- *Corresponding Author:
- Xia Wang
Department of Oncology, West Anhui Health Vocational College, Lu’an, Anhui Province 237005, China
E-mail: lawangxia@126.com
| Date of Received | 19 December 2022 |
| Date of Revision | 10 July 2023 |
| Date of Acceptance | 21 December 2023 |
| Indian J Pharm Sci 2023;85(6):1746-1754 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The aim of this study was to research the association between lysyl oxidase-like 1-antisense 1 and microRNA-761, and explore the molecular mechanisms governing the growth and programmed cell death in ovarian cancer cells. To identify the targeting association between long noncoding RNA lysyl oxidase-like 1-antisense 1 and microRNA-761, the LncBase Predicted v.2 and dual luciferase report examination were employed. The 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide assay and flow cytometry were used to examine the apoptosis and proliferation of ovarian cancer cells, respectively. Western blot was performed to test the levels of cyclin D1, B-cell lymphoma-2, and Bcl-2-associated X protein expressions. SKOV-3 cells exhibited a notable increase in the expression of lysyl oxidase-like 1-antisense 1 compared to normal ovarian epithelial cells HOSE, while displaying a significant reduce in the expression of microRNA-761 than normal HOSE cells. The transfection of microRNA-761 caused a significant reduce in the relative luciferase activity of lysyl oxidase-like 1-antisense 1-WT. Suppression of lysyl oxidase-like 1-antisense 1 and upregulation of microRNA-761 resulted in a substantial reduce in the optical density value and horizontal of cyclin D1 and B-cell lymphoma-2 albumen expression in SKOV-3 cells after 24, 48, and 72 h. Additionally, it led to a raise in the rate of apoptosis and the expression level of Bcl-2-associated X protein albumen in SKOV-3 cells. Co-transfection of si-lysyl oxidase-like 1-antisense 1 and anti-microRNA-761 resulted in a significant decrease in microRNA-761 expression, apoptosis rate, and Bcl-2-associated X protein levels in SKOV-3 cells. Additionally, it led to an increase in optical density value and cyclin D1 horizontals in SKOV-3 cells at 24, 48, and 72 h. In conclusion, the elevated expression of long noncoding RNA lysyl oxidase-like 1-antisense 1 in ovarian cancer cells is associated with its close interaction with the targeted negative regulation of microRNA-761 expression, and knocking down long noncoding RNA lysyl oxidase-like 1-antisense 1 expression can enhance the apoptosis of ovarian cancer cells.
Keywords
Long noncoding RNA lysyl oxidase-like 1-antisense 1, microRNA-761, ovarian cancer cell, radiotherapy, mortality
Ovarian cancer, a highly fatal gynecological malignancy, exhibits significant morbidity and mortality globally[1]. Due to the absence of usual symptoms, the majority of instances are identified at a late stage, with the presence of metastasis already established upon diagnosis of the advanced condition, resulting in an unfavorable prognosis[2]. Regrettably, even with the positive advancements in chemotherapy, surgical procedures, and occasional use of radiotherapy, the estimated 5 y survival rate for ovarian cancer at any stage is approximately 35 %-38 %[3]. Postmenopausal women primarily suffer from ovarian cancer, making up over 90 % of all cases of malignant tumors in the ovaries. Ovarian cancer is most prevalent among women in their sixties and seventies, leading to approximately 13 850 female deaths annually. This cancer type is the primary cause of mortality among women diagnosed with malignant tumors in the field of gynecology[4]. Hence, it is imperative that we promptly discover novel biomarkers for forecasting tumor occurrence and clinical diagnosis, gain profound insights into the molecular mechanism underlying the initiation, advancement, and progression of ovarian cancer, and investigate fresh therapeutic approaches and targets for combating ovarian cancer[5].
Non-coding Ribonuclic Acid (RNA) plays a crucial role in various biological processes, encompassing microRNA (miRNAs) and long noncoding RNA (lncRNA). The process of malignant tumor is significantly influenced by lncRNA. Lysyl Oxidase-Like 1-Antisense 1 (LOXL1-AS1) has been evidenced to function as an oncogene in various types of tumors. Numerous studies in the cancer domain have demonstrated that lncRNA manipulates the horizontal of target miRNA by deceiving miRNA into competing as endogenous noncoding RNA.
