- *Corresponding Author:
- S. K. Dunga
Department of Pharmacy, Annamalai University, Chidambaram, Tamilnadu 608002, India
E-mail: dungakiran@gmail.com
| Date of Received | 21 December 2023 |
| Date of Revision | 15 May 2024 |
| Date of Acceptance | 24 June 2024 |
| Indian J Pharm Sci 2024;86(3):1102-1109 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The purpose of this study was to examine how rats might be protected from stone formation by using a hydroalcoholic extract of Litchi chinensis fruit. Adult male albino Wistar rats were given 0.75 % ethylene glycol water orally for 28 d to produce lithiasis. Cystone (750 mg/kg, p.o.) was utilized as a reference standard to assess the action against ethylene glycol-mediated lithiasis and rats were administered oral dosages of 200 and 400 mg/kg of hydroalcoholic extract of Litchi chinensis fruit once daily. In the study, it was shown that lithiasis led to a rise in serum total protein, creatinine, uric acid, blood urea nitrogen, bilirubin, calcium, oxalate and lactate dehydrogenase, as well as an increase in urine creatinine, uric acid, urea, calcium and oxalate levels. In contrast, magnesium and citrate levels were found to decrease. At the end of the 28th d, the investigation revealed changes in pancreatic and liver histology, an increase in inflammatory mediators (tumor necrosis factor alpha and interleukin-6) and changes in enzymatic and non-enzymatic oxidative stress markers (glutathione, prothrombin complex concentrate, and mass drug administration). The biomarkers were found to be significantly elevated in lithiatic rats before treatment with hydroalcoholic extract of Litchi chinensis fruit, suggesting that this compound may have stone-inhibiting properties. This could be attributed to the presence of polyphenols in Litchi chinensis, which can protect against the formation of stones in the pancreas (pancreatolithiasis) and kidneys (nephrolithiasis) of animals with lithiasis.
Keywords
Litchi chinensis, ethylene glycol, oxidative stress, inflammation
A medical disorder that has been known for centuries is kidney stone disease, which is also called nephrolithiasis or urolithiasis. A growing number of people around the world are experiencing the discomfort of kidney stones, which affect an estimation of 1 %-15 % of the population[1]. Kidney stones, if not addressed, can cause a ureteral blockage, blood in urine, recurrent Urinary Tract Infections (UTI), nausea, vomiting and agonizing pain when urinating. An increased risk of cardiovascular disease, diabetes, high blood pressure, chronic kidney disease, end-stage renal disease and kidney stones is also linked to kidney stones[2]. Calcium Oxalate (CaOx), carbapatite, urate, struvite and brushite are the five most common kinds of kidney stones. The systemic disease known as metabolic syndrome is thought to have a connection to kidney stones[3]. Extracorporeal Shockwave Lithotripsy (ESWL) and conventional medication are the usual tools for the treatment of kidney stones. Nevertheless, there are several potential side effects associated with these procedures, and there is also a chance of infection and residual stone fragments following ESWL. In recent years, many nations have begun to see medicinal plants as a viable substitute for traditional pharmaceuticals[4]. Many different treatment types rely on medicinal plants as a natural medicine source because of their low risk of adverse effects, high effectiