- *Corresponding Author:
- Y. Kamisah
Department of Pharmacology, Faculty of Medicine, Universiti Kebangsaan Malaysia, 56000 Cheras, Malaysia
E-mail: kamisah_y@yahoo.com
| Date of Submission | 02 March 2019 |
| Date of Revision | 21 June 2019 |
| Date of Acceptance | 23 September 2019 |
| Indian J Pharm Sci 2019;81(6):1029-1035 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Parkia speciosa, a plant that grows abundantly in Southeast Asia region, has been reported to exhibit antioxidant property. This study aimed to investigate the mechanism of antioxidant effects of empty pod extract of Parkia speciosa on hydrogen peroxide-induced oxidative stress in H9c2 cardiomyocytes. There were 4 groups of cardiomyocytes, the negative control, hydrogen peroxide, extract of Parkia speciosa+hydrogen peroxide and quercetin+hydrogen peroxide. Cardiomyocytes of groups 3 and 4 were preincubated for 1 h with 500 µg/ml of the ethyl acetate fraction of the Parkia speciosa extract or 1000 µM quercetin prior to exposure to 500 μM hydrogen peroxide for 1 h. Hydrogen peroxide significantly increased the protein expression of NADPH oxidase 4, superoxide dismutase 1 and p38 mitogen-activated protein kinase, in comparison to the negative control. NADPH oxidase 4 and superoxide dismutase 1 activities as well as reactive oxygen species level were also elevated. These elevations were significantly decreased in the pretreated groups. The Parkia speciosa extract group had comparable effects to that of quercetin. Parkia speciosa extract showed antioxidant property against hydrogen peroxide-induced oxidative stress in H9c2 cardiomyocytes by regulating mitogen-activated protein kinase and NADPH oxidase 4 signaling pathways.
Keywords
NADPH oxidase, MAPK, polyphenol, superoxide dismutase
Oxidative stress is involved in the development of diseases, which include cardiovascular diseases[1]. Increased oxidative stress in the cardiovascular system causes biochemical modification and oxidative injuries in the cells. There are many pathways involved in oxidative stress such as NADPH oxidase (NOX) pathways[2] and phospho-p38 mitogen-activated protein kinase (MAPK)[3]. Activation of NOX4 would increase reactive oxygen species (ROS) generation, in particular superoxide anion (O2-•), which then stimulates superoxide dismutase 1 (SOD1) to dismutase the anion[4,5]. ROS acts as a second messenger that stimulates MAPK activity[6]. Hydrogen peroxide (H2O2) is one of the signaling molecules that can permeate the cell membrane and activate NOX. NOX4, but not NOX1 and NOX2 is upregulated in cardiac cells with increased oxidative stress[7]. Clinically, an increase in oxidative stress was reported in patients with ST-elevation myocardial infarction[8].
Parkia speciosa Hassk, called as petai papan by the locals, of Leguminosae family, grows abundantly in Southeast Asia region including Indonesia, Thailand, Philippines and Malaysia[9]. Its empty pods with or without seeds are used in folk medicine to help control heart problems[10]. The empty pods contain higher amounts of antioxidants than the seeds[11], believed to be attributable to presence of quercetin, ellagic acid, gallic acid and catechin[12,13]. The pods were also reported to possess antiinflammatory property[13,14].
The antioxidant property of P. speciosa extract (PSE) has been reported in many studies owing to the presence of polyphenols[15-17]. However, information on its antioxidant mechanism is still lacking. It might have a beneficial role in alleviating cardiovascular disease, thus, this study was conducted to further understand its antioxidant mechanism based on MAPK and NOX signaling pathways in H9c2 cardiomyocytes that were induced by H2O2 as a model to enhance oxidative stress.
Materials and Methods
Preparation of PSE:
Pods of P. speciosa plant were bought from a plantation in Bidor, Perak, Malaysia (Dec 2014). A voucher specimen (UKMB 40239) of the plant was located in the Universiti Kebangsaan Malaysia Herbarium (Bangi, Selangor, Malaysia) after its identification by a botanist. Preparation of ethyl acetate fraction from ethanol PSE was done according to a previously described method[13]. The fraction was then kept at 4°. The yield based on dry weight of the pods was 0.938 %.