LncRNAs do not have the ability to code for proteins. According to previous research, the disruption of lncRNA expression is significant in the development of tumors[6]. In different phases of cancer progression, LncRNA exhibits diverse biological roles such as cell growth and immune response, cell division and specialization, control of cell death, identification as a genomic imprinting indicator, and functioning as a Competitive Endogenous RNA (CERNAs) that competes for micro-RNAs binding sites[7-9]. An increasing number of researches on ovarian cancer are centered around non-protein coding genes, and the significant alteration in lncRNA regulation greatly contributes to the development of malignant phenotypic changes in ovarian cancer[10-12]. Hence, lncRNA could serve as a diagnostic tool for managing ovarian cancer.
miRNAs are small RNA molecules that are endogenous and non-coding. They have the ability to adjust the instability and translation inhibition of miRNAs by binding to specific complementary sites located in the 3-untranslated region of target genes, as stated in reference[13]. Research on growth indicates that miRNA plays a role in numerous cellular activities, such as growth, specialization, cell death, movement, infiltration, and multiplication[14]. Furthermore, mounting proof suggests that miRNAs undergo abnormal regulation in diverse types of cancer[15]. Numerous studies indicate that the dysregulation of miRNA in cancer is imbalanced, serving as both a tumor suppressor and an oncogene. The close association between miR-761 and the onset and advancement of various forms of cancer has been demonstrated. Despite the difficulties posed by alterations in downstream genes, the utilization of miRNAs holds great significance in the management of ovarian cancer[16,17].
Hence, the discovery of novel biomarkers will enhance the well-being of individuals with cancer. miRNAs, are a type of RNA that can control the expression of target genes by binding to the 3-Untranslated Regions (UTR), 5’-UTR, or open reading frame. Numerous studies indicate that the dysregulation of miRNA in cancer is imbalanced, serving as both a tumor suppressor and an oncogene. The close association between miR-761 and the onset and advancement of various forms of cancer has been demonstrated. According to reports, the horizontal of miR-761 expression in ovarian cancer tissue is notably reduced compared to its corresponding non-cancerous tissue. The functional tests indicate that miR-761 hinders the growth and infiltration of ovarian cancer through its interaction with Musashi-2 (MSI2)[18,4].
Materials and Methods
Instruments and reagents:
The items used in the experiment included SKOV-3 cells (a type of ovarian cancer cell), HOSE cells (a type of normal ovarian epithelial cell), trypsin, miR-761 mimics, a Negative Control (NC), a plasmid extraction kit, a fluorescence quantitative kit, penicillin, Roswell Park Memorial Institute (RPMI) 1640 medium, and Lipofectamine 2000 transfection reagent.
The SKOV-3 cell line was preserved in McCoys 5A altered solution (Gibco, Invitrogen), which was enhanced with 10 % Fetal Bovine Serum (FBS) that had been heat inactivated.
Cell culture:
HOSE, a normal cell of the ovary, and SKOV-3, a cell of ovarian cancer, were cultivated in RPMI 1640 medium. The medium was supplemented with 10 % FBS from fetuses. The culture was conducted at a temperature of 37°, with a Carbon dioxide (CO2) concentration of 5 % and humidity ranging from 70 % to 80 %. Next, the cells in the phase of exponential growth were introduced into a 6-well plate and incubated for a specific duration. Subsequently, the cells were examined in an inverted manner using a microscope and synchronized with a medium devoid of serum for 12 h once the fusion of cells reached approximately 50 %. Following this, the experiment of cell transfection was conducted.
Cell transfection:
Following the guidelines provided by the maker, the cells underwent transfection using Lipofectamine 2000 transfection reagent. Specifically, the cells were transfected with si-NC, si-LOXL1-AS1, miR-NC, miR-761, anti-miR-NC, and anti-miR-761. After 48 h of transfection, the cells were gathered. After transfection, it was necessary to change a new medium to continue culture for subsequent experiments.
The expression of LncRNA LOXL1-AS1 and miR-761 was tested by quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR):
TRIzol reagent was used to extract total RNA from HOSE and SKOV-3. Prime Script one step RT-PC kit was utilized to convert Protected Natural Areas (PNAs) into cDNA, followed by conducting the procedure as per the guidelines of the fluorescence quantitative kit (Table 1). The cycling parameters included heating at 95° for 30 s, cooling at 60° for 30 s, and elongation at 72° for 30 s, with a total of 40 cycles. Lastly, there was an additional extension step at 60° for 5 min. PCR was used to amplify LOXL1-AS1, using Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) as the internal reference, and miR-761 was amplified using U6 as the internal reference. The 2-△△Ct method was used to determine the relative expression.
| Gene | Primer Sequence 5’-3’ | |
|---|---|---|
| LOXL1-AS1 | Upstream | 5′-Gatatgttggatgatgga-3′ |
| Downstream | 5′-Gatatgttggatggatga-3′ | |
| miR-76 | Upstream | 5′-Acagcaggcacagac-3′ |
| Downstream | 5′-Gagcaggctggagaa-3′ | |
| U6 | Upstream | 5′-Ctcgcttcggcagcaca-3′ |
| Downstream | 5′-Aacgcttcacgaatttgcgt-3′ | |
| GAPDH | Upstream | 5′-Gaaggtgaaggtcggagtc-3′ |
| Downstream | 5′-Gaagatggtgatgggatttc-3′ | |
Table 1: Primer sequence table.
