailing hypothesis. FASEB J 1990;4:3076-86.
- Alderton PM, Gross J, Green MD. Comparative study of doxorubicin, mitoxantrone, and epirubicin in combination with ICRF-187 (ADR-529) in a chronic cardiotoxicity animal model. Cancer Res 1992;52:194-201.
- Rossi F, Filippelli W, Russo S, Filippelli A, Berrino L. Cardiotoxicity of doxorubicin: effects of drugs inhibiting the release of vasoactive substances. Pharm Tox 1994;75:99-107.
- Vásquez-Vivar J, Martasek P, Hogg N, Masters BS, Pritchard KA Jr, Kalyanaraman B. Endothelial nitric oxide synthase-dependent superoxide generation from adriamycin. Biochem Pharmacol 1997;36:11293-7.
- Rajagopalan S, Politi PM, Sinha BK, Myers CE. Adriamycin-induced free radical formation in the perfused rat heart: implications for cardiotoxicity. Cancer Res 1988;48:4766-9.
- Keizer HG, Pinedo HM, Schuurhuis GJ, Joenje H. Doxorubicin (adriamycin): a critical review of free radical-dependent mechanisms of cytotoxicity. Pharmacol Ther 1990;47:219-31.
- Luo X, Evrovsky Y, Cole D, Trines J, Benson LN, Lehotay DC. Doxorubicin-induced acute changes in cytotoxic aldehydes, antioxidant status and cardiac function in the rat. Biochim Biophys Acta 1997;1360:45-52.
- Wojnowski L, Kulle B, Schirmer M, Schlüter G, Schmidt A, Rosenberger A, et al. NAD(P)H oxidase and multidrug resistance protein genetic polymorphisms are associated with doxorubicin-induced cardiotoxicity. Circulation 2005;112:3754-62.
- Blanco JG, Leisenring WM, Gonzalez-Covarrubias VM, Kawashima TI, Davies SM, Relling MV, et al. Genetic polymorphisms in the carbonyl reductase 3 gene CBR3 and the NAD(P)H: quinone oxidoreductase 1 gene NQO1 in patients who developed anthracycline-related congestive heart failure after childhood cancer. Cancer 2008;112:2789-95.
- Nohl H, Gille L, Staniek K. The exogenous NADH dehydrogenase of heart mitochondria is the key enzyme responsible for selective cardiotoxicity of anthracyclines. Z Naturforsch C. 1998;53:279-85.
- Goormaghtigh E, Huart P, Praet M, Brasseur R, Ruysschaert JM. Structure of the adriamycin-cardiolipin complex. Role in mitochondrial toxicity. Biophys Chem 1990;35:247-57.
- Zhou S, Starkov A, Froberg MK, Leino RL, Wallace KB. Cumulative and irreversible cardiac mitochondrial dysfunction induced by doxorubicin. Cancer Res 2001;61:771-7.
- Neilan TG, Blake SL, Ichinose F, Raher MJ, Buys ES, Jassal DS, et al. Disruption of nitric oxide synthase 3 protects against the cardiac injury, dysfunction, and mortality induced by doxorubicin. Circulation 2007;116:506-14.
- Halestrap AP. Calcium, mitochondria, and reperfusion injury: a pore way to die. Biochem Soc Trans 2006;34:232-7.
- Frits A. de Wolf. Binding of doxorubicin to cardiolipin as compared to other anionic phospholipids-an evaluation of electrostatic effects. Biosci Rep 1991;11:275-84.
- Cui H, Schroering A, Ding HF. P53 mediates DNA damaging drug-induced apoptosis through a caspase-9-dependent pathway in SH-SY5Y neuroblastoma cells. Mol Cancer Ther 2002;1:679-86.
- Tacar O, Sriamornsak P, Dass CR. Doxorubicin: an update on anticancer molecular action, toxicity, and novel drug delivery systems. Pharm Pharmacol 2013;65:157-70.
- Kashfi K, Israel M, Sweatman TW, Seshadri R, Cook GA. Inhibition of mitochondrial carnitine palmitoylt ransferases by adriamycin and adriamycin analogues. Biochem Pharmacol 1990;40:1441-8.
- Ventura-Clapier R, Garnier A, Veksler V. Energy metabolism in heart failure. J Physiol 2004;555:1-13.
- Cole MP, Chaiswing L, Oberley TD, Edelmann SE, Piascik MT, Lin SM, et al. The protective roles of nitric oxide and superoxide dismutase in adriamycin-induced cardiotoxicity. Cardiovasc Res 2006;69:186-97.