Phytochemical analysis of PSE:
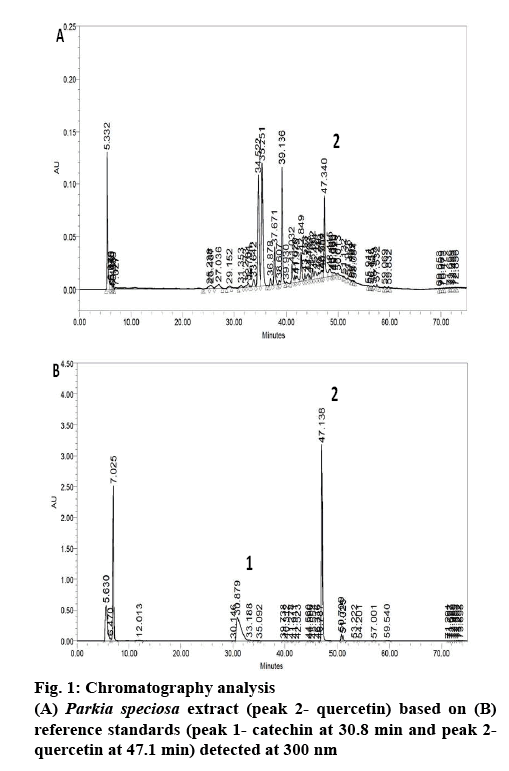
Phytochemical analysis of the PSE was conducted by the means of high-performance liquid chromatography (HPLC) according to a technique previously reported[18] with some modifications[13]. The chromatographic system used was Waters 2535 with a photodiode array detector (Waters 2998, Waters Corp., Milford, MA, USA). A C-18 reversed-phased column was used (5 μm, 4.6×250 mm; XBridge, Waters, Dublin, Ireland). The system was set at 300 nm. The mobile phases were 1 % aqueous acetic acid solution (A) and acetonitrile (B), with the following gradient at ambient temperature, 13 % B for the first 10 min, then changed to 20 % at 20 min, 30 % at 30 min, 50 % at 40 min and to 70 % at 60 min. At 80 min, the gradient was reduced to 20 % B. The flow rate was 0.5 ml/min. The obtained peaks were then compared against the retention time of catechin (30.8 min) and quercetin (47.1 min), the reference standards.
Cell culture and treatment:
H9c2 cardiomyocytes (ATCC, Manassas, Virginia, USA) were grown in Dulbecco’s modified Eagle medium (DMEM) containing 1 % penicillin/ streptomycin/amphotericin B solution (Gibco, Grand Island, NY, USA) and 10 % fetal bovine serum (PAA Laboratories GmbH, Pasching, Austria) in a humidified incubator (5 % CO2 and 95 % air) at 37°. Cells at passages 4-7 were used in the study. There were 4 groups of cardiomyocytes, which were H2O2 (500 μM), PSE (500 μg/ml)+H2O2 (500 μM), quercetin (1000 μM)+H2O2 (500 μM) serving as positive control[19] and a negative control (0.1 % dimethyl sulfoxide, DMSO in DMEM). The cells were pretreated for 1 h and exposure to H2O2 was for another hour. The extract was dissolved in 0.1 % DMSO which gave final concentration of 0.01 % DMSO in the culture.
Cell viability assay:
Seeded cells (3×104 cells/well) were used to measure the effects of various concentrations of H2O2 (50-550 μM) on cell viability after 1 h using CellTiter 96 aqueous One Solution Proliferation Assay kit (Promega, Madison, Wisconsin, USA). The viability of the cells pretreated with various concentrations of the extract fraction (31.25-1000 μg/ml) for 1 h, and then treated with median inhibitory concentration (IC50) of H2O2 for another 1 h, was also measured.
Western blot analysis:
The cells (1.2×105 cell/ml) were seeded in T-75 flask. Extraction of protein samples from cultured cells and western blotting methods were according to previously described protocols[20,21]. The primary antibodies used were NOX4, β-actin (Santa Cruz Biotech, Dallas, Texas, USA), SOD1 and p38 MAPK with 1:100, 1:1000, 1:1000 and 1:500 dilutions, respectively. The secondary antibodies were goat antirabbit IgG-HRP (Cell Signaling, Danvers, Massachusetts, USA) or goat antimouse IgG-HRP (Santa Cruz Biotech, Dallas, Texas, USA) at 1:3000 dilution. The intensity of the oxidative stress related proteins were determined relatively to the intensity of the β-actin and all results were analysed using Image-J software.