Double luciferase report experiment to analyze the targeting relationship between LOXL1-AS1 and miR-761:
LncBase Predicted v.2 was used to predict the potential binding locations of LOXL1-AS1 and miR-761. Subsequently, primers were developed to amplify the anticipated binding sequence of LOXL1-AS1 and miR-761 through PCR technology. The resulting fragments were then inserted into a luciferase reporter gene plasmid, resulting in the acquisition of the Wild-Type vector (LOXL1-AS1-WT) for LOXL1-AS1. SKOV-3 cells were transfected with LOXL1-AS1-WT and LOXL1-AS1-Mut along with miR-NC and miR-761 using Lipofectamine 2000. Following a 48 h transfection, luciferase activity was observed as per the guidelines of the dual luciferase reporter gene detection kit, and the obtained data were analyzed.
Cell proliferation experiment:
3-(4,5-Dimethylthiazol-2-yl)-2,5 Diphenyl Tetrazolium Bromide (MTT) assay was used to measure cell proliferation. Inoculating 96-well plates, two sets of cells in the logarithmic growth phase were added, with 5000 cells per 100 l in every well. Following 24, 48, and 72 h of incubation, 20 ml of 5 mg per milliliter MTT were introduced into each well. After a 4 h period, the liquid above the cells was removed, and subsequently, 200 microliters of dimethyl sulfoxide were added. The OD was tested at 490 nm by enzyme-labeled instrument.
Flow cytometry experiment:
We utilized the Annexin V-Fluorescein Isothiocyanate (FITC)/Propidium Iodide (PI) cell apoptosis detection kit and adhered to the provided instructions. After transfection, obtain 1×106 SKOV-3 cells/ml, transfer them to a 6-well plate, trypsinize and collect the cells, rinse the cells twice with pre-chilled PBS buffer, introduce 500 μl buffer, and subsequently apply 5 μl Annexin V-FITC and 5 μl cells stained with PI working solution. Incubate the mixture in darkness for 15 min prior to apoptosis detection using flow cytometry.
Western blot experiment:
Obtain cells transfected for 48 h, extract all proteins from both sets of cells using PIPA lysate, determine the protein concentration following the instructions of the protein assay kit, and modify the protein concentration to 3 mg/ml. Next, heat the protein in a boiling water bath to denature it, and finally, store it at -80° for future utilization. A conventional wet membrane transfer device is used to transfer 40 g of cell protein to Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE). Place it in 5 % skimmed milk for 2 h, rinse the membrane with Tris-Buffered Saline with 0.1 % Tween® 20 Detergent (TBST) for 3 min, and introduce a primary antibody solution diluted 1:1000 for incubation. Leave it overnight at 4°. Wash the TBST membrane thrice for 10 min each, followed by incubation for 2 h in a secondary antibody solution diluted 1:5000. TBST filter was rinsed three times, with each rinse lasting 10 min. For color development, Enhanced Chemiluminescence (ECL) improved luminescence liquid was utilized, and the expression of cyclin D1, p21, B-cell lymphoma-2 (Bcl-2), Bcl-2-associated X protein (BAX), and p21 proteins was analyzed using Quantity One 4.40 software.
Statistical analysis:
The data processing was done using Statistical Package for the Social Sciences (SPSS) 21.0 software for statistical analysis. The measurement data were represented as x±s and followed a normal distribution. The t-test was utilized to compare the two groups, while one-way Analysis Of Variance (ANOVA) was employed for data analysis among multiple groups. A difference that was statistically significant was indicated by p<0.05.
Results and Discussion
The qRT-PCR findings indicated a notable up-regulation in LOXL1-AS1 expression and a significant down-regulation in miR-761 expression in SKOV-3 ovarian cancer cells when compared to HOSE normal ovarian epithelial cells (p<0.05). Table 2 displayed the findings. The expression of LOXL1-AS1 and miR-761 in SKOV-3 cells and HOSE cells was observed in Table 2.
| Group | LOXL1-AS1 | miR-761 |
|---|---|---|
| HOSE | 1.00±0.07 | 1.00±0.05 |
| SKOV-3 | 2.78±0.32* | 0.53±0.04* |
Note: Compared with HOSE group, * p<0.05
Table 2: Expression of LOXL1-AS1 and miR-761 in SKOV-3 cells and HOSE cells (x±s, n=3).