- Tokarska-Schlattner M, Zaugg M, Zuppinger C, Wallimann T, Schlattner U. New insights into doxorubicin-induced cardiotoxicity: the critical role of cellular energetics. J Mol Cell Cardiol 2006;41:389-405.
- Suliman HB, Carraway MS, Ali AS, Reynolds CM, Welty-Wolf KE, Piantadosi CA. The CO/HO system reverses inhibition of mitochondrial biogenesis and prevents murine doxorubicin cardiomyopathy. J Clin Invest 2007;117:3730-41.
- Piantadosi CA, Carraway MS, Babiker A, Suliman HB. Heme oxygenase-1 regulates cardiac mitochondrial biogenesis via Nrf2-mediated transcriptional control of nuclear respiratory factor-1. Circ Res 2008;103:1232-40.
- Kim DS, Kim HR, Woo ER, Kwon DY, Kim MS, Chae SW, et al. Protective effect of calceolarioside on adriamycin-induced cardiomyocyte toxicity. Eur J Pharmacol 2006;541:24-32.
- Sawyer DB, Peng X, Chen B, Pentassuglia L, Lim CC. Mechanisms of anthracycline cardiac injury: can we identify strategies for cardioprotection? Prog Cardiovasc Dis 2010;53:105-13.
- Zhang YW, Shi J, Li YJ, Wei L. Cardiomyocyte death in doxorubicin-induced cardiotoxicity. Arch Immunol Ther Exp (Warsz) 2009;57:435-45.
- Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev 2004;56:185-229.
- Meinardi MT, van der Graaf WT, van Veldhuisen DJ, Gietema JA, de Vries EG, Sleijfer DT. Detection of anthracycline-induced cardiotoxicity. Cancer Treat Rev 1999;25:237-47.
- Rowan RA, Masek MA, Billingham ME. Ultrastructural morphometric analysis of endomyocardial biopsies. Idiopathic dilated cardiomyopathy, anthracycline cardiotoxicity, and normal myocardium. Am J Cardiovasc Pathol 1988;2:137-44.
- Chen B, Peng X, Pentassuglia L, Lim CC, Sawyer DB. Molecular and cellular mechanisms of anthracycline cardiotoxicity. Cardiovasc Toxicol 2007;7:114-21.
- Berthiaume JM, Wallace KB. Adriamycin-induced oxidative mitochondrial cardiotoxicity. Cell Biol Toxicol 2007;23:15-25.
- Wallace KB. Adriamycin-induced interference with cardiac mitochondrial calcium homeostasis. Cardiovasc Toxicol 2007;7:101-7.
- Ueno M, Kakinuma Y, Yuhki K, Murakoshi N, Iemitsu M, Miyauchi T, et al. Doxorubicin induces apoptosis by activation of caspase-3 in cultured cardiomyocytes in vitro and rat cardiac ventricles in vivo. J Pharmacol Sci 2006;101:151-8.
- Minotti G, Ronchi R, Salvatorelli E, Menna P, Cairo G. Doxorubicin irreversibly inactivates iron regulatory proteins 1 and 2 in cardiomyocytes: evidence for distinct metabolic pathways and implications for iron-mediated cardiotoxicity of antitumor therapy. Cancer Res 2001;61:8422-8.
- Bernardi P, Krauskopf A, Basso E, Petronilli V, Blachly-Dyson E, Di Lisa F. The mitochondrial permeability transition from in vitro artifact to disease target. FEBS J 2006;273:2077-99.
- Halestrap AP. What is the mitochondrial permeability transition pore? J Mol Cell Cardiol 2009;46:821-31.
- Baines CP. The mitochondrial permeability transition pore and ischemia-reperfusion injury. Basic Res Cardiol 2009;104:181-8.
- Nakayama H, Chen X, Baines CP, Klevitsky R, Zhang X, Zhang H, et al. Ca2+ and mitochondrial-dependent cardiomyocyte necrosis as a primary mediator of heart failure. J Clin Invest 2007;117:2431-44.
- Arbustini E, Brega A, Narula J. Ultrastructural definition of apoptosis in heart failure. Heart Fail Rev 2008;13:121-35.
- Aleman BM, van den Belt-Dusebout AW, De Bruin ML, van 't Veer MB, Baaijens MH, de Boer JP, et al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood 2007;109:1878-86.