Preparation of cell lysate:
The cells (1.2×105 cell/ml) were seeded in T-75 flask to obtain cell lysate. After being treated according to the groups, media in the culture flask were removed and the flask was rinsed with phosphate-buffered saline (PBS) solution twice. Then, the cells that attached to the surface of the flask were removed by using cell scraper. PBS solution containing the cells was centrifuged at 2500 rpm for 5 min. The cell pellet was resuspended in 1 ml of PBS solution, before being centrifuged again at 2500 rpm for 5 min at 4°. The resulting pellet was resuspended in 100 μl of lysis buffer containing protease inhibitor (Complete Mini EDTA-free; Roche, Mannheim, Germany) and dithiothreitol (0.154 mg/ml) in radioimmunoprecipitation assay buffer (RIPA buffer 1x; Sigma-Aldrich, St. Louis, Missouri, USA). The tube was vortexed every 5 min thrice. Then, the tube was centrifuged at 12 000 rpm for 15 min at 4°. The resulting supernatant was the cell lysate.
Determination of NOX activity:
NOX activity assay was carried out using cell lysate following an established method[22]. Briefly, 50 μg cell lysate was added into a reaction mixture (final volume of 200 μl) that consisted of 250 μg/l cytochrome C (Sigma-Aldrich, St Louis, Minnesota, USA), 100 μM NADPH (Sigma-Aldrich, St Louis, Minnesota USA), with or without 100 μM diphenyleneiodonium chloride (Sigma-Aldrich, St Louis, Minnesota USA). The mixture was incubated for 120 min at 37°. Then, the absorbance was measured at 550 nm in ELISA microtiter plate reader. The difference between the sample absorbance values at 0 and 120 min, was taken to calculate the NOX activity with the extinction coefficient 21 mmol/l/cm. It was expressed as percentage of control.
Determination of intracellular ROS level:
Cell-permeant fluorescent probe, 2’,7’-dichlorodihydrofluorescein (DCFH-DA, Sigma- Aldrich, St Louis, Minnesota, USA)[23] was used to determine ROS level in the cells (5×104 cell/ml). The absorbance was read at 485 nm excitation and 535 nm emission by utilizing a fluorescence plate reader (EnSpire Multimode Plate Reader, PerkinElmer, USA). ROS level was expressed as percentage of control.
Determination of SOD activity:
SOD activity was determined[24-26] in the cell lysate. Lysate mixed with 50 mM PBS (pH 7.8), 201.06 mM L-methionine (Sigma-Aldrich, St Louis, Minnesota, USA), 1.71 mM nitro blue tetrazolium (Sigma-Aldrich, St Louis, Minnesota, USA), 1 % Triton X-100 (Sigma- Aldrich, St Louis, Minnesota, USA) and 106.28 μM riboflavin (Sigma-Aldrich, St Louis, Minnesota, USA) was placed in an illuminated aluminium box with two 20-watt Sylvania Arolux fluorescent lamps for 7 min. The absorbance was read at 560 nm by a spectrophotometer (Shimadzu, Kyoto, Japan). SOD activity was calculated as % inhibition of blue formazan formed from the reaction of nitro blue tetrazolium and superoxide produced. The enzyme activity was depicted as U/mg protein. The content of the protein was evaluated using Bradford reagent (Sigma-Aldrich, St Louis, Minnesota, USA).
Statistical analysis:
The experiments were conducted in triplicate. The data were expressed as mean±standard error of mean (SEM). Shapiro-Wilk test was used to evaluate the normality of the data. For normally distributed data, one way analysis of variance (ANOVA) and Tukey’s post-hoc test were applied, while for non-normally distributed data, Kruskal-Wallis test was conducted. P values <0.05 were considered statistically significant.
Results and Discussion
P. speciosa empty pods has been reported to contain polyphenols[12,13]. In the present study, the PSE showed 5 main peaks in the chromatogram (figure 1). However, only quercetin was detected in the extract. The quercetin content in the extract was about 5.74 mg quercetin per 100 g dried extract. In our previous study, PSE ethyl acetate fraction contained the largest amount of quercetin compared to other fractions (ethanol and hexane)[13]. Based on this, the ethyl acetate fraction was selected for further study and quercetin was chosen as the positive control.