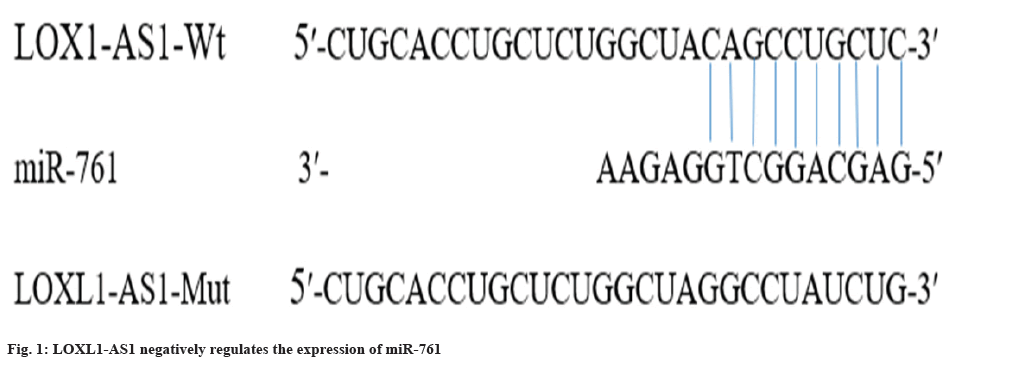
To gain a deeper comprehension of the functioning mechanism of LOXL1-AS1, LncBase Predicted v. 2 was employed to anticipate the presence of a nucleotide sequence that is complementary to miR-761 within LOXL1-AS1. The outcomes are illustrated in fig. 1. According to the findings from the dual luciferase assay, the introduction of miR-761 resulted in a significant decrease in the relative luciferase activity of LOXL1-AS1-WT (p<0.01), while it did not have a notable impact on the relative luciferase activity of LOXL1-AS1-Mut (Table 3). Simultaneously, based on the qRT-PCR findings, it was observed that the introduction of pcDNA-LOXL1-AS1 (plasmid for overexpressing LncRNA LOXL1-AS1) notably diminished the miR-761 expression in comparison to the pcDNA group. In comparison to the si-NC group, the introduction of si-LOXL1-AS1 bring about a significant enhancement in the levels of miR-761 expression (p<0.05) (Table 4). Hence, the lncRNA LOXL1-AS1 exhibits the ability to exert a negative regulatory effect on the expression of miR-761 (p<0.05).
| Group | LOXL1-AS1-WT | LOXL1-AS1-Mut |
|---|---|---|
| miR-NC | 1.00±0.07 | 0.85±0.09 |
| miR-761 | 0.51±0.03* | 0.96±0.09 |
Note: Compared with miR-NC group, *p<0.01
Table 3: Double luciferase report experiment (x±s, n=3).
| Group | miR-761 |
|---|---|
| pcDNA | 1.00±0.07 |
| pcDNA-LOXL1-AS1 | 0.47±0.03a |
| si-NC | 1.15±0.09 |
| si-LOXL1-AS1 | 2.94±0.26b |
Note: Compared with pcDNA group, ap<0.05 and compared with si-NC group, bp<0.05
Table 4: Targeted regulation of miR-761 expression by lncRNA LOXL1-AS1 (x±s, n=3).
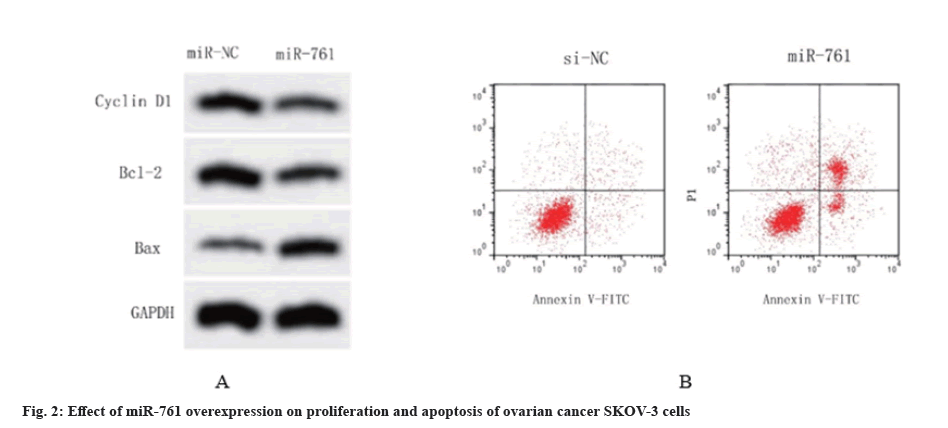
Table 5 demonstrates that the miR-761 transfected SKOV-3 cells exhibited a noteworthy increase in miR-761 expression when compared to the miR-NC group, firming the successful creation of SKOV-3 cells with miR-761 overexpression (p<0.05). In the meantime, the MTT test outcomes indicated that the excessive expression of miR-761 notably decreased the Optical Density (OD) measurements of SKOV-3 cells after 24, 48, and 72 h (p<0.05), consequently enhancing the rate of proliferation inhibition in SKOV-3 cells. Flow cytometry experiments demonstrated that the transfection of miR-761 into SKOV-3 cells resulted in a significant raise in the apoptosis rate compared to the miR-NC group (p<0.05). The Western blot analysis revealed a significant decrease in the expression levels of cyclin D1 and Bcl-2 proteins in the ovarian cancer cell group that underwent miR-761 transfection. Conversely, the expression levels of BAX albumen were notably raised (p<0.05) (fig. 2). The aforementioned trials demonstrated that the excessive expression of miR-761 impeded the growth of SKOV-3 cells, a type of ovarian cancer, and stimulated apoptosis in these cells.