- Nitobe J, Yamaguchi S, Okuyama M, Nozaki N, Sata M, Miyamoto T, et al. Reactive oxygen species regulate FLICE inhibitory protein (FLIP) and susceptibility to Fas-mediated apoptosis in cardiac myocytes. Cardiovasc Res 2003;57:119-28.
- Liu B, Bai QX, Chen XQ, Gao GX, Gu HT. Effect of curcumin on expression of survivin, Bcl-2, and Bax in human multiple myeloma cell line. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2007;15:762-6.
- Shan YX, Liu TJ, Su HF, Samsamshariat A, Mestril R, Wang PH. Hsp10 and Hsp60 modulate Bcl-2 family and mitochondria apoptosis signaling induced by doxorubicin in cardiac muscle cells. J Mol Cell Cardiol 2003;35:1135-43.
- Fan GC, Zhou X, Wang X, Song G, Qian J, Nicolaou P, et al. Heat shock protein 20 interacting with phosphorylated Akt reduces doxorubicin-triggered oxidative stress and cardiotoxicity. Circ Res 2008;103:1270-9.
- Rayner K, Chen YX, McNulty M, Simard T, Zhao X, Wells DJ, et al. Extracellular release of the atheroprotective heat shock protein 27 is mediated by estrogen and competitively inhibits acLDL binding to scavenger receptor-A. Circ Res 2008;103:133-41.
- Rayner K, Chen YX, McNulty M, Simard T, Zhao X, Wells DJ, et al. Extracellular release of the atheroprotective heat shock protein 27 is mediated by estrogen and competitively inhibits acLDL binding to scavenger receptor-A. Circ Res 2008;103:133-41.
- Riad A, Bien S, Gratz M, Escher F, Westermann D, Heimesaat MM, et al. Toll-like receptor-4 deficiency attenuates doxorubicin-induced cardiomyopathy in mice. Eur J Heart Fail 2008;10:233-43.
- Ueno M, Kakinuma Y, Yuhki K, Murakoshi N, Iemitsu M, Miyauchi T, et al. Doxorubicin induces apoptosis by activation of caspase-3 in cultured cardiomyocytes in vitro and rat cardiac ventricles in vivo. J Pharmacol Sci 2006;101:151-8.
- Maejima Y, Adachi S, Morikawa K, Ito H, Isobe M. Nitric oxide inhibits myocardial apoptosis by preventing caspase-3 activity via S-nitrosylation. J Mol Cell Cardiol 2005;38:163-74.
- L'Ecuyer T, Allebban Z, Thomas R, Vander Heide R. Glutathione S-transferase overexpression protects against anthracycline-induced H9C2 cell death. Am J Physiol Heart Circ Physiol 2004;286:H2057-64.
- Lu L, Wu W, Yan J, Li X, Yu H, Yu X. Adriamycin-induced autophagic cardiomyocyte death plays a pathogenic role in a rat model of heart failure. Int J Cardiol 2009;134:82-90.
- Tokarska-Schlattner M, Zaugg M, da Silva R, Lucchinetti E, Schaub MC, Wallimann T, et al. Acute toxicity of doxorubicin on isolated perfused heart: response of kinases regulating energy supply. Am J Physiol 2005;289:H37-47.
- Langer SW. Dexrazoxane for the treatment of chemotherapy-related side effects. Cancer Manag Res 2014;6:357-63.
- Jensen BC, McLeod HL. Pharmacogenomics as a risk mitigation strategy for chemotherapeutic cardiotoxicity. Pharmacogenomics 2013;14:205-13.
- Aminkeng F, Bhavsar AP, Visscher H, Rassekh SR, Li Y, Lee JW, et al. A coding variant in RARG confers susceptibility to anthracycline-induced cardiotoxicity in childhood cancer. Nat Genet 2015;47:1079-84.
- Reichwagen A, Ziepert M, Kreuz M, Gödtel-Armbrust U, Rixecker T, Poeschel V, et al. Association of NADPH oxidase polymorphisms with anthracycline-induced cardiotoxicity in the RICOVER-60 trial of patients with aggressive CD20(+) B-cell lymphoma. Pharmacogenomics 2015;16:361-72.
- Krajinovic M, Elbared J, Drouin S, Bertout L, Rezgui A, Ansari M, et al. Polymorphisms of ABCC5 and NOS3 genes influence doxorubicin cardiotoxicity in survivors of childhood acute lymphoblastic leukemia. Pharmacogenomics J 2016;16:530-5.