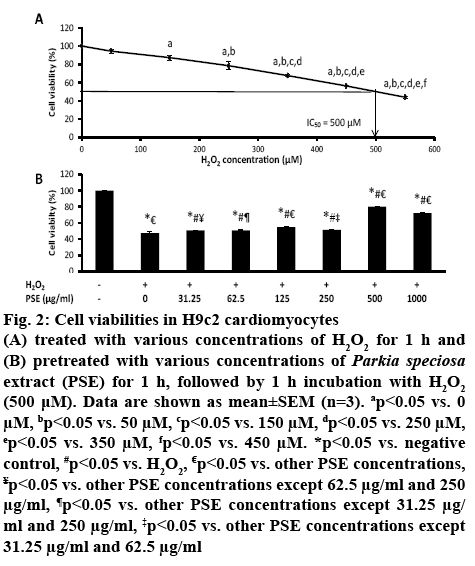
H9c2 cardiomyocytes were cultured with increasing concentrations of H2O2 (50-550 μM) for 1 h. The exposure to H2O2 had decreased H9c2 cell viability concentration-dependently. The median inhibitory concentration (IC50) value of H2O2 obtained was 500 μM (figure 2A). Pretreatment with PSE (31.25 μg/ml to 1000 μg/ml) prior to exposure to 500 μM H2O2 for 1 h had increased cell viability concentration-dependently compared to non-pretreated group (0 μg/ml) that was exposed to H2O2 (p<0.05, figure 2B). The cell viabilities in groups pretreated with 31.25, 62.5 and 250 μg/ml PSE were not different. The group pretreated with 500 μg/ml PSE had the highest cell viability, which was 80.08±0.41 %, while in the group pretreated with 1000 μg/ml PSE was 72.14±0.64 %. The concentration of 500 μg/ml of PSE was selected to be used in subsequent experiments. The significant reduction in cell viability after 1 h exposure of H2O2 showed cytotoxic effect of H2O2. The IC50 of H2O2 obtained in the present study was similarly reported[27]. The peroxide produces hydroxyl radicals in the presence on ferric ion, and superoxide anion which are toxic to cells and increases oxidative stress[28], leading to cell death. The protective effects of PSE against cell death induced by H2O2 most probably owing to high content of flavonoid and phenolic compounds present in the empty pods[15].
Figure 2: Cell viabilities in H9c2 cardiomyocytes
(A) treated with various concentrations of H2O2 for 1 h and (B) pretreated with various concentrations of Parkia speciosa extract (PSE) for 1 h, followed by 1 h incubation with H2O2 (500 μM). Data are shown as mean±SEM (n=3). ap<0.05 vs. 0 μM, bp<0.05 vs. 50 μM, cp<0.05 vs. 150 μM, dp<0.05 vs. 250 μM, ep<0.05 vs. 350 μM, fp<0.05 vs. 450 μM. *p<0.05 vs. negative control, #p<0.05 vs. H2O2, €p<0.05 vs. other PSE concentrations, ¥p<0.05 vs. other PSE concentrations except 62.5 μg/ml and 250 μg/ml, ¶p<0.05 vs. other PSE concentrations except 31.25 μg/ml and 250 μg/ml, ‡p<0.05 vs. other PSE concentrations except 31.25 μg/ml and 62.5 μg/ml
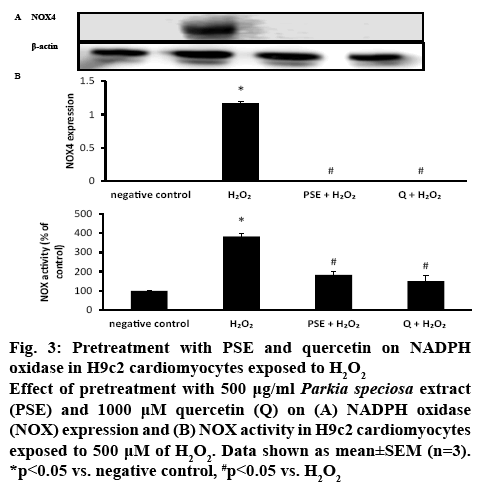
H9c2 cells exposed to H2O2 have been used as a model for evaluating the effects of natural compounds on cardiovascular diseases that are caused by oxidative stress[5,25]. Oxidative stress enhances ROS production and increases the risk for cardiovascular disease[26]. Overproduction of ROS is believed to contribute to the declining cardiac function in patients with cardiac disease[8]. It is known that NOX is involved in one of the pathways in oxidative stress. Induction with H2O2 elevated the NOX4 protein expression significantly (1.17±0.02, p<0.05) compared to the negative control. PSE and quercetin pretreatments significantly blocked the elevation of the NOX4 induced by H2O2. In the negative control, PSE+H2O2 and quercetin+H2O2 groups, there was no detectable NOX4 expression was observed (figure 3A). NOX activity was significantly more than two-fold higher (383.33±16.67 %, p<0.05) in H9c2 cells that were exposed to H2O2 than the negative control. PSE (183.33±16.67 %) and quercetin (150.00±28.87 %) pretreatments had reduced NOX activity significantly compared to H2O2 group (p<0.05).