| Group | miR-761 | 24 h | 48 h | 72 h | Apoptosis rate (%) | Cyclin D1 protein | Bcl-2 protein | BAX protein |
|---|---|---|---|---|---|---|---|---|
| miR-NC | 1.00±0.07 | 0.51±0.03 | 0.83±0.04 | 1.32±0.08 | 7.54±0.77 | 0.59±0.06 | 0.72±0.04 | 0.26±0.05 |
| miR-761 | 2.59±0.18* | 0.35±0.05* | 0.52±0.04* | 0.76±0.06* | 19.48±1.03* | 0.21±0.04* | 0.35±0.03* | 0.64±0.07* |
Note: Compared with miR-NC group, *p<0.05
Table 5: Effects of miR-761 overexpression on proliferation and apoptosis of SKOV-3 cells (x±s, n=3).
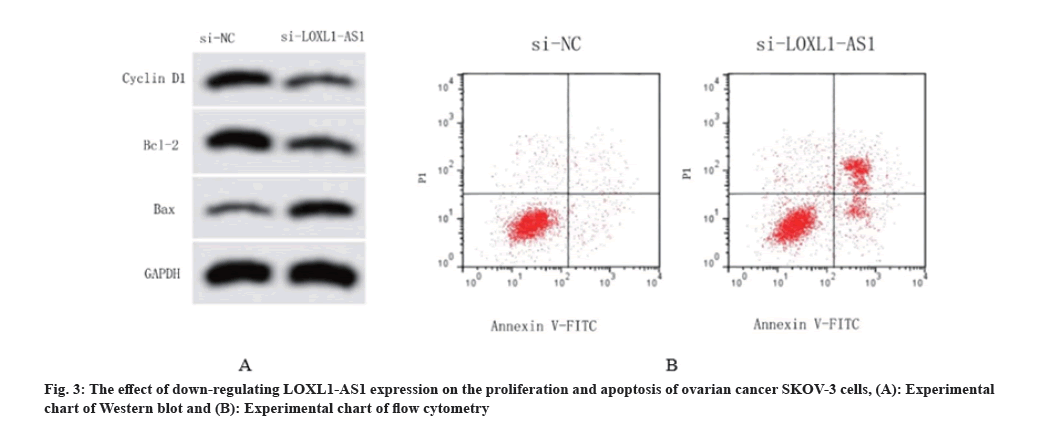
According to Table 6, the si-NC group was compared to SKOV-3 cells transfected with si-LOXL1-AS1, revealing a significant reduce in the expression of LOXL1-AS1 (p<0.05). This suggests the successful creation of ovarian cancer cells with reduced LOXL1-AS1 expression. Based on the findings from MTT and flow cytometry tests, it is evident that suppressing the expression of LOXL1-AS1 considerably reduced the OD values of SKOV-3 cells after 24, 48, and 72 h (p<0.05). Additionally, it enhanced the rate of proliferation inhibition and significantly elevated the rate of cell apoptosis in SKOV-3 cells. The Western blot experiments revealed that the expression horizontals of cyclin D1 and Bcl-2 were significantly decreased in SKOV-3 cells transfected with si-LOXL1-AS1 than the si-NC group. Additionally, there was a significant raise in the expression levels of BAX protein (p<0.05). Fig. 3 displayed the findings. Thus, the inhibition of LOXL1-AS1 can enhance apoptosis in SKOV-3 cells of ovarian cancer.
| Group | miR-761 | 24 h | 48 h | 72 h | Apoptosis rate (%) | Cyclin D1 protein | Bcl-2 protein | BAX protein |
|---|---|---|---|---|---|---|---|---|
| si-NC | 1.00±0.07 | 0.49±0.03 | 0.82±0.04 | 1.28±0.08 | 7.52±0.81 | 0.56±0.05 | 0.71±0.04 | 0.24±0.05 |
| si-LOXL1-AS1 | 0.58±0.04* | 0.33±0.03* | 0.47±0.04* | 0.74±0.05* | 20.73±1.26* | 0.25±0.03* | 0.37±0.03* | 0.67±0.07* |
Note: Compared with miR-NC group, *p<0.05
Table 6: Effects of down-regulation of LOXL1-AS1 expression on proliferation and apoptosis of SKOV-3 cells (x±s, n=3).
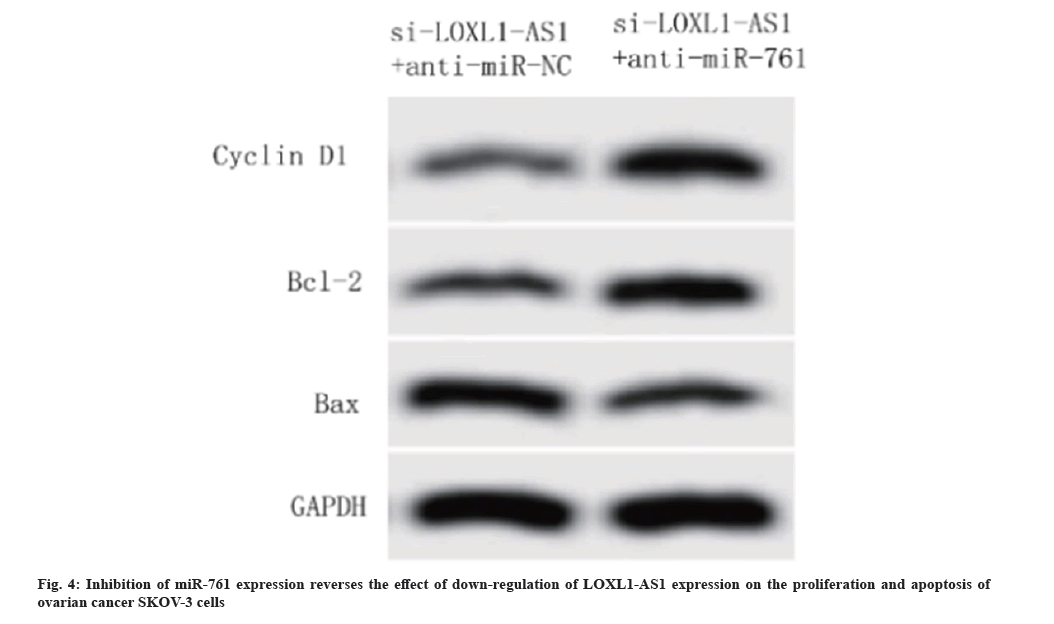
Compared with the co-transfection of si-LOXL1-AS1 and anti-miR-NC, the co-transfection of si-LOXL1-AS1 and anti-miR-761 significantly reduced the expression of miR-761 and BAX protein in SKOV-3 cells, and raised the OD value of SKOV-3 cells at 24 h, 48 h and 72 h and the protein horizontals of cyclin D1 and Bcl-2 (p<0.05) as shown in Table 7 and fig. 4.
| Group | miR-761 | 24 h | 48 h | 72 h | Apoptosis rate (%) | Cyclin D1 protein | Bcl-2 protein | BAX protein |
|---|---|---|---|---|---|---|---|---|
| si-LOXL1-AS1+anti-miR-NC | 2.74±0.25 | 0.39±0.04 | 0.52±0.05 | 0.64±0.07 | 25.48±2.03 | 0.37±0.05 | 0.43±0.04 | 0.78±0.08 |
| si-LOXL1-AS1+anti-miR-761 | 1.38±0.16* | 0.45±0.05* | 0.69±0.03* | 1.26±0.03* | 14.92±1.19* | 0.65±0.03* | 0.79±0.03* | 0.37±0.04* |
Note: Compared with Si-LOXL1-AS1+anti-miR-NC group, *p<0.05
Table 7: Effects of down-regulation of LOXL1-AS1 expression on proliferation and apoptosis of SKOV-3 cells (x±s, n=3).
Currently, ovarian cancer ranks among the most lethal types of gynecological malignancies. Recent statistics indicate that the fatality rate of ovarian cancer among women in China has been on the rise, posing a significant threat to the safety of individuals’ lives[19,20]. Hence, it is imperative to acquire an in-depth comprehension of the framework underlying the onset and progression of ovarian cancer, and discover efficacious therapies to enhance the present scenario.