Figure 3: Pretreatment with PSE and quercetin on NADPH oxidase in H9c2 cardiomyocytes exposed to H2O2
Effect of pretreatment with 500 μg/ml Parkia speciosa extract (PSE) and 1000 μM quercetin (Q) on (A) NADPH oxidase (NOX) expression and (B) NOX activity in H9c2 cardiomyocytes exposed to 500 μM of H2O2. Data shown as mean±SEM (n=3). *p<0.05 vs. negative control, #p<0.05 vs. H2O2
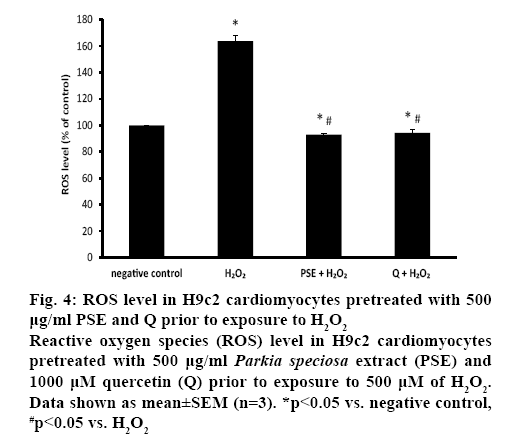
NOX activity was similar in both pretreated groups, which was also comparable to the negative control (figure 3B). The ROS level was measured to confirm the presence of oxidative stress. Exposure to H2O2 significantly augmented intracellular ROS level (163.76±4.23 %) in H9c2 cells. Both PSE (92.85± 0.98 %) and quercetin (94.34±2.87 %) groups that were treated with H2O2 had significantly lower intracellular ROS level than the negative control and H2O2 groups. In the pretreated groups, the ROS levels were similar (figure 4).
Figure 4: ROS level in H9c2 cardiomyocytes pretreated with 500 μg/ml PSE and Q prior to exposure to H2O2
Reactive oxygen species (ROS) level in H9c2 cardiomyocytes pretreated with 500 μg/ml Parkia speciosa extract (PSE) and 1000 μM quercetin (Q) prior to exposure to 500 μM of H2O2. Data shown as mean±SEM (n=3). *p<0.05 vs. negative control, #p<0.05 vs. H2O2
Elevation of oxidative stress induced by H2O2 promotes NOX4 expression, and then its activity as seen in this study. NOX4 is the main isoform of NOX expressed in the cardiomyocytes and the major source of O2•- in the heart[29-30]. Its activation involves p22phox and polymerase delta interacting protein 2 (Poldip2)[31].
When induced with H2O2, p22phox forms a stabilizing complex with NOX4, followed by the translocation of Poldip2 to form an active complex[31,32]. Then, NOX4 produces O2•- by transferring electrons from NADPH to O2 and O2•-[33]. The effect of H2O2 on NOX4 expression was totally blocked by the PSE and quercetin. It was similarly demonstrated[34] that showed reduction of NOX4 expression by ZYZ-772, a quercetin metabolite from Zanthoxylum bungeanum in H9c2 cells. On the other hand, the NOX activity was not similarly blocked in both groups (PSE and quercetin) because the activity measured was not NOX4-specific, but measured total activity of other NOXs as well (NOX1, NOX2, NOX3, NOX5, DUOX1 and DUOX2). The protective effect of PSE was most likely afforded by its quercetin content. It is postulated that PSE and quercetin inhibited Poldip2 translocation to p22phox in the membrane, as well as inhibited translocation of cytosolic subunit (p40phox, p47phox and p67phox) to the membrane and hence, the formation of active oxidase to form O2•[35].
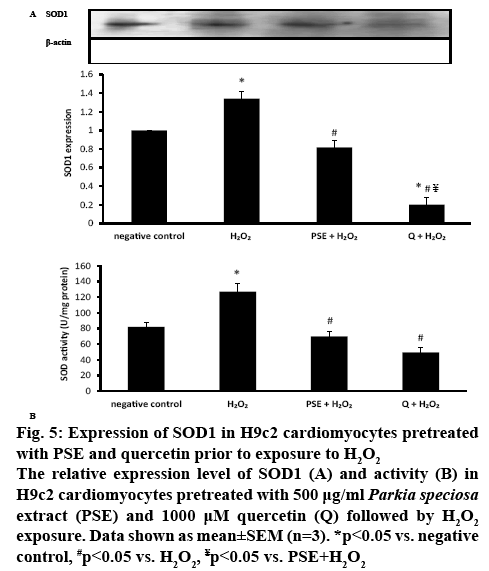
In H2O2-treated H9c2 cells, SOD1 protein expression was significantly increased (1.34±0.08, p<0.05). The increment was significantly attenuated in the groups pretreated with PSE (0.82±0.07) and quercetin (0.20±0.08, p<0.05). Quercetin pretreatment had significantly diminished SOD1 protein expression, which expression was significantly smaller than the negative control group and PSE-pretreated group (p<0.05). The protein expression observed between the negative control group and PSE-pretreated group was not different (figure 5A). Incubation with H2O2 significantly elevated SOD activity (127.01±10.73 U/mg protein) in the cells compared to the negative control (81.97±5.60 U/mg protein). The SOD activity was decreased to 69.86±6.87 U/mg protein in the PSE-pretreated and 49.46±5.78 U/mg protein in the quercetin-pretreated groups, compared to the H2O2 group (p<0.05). The activity of the enzyme was not different in both pretreated groups, and comparable to the activity of the negative control group (figure 5B).