Due to its crucial involvement in transcription and translation processes, lncRNA has garnered significant attention in the field of cancer gene investigation. Numerous studies have proved that lncRNA plays a crucial part in the onset, cellular growth, and programmed cell death of ovarian cancer, gastric cancer, and various other malignancies. Based on relevant reports, the expression of LOXL1-AS1 in gastric cancer is considerably increased when compared to the surrounding tissues. Furthermore, its expression is strongly associated with the size, proliferation, apoptosis, local lymph node metastasis, and prognosis of patients. The inhibition of LOXL1-AS1 expression significantly enhances the apoptosis of gastric cancer cells and suppresses the invasion and migration of cancer cells[21]. Additionally, the findings from this research indicated a significant reduce in the proliferation rate of SKOV-3 ovarian cancer cells and a notable increase in the rate of apoptosis following the inhibition of LOXL1-AS1 expression. Simultaneously, there was reduction in the horizontals of cyclin D1 and the anti-apoptosis albumen Bcl-2, while the expression horizontal of the apoptosis-promoting albumen BAX showed an increase. Hence, LOXL1-AS1 can be utilized as a fresh molecular objective for combating ovarian cancer.
Research has indicated that miR-761 functions as a gene that inhibits tumor growth in malignant cancers like ovarian cancer and glioma. In a study conducted by Wang et al., it was discovered that miR-761 caused the down-regulation of CRKL, leading to the promotion of apoptosis in SKOV-3 cells of ovarian cancer[22]. In their study, Cao et al.[23] discovered a reduce in the expression of miR-761 in colorectal cancer. Furthermore, they observed that the upregulation of miR-761 facilitated the apoptosis of colorectal cancer cells and heightened their responsiveness to 5-fluorouracil. In a study by Chen et al., it was demonstrated that LNC00665 specifically interacts with miR-761 and modulates the Wnt/β-catenin signaling pathway. This interaction has significant implications on the proliferation, cell cycle distribution of glioma cells[24]. The present investigation observed a decrease in the expression of miR-761 in SKOV-3 cells, a type of ovarian cancer cells. Additionally, the outcomes obtained from MTT and flow cytometry experiments indicated that the overexpression of miR-761 enhanced the apoptosis of SKOV-3 cells. And the upregulation of miR-761 brings out a notable reduce in the levels of cyclin D1 and Bcl-2 proteins, while it enhanced the expression of BAX protein. These findings further validated the outcomes obtained from the flow cytometry investigations. Simultaneously, LncBase Predicted v.2 indicated that miR-761 could potentially be targeted by lncRNA LOXL1-AS1, a finding that was subsequently validated in the double luciferase report assay. Furthermore, when considering the qRT-PCR findings, it was observed that the lncRNA LOXL1-AS1 has the ability to inhibit the expression of miR-761. Reversing the inhibition of miR-761 expression counteracted the down-regulation of lncRNA LOXL1-AS1 expression, which was inhibiting SKOV-3 cell multiplication and the expression of cyclin D1 and Bcl-2. Additionally, it also counteracted the promotion of apoptosis and BAX protein expression. The findings suggested that the growth and programmed cell death of ovarian cancer cells were enhanced by lncRNA LOXL1-AS1 through specific control of miR-761.
In summary, the up-regulation of lnc RNA LOXL1-AS1 is accompanied by the down-regulation of miR-761.The excessive expression of miR-761 and the decrease in LOXL1-AS1 can effectively hinder the growth and enhance the cell death of ovarian cancer cells. Furthermore, the lncRNA LOXL1-AS1 has the ability to inhibit the expression of miR-761, thereby impacting the growth and programmed cell death of ovarian cancer cells. Hence, this research offers a potent biomarker and molecular objective for combating ovarian cancer[25].
Conflict of interests:
The authors declared no conflict of interests.
References
- Shi Y, Gao S, Zheng Y, Yao M, Ruan F. LncRNA CASC15 functions as an unfavorable predictor of ovarian cancer prognosis and inhibits tumor progression through regulation of miR-221/ARID1A axis. Onco Targets Ther 2019;12:8725-36.
[Crossref] [Google Scholar] [PubMed]
- Dong R, Liu X, Zhang Q, Jiang Z, Li Y, Wei Y, et al. miR-145 inhibits tumor growth and metastasis by targeting metadherin in high-grade serous ovarian carcinoma. Oncotarget 2014;5(21):10816-29.
[Crossref] [Google Scholar] [PubMed]
- Cui J, Eldredge JB, Xu Y, Puett D. microRNA expression and regulation in human ovarian carcinoma cells by luteinizing hormone. PLoS One 2011;6(7):e21730.
[Crossref] [Google Scholar] [PubMed]
- Jelovac D, Armstrong DK. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J Clin 2011;61(3):183-203.
[Crossref] [Google Scholar] [PubMed]
- Morice P, Gouy S, Leary A. Mucinous ovarian carcinoma. N Engl J Med 2019;380:1256-66.