Figure 5: Expression of SOD1 in H9c2 cardiomyocytes pretreated with PSE and quercetin prior to exposure to H2O2
The relative expression level of SOD1 (A) and activity (B) in H9c2 cardiomyocytes pretreated with 500 μg/ml Parkia speciosa extract (PSE) and 1000 μM quercetin (Q) followed by H2O2 exposure. Data shown as mean±SEM (n=3). *p<0.05 vs. negative control, #p<0.05 vs. H2O2, ¥p<0.05 vs. PSE+H2O2
Upregulation of NOX4 and elevated NOX activity in the present study increased production of ROS in the cells, which was mitigated by PSE and quercetin. O2•- is importantly involved in the pathogenesis of many diseases, like hypertension and atherosclerosis[36]. Quercetin in the PSE might be neutralizing ROS by donating electrons or hydrogen atoms. Produced O2•- is subsequently converted to H2O2 by SOD[33]. Therefore, increased production of the anion following exposure to H2O2 had increased the expression and activity of the SOD1, as observed in the present study, in turn to increase the synthesis of functional SOD enzyme to convert O2•- anion to less reactive H2O2 [4], which is then converted into water and oxygen by catalase and glutathione peroxidase[37]. SOD is the primary defense mechanism and plays more important role in detoxifying ROS than catalase and glutathione peroxidase[4].
Reduced formation of ROS by both PSE and quercetin had reduced the demand to increase SOD1 synthesis in the cells, as proven by decreased expression and activity of the antioxidant enzyme. Quercetin had stronger effect on SOD1 downregulation than PSE, possibly attributable to its higher concentration (1000 μM) than in the latter (2.12 μM). However, its effect on the SOD activity was comparable with PSE, which might be due to the post-translational modification that leads to decreased SOD enzyme synthesis by the quercetin.
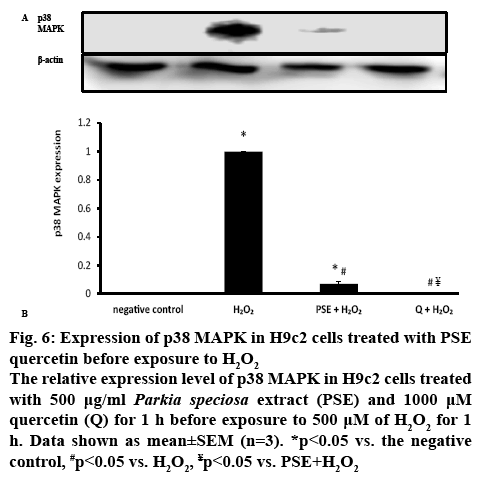
p38 MAPK is one of the pathways known to be involved in oxidative stress. Therefore, the protein expression of MAPK was determined to confirm the involvement of the kinase in the protective effect of PSE. H2O2 induction in H9c2 cells significantly (p<0.05) increased p38 MAPK protein expression in comparison to the negative control group (figure 6). p38 MAPK protein expression was significantly reduced in with PSE- (0.07±0.02) and quercetin-pretreated groups compared to the H2O2 group (p<0.05). PSE-pretreated group had significantly greater p38 MAPK protein expression than the negative control. Quercetin-pretreated group had comparable protein expression with the negative control group and significantly lower protein expression than PSE-pretreated group. No p38 MAPK expression was detected in the quercetin-pretreated and negative control groups.
Figure 6: Expression of p38 MAPK in H9c2 cells treated with PSE quercetin before exposure to H2O2
The relative expression level of p38 MAPK in H9c2 cells treated with 500 μg/ml Parkia speciosa extract (PSE) and 1000 μM quercetin (Q) for 1 h before exposure to 500 μM of H2O2 for 1h. Data shown as mean±SEM (n=3). *p<0.05 vs. the negative control, #p<0.05 vs. H2O2, ¥p<0.05 vs. PSE+H2O2
ROS triggers phosphorylation and activation of p38 MAPK, evidenced by augmented expression of the kinase and such effect was attenuated by PSE and quercetin. H2O2 might cause oxidative modification of the intracellular kinase (MAP kinase kinase kinase, MAP3Ks), which is involved in the first step for p38 MAPK activation[38], or deactivation of MAPK phosphatases (MKPs)[19]. Few other studies had previously shown that elevated ROS produced by H2O2 activated p38 MAPK phosphorylation[6,34]. PSE and quercetin could be triggering MKPs deactivation of MAPK by dephosphorylating residues of tyrosine and threonine at the MAPK activation site[39]. More prominent inhibitory effect of quercetin on p38 MAPK expression could be due to its higher concentration. The current in vitro study indicated the protective effects of PSE in impeding oxidative stress-associated cardiovascular diseases. It’s in vitro beneficial effects needs to be further confirmed by in vivo studies, before its use as a supplement to reduce the risk of cardiovascular diseases. In conclusion, the current findings suggested that PSE acted as an antioxidant and protected H9c2 cardiomyocytes against H2O2-induced oxidative stress by modifying NOX4 and MAPK signalling pathways. Its effects were comparable to that of quercetin, but less effective in downregulating SOD1 and p38 MAPK. The beneficial effects of this plant could be most probably owing to the polyphenol content, particularly quercetin.
Acknowledgments:
The authors thanked technical aid provided by Encik Fadhlullah Zuhair Japar Sidik, Puan Juliana Abdul Hamid and Puan Nurul Hafizah Abas. This work was financially supported by Universiti Kebangsaan Malaysia (AP-2014-013).
References
- Siti HN, Kamisah Y, Kamsiah J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vascul Pharmacol2015;71:40-56.
- Cao YJ, Zhang YM, Qi JP, Liu R, Zhang H, He LC. Ferulic acid inhibits H2O2-induced oxidative stress and inflammation in rat vascular smooth muscle cells via inhibition of the NADPH oxidase and NF-κB pathway. Int Immunopharmacol 2015;28:1018-25.
- Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev 2002;23:599-622.
- Dhawan V. Reactive oxygen and nitrogen species: General considerations. In: Saha GK, Jindal SK, Biswal S, Barnes PJ, Pawankar R, editors. Studies on Respiratory Disorders. New York: Humana Press; 2014. p. 27-47.
- Zhang F, Huang B, Zhao Y, Tang S, Xu H, Wang L, et al. BNC protects H9c2 cardiomyoblasts from H2O2-induced oxidative injury through ERK1/2 signaling pathway. Evid Based Complement Altern Med 2013;2013:1-12.
- Daubney J, Bonner PL, Hargreaves AJ, Dickenson JM. Cardioprotective and cardiotoxic effects of quercetin and two of its in vivo metabolites on differentiated H9c2 cardiomyocytes. Basic Clin Pharmacol Toxicol 2015;116:96-109.
- Varga ZV, Kupai K, Szűcs G, Gáspár R, Pálóczi J, Faragó N, et al. MicroRNA-25-dependent up-regulation of NADPH oxidase 4 (NOX4) mediates hypercholesterolemia-induced oxidative/nitrative stress and subsequent dysfunction in the heart. J Mol Cell Cardiol 2013;62:111-21.
- Ekeløf S, Jensen SE, Rosenberg J, Gögenur I. Reduced oxidative stress in STEMI patients treated by primary percutaneous coronary intervention and with antioxidant therapy: A systematic review. Cardiovasc Drugs Ther 2014;28:173-81.
- Balaji K, Nedumaran SA, Devi T, Sikarwar MS, Fuloria S. Phytochemical analysis and in vitro antioxidant activity of Parkia speciosa. Int J Green Pharm 2015;9:S50-4.
- Yullia T. Prakata. In: Petai VM, Jengkol, editors. Tim Dapur DeMedia, DeMedia Pustaka. 1st ed. Indonesia: Jakarta Selatan; 2008. p. 2.
- Kamisah Y, Othman F, Qodriyah HMS, Jaarin K. Parkia speciosa Hassk.: A potential phytomedicine. Evid Based Complement Altern Med2013;2013:1-9.
- Ko HJ, Ang LH, Ng LT. Antioxidant activities and polyphenolic constituents of bitter bean Parkia speciosa. Int J Food Prop 2014;17:1977-86.
- Mustafa NH, Ugusman A, Jalil J, Kamisah Y. Anti-inflammatory properties of Parkia speciosa empty pod extract in human umbilical vein endothelial cells. J Appl Pharm Sci 2018;8:152-8.