- Qi P, Du X. The long non-coding RNAs, a new cancer diagnostic and therapeutic gold mine. Mod Pathol 2013;26(2):155-65.
[Crossref] [Google Scholar] [PubMed]
- Kogo R, Shimamura T, Mimori K, Kawahara K, Imoto S, Sudo T, et al. Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res 2011;71(20):6320-6.
[Crossref] [Google Scholar] [PubMed]
- Struhl K. Transcriptional noise and the fidelity of initiation by RNA polymerase II. Nat Struct Mol Biol 2007;14(2):103-5.
[Crossref] [Google Scholar] [PubMed]
- Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 2010;464(7291):1071-6.
[Crossref] [Google Scholar] [PubMed]
- Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: The Rosetta stone of a hidden RNA language? Cell 2011;146(3):353-8.
[Crossref] [Google Scholar] [PubMed]
- Mitra R, Chen X, Greenawalt EJ, Maulik U, Jiang W, Zhao Z, et al. Decoding critical long non-coding RNA in ovarian cancer epithelial-to-mesenchymal transition. Nat Commun 2017;8(1):1604.
[Crossref] [Google Scholar] [PubMed]
- Li Z, Lei H, Luo M, Wang Y, Dong L, Ma Y, et al. DNA methylation downregulated mir-10b acts as a tumor suppressor in gastric cancer. Gastric Cancer 2015;18(1):43-54.
[Crossref] [Google Scholar] [PubMed]
- Li Z, Yu X, Shen J, Wu WK, Chan MT. microRNA expression and its clinical implications in Ewing's sarcoma. Cell Prolif 2015;48(1):1-6.
[Crossref] [Google Scholar] [PubMed]
- Zhao Y, Li Y, Lou G, Zhao L, Xu Z, Zhang Y, et al. miR-137 targets estrogen-related receptor alpha and impairs the proliferative and migratory capacity of breast cancer cells. PloS One 2012;7(6):e39102.
[Crossref] [Google Scholar] [PubMed]
- Li Z, Yu X, Shen J, Chan MT, Wu WK. micro RNA in intervertebral disc degeneration. Cell Prolif 2015;48(3):278-83.
- Tripathi MK, Doxtater K, Keramatnia F, Zacheaus C, Yallapu MM, Jaggi M, et al. Role of lncRNAs in ovarian cancer: Defining new biomarkers for therapeutic purposes. Drug Discov Today 2018;23(9):1635-43.
[Crossref] [Google Scholar] [PubMed]
- Zhou X, Zhang L, Zheng B, Yan Y, Zhang Y, Xie H, et al. micro RNA-761 is upregulated in hepatocellular carcinoma and regulates tumorigenesis by targeting Mitofusin-2. Cancer Sci 2016;107(4):424-32.
[Crossref] [Google Scholar] [PubMed]
- Shi C, Zhang Z. miR-761 inhibits tumor progression by targeting MSI1 in ovarian carcinoma. Tumor Biol 2016;37:5437-43.
[Crossref] [Google Scholar] [PubMed]
- Torre LA, Trabert B, de Santis CE, Miller KD, Samimi G, Runowicz CD, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin 2018;68(4):284-96.
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71(3):209-49.
[Crossref] [Google Scholar] [PubMed]
- Zhu H, Lu J, Zhao H, Chen Z, Cui Q, Lin Z, et al. Functional long noncoding RNAs (lncRNAs) in clear cell kidney carcinoma revealed by reconstruction and comprehensive analysis of the lncRNA–miRNA–mRNA regulatory network. Med Sci Monit 2018;24:8250.
[Crossref] [Google Scholar] [PubMed]
- Wang S, Zhang J, Chen G, Dong S. miR-761 inhibits human osteosarcoma progression by targeting CXCR1. Int J Clin Exp Pathol 2018;11(11):5327.
[Google Scholar] [PubMed]
- Cao S, Lin L, Xia X, Wu H. microRNA-761 promotes the sensitivity of colorectal cancer cells to 5-Fluorouracil through targeting FOXM1. Oncotarget 2018;9(1):321-31.
[Crossref] [Google Scholar] [PubMed]
- Lv Z, Ma J, Wang J, Lu J. microRNA-761 targets FGFR1 to suppress the malignancy of osteosarcoma by deactivating PI3K/Akt pathway. Onco Targets Ther 2019;12(1):8501-3.
[Crossref] [Google Scholar] [PubMed]
- Cao MX, Jiang YP, Tang YL, Liang XH. The crosstalk between lncRNA and microRNA in cancer metastasis: Orchestrating the epithelial-mesenchymal plasticity. Oncotarget 2017;8(7):12472-83.
[Crossref] [Google Scholar] [PubMed]