- Gui JS, Jalil J, Jubri Z, Kamisah Y. Parkia speciosa empty pod extract exerts anti-inflammatory properties by modulating NFκB and MAPK pathways in cardiomyocytes exposed to tumor necrosis factor-α. Cytotechnology 2019;71:79-89.
- Kamisah Y, Zuhair JSF, Juliana AH, Jaarin K. Parkia speciosa empty pod prevents hypertension and cardiac damage in rats given N(G)-nitro-l-arginine methyl ester. Biomed Pharmacother 2017;96:291-8.
- Reihani SFS, Azhar ME. Antioxidant activity and total phenolic content in aqueous extracts of selected traditional Malay salads (Ulam). Int Food Res J 2012;19:1439-44.
- Tunsaringkarn T, Soogarun S, Rungsiyothin A, Palasuwan A. Inhibitory activity of Heinz body induction in vitro antioxidant model and tannin concentration of Thai mimosaceous plant extracts. J Med Plant Res 2012;6:4096-101.
- Komolafe K, Olaleye TM, Omotuyi OI, Boligon AA, Athayde ML, Akindahunsi AA, et al. In vitro antioxidant activity and effect of Parkia biglobosa bark extract on mitochondrial redox status. J Acupunct Meridian Stud 2014;7:202-10.
- Chen YW, Chou HC, Lin ST, Chen YH, Chang YJ, Chen L, et al. Cardioprotective effects of quercetin in cardiomyocyte under ischemia/reperfusion injury. Evid Based Complement Altern Med2013;2013:1-16.
- Mahmood T, Yang PC. Western blot: technique, theory and troubleshooting. N Am J Med Sci 2012;4:429-34.
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: A laboratory manual. Vol 3. 3rd ed. New York: Cold Spring Harbor Laboratory Inc.; 2001.
- Mustapha NM, Tarr JM, Kohner EM, Chibber R. NADPH oxidase versus mitochondria-derived ROS in glucose-induced apoptosis of pericytes in early diabetic retinopathy. J Ophthalmol 2010;2010:1-10.
- Yang Y, Kim SC, Yu T, Yi YS, Rhee MH, Sung GH, et al. Functional roles of p38 mitogen-activated protein kinase in macrophage-mediated inflammatory responses. Mediators Inflamm2014;2014:1-13.
- Beyer WF, Fridovich I. Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem 1987;161:559-66.
- Mojarrab M, Jamshidi M, Ahmadi F, Alizadeh E, Hosseinzadeh L. Extracts of Artemisia ciniformis protect cytotoxicity induced by hydrogen peroxide in H9c2 cardiac muscle cells through the inhibition of reactive oxygen species. Adv Pharmacol Sci 2013;2013:1-5.
- Dhalla NS, Temsah RM, Netticadan T. Role of oxidative stress in cardiovascular diseases. J Hypertens 2000;18:655-73.
- Ariumi H, Aso K, Yoshiyama Y. Hydrogen gas protects H9c2 cardiomyocytes from H2O2-induced oxidative stress. AATEX 2014;19:51-4.
- Imlay JA. Cellular defenses against superoxide and hydrogen peroxide. Annu Rev Biochem 2008;77:755-76.
- Ago T, Matsushima S, Kuroda J, Zablocki D, Kitazono T, Sadoshima J. The NADPH oxidase Nox4 and aging in the heart. Aging2010;2:1012-6.
- Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci U S A 2010;107:15565-70.
- Miller FJ Jr. Nox4-walking the walk with Poldip2. Circ Res 2009;105:209-10.
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 2007;87:245-313.
- Brown DI, Griendling KK. Nox proteins in signal transduction. Free Radic Biol Med2009;47:1239-53.
- Wang Y, Zhong L, Liu X. ZYZ-772 prevents cardiomyocyte injury by suppressing Nox4-derived ROS production and apoptosis. Molecules 2017;22:E331.
- Maraldi T. Natural compounds as modulators of NADPH oxidases. Oxid Med Cell Longev 2013;2013:1-10.
- Fukai T, Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal 2011;15:1583-606.
- Paravicini TM, Touyz RM. NADPH oxidases, reactive oxygen species, and hypertension: clinical implications and therapeutic possibilities. Diab Care 2008;31:S170-80.
- Son Y, Cheong YK, Kim NH, Chung HT, Kang DG, Pae H. Mitogen-activated protein kinases and reactive oxygen species: how can ROS activate MAPK pathways? J Signal Transduct 2011;2011:792639.
- Kondoh K, Nishida E. Regulation of MAP kinases by MAP kinase phosphatases. Biochim Biophys Acta 2007;1773:1227-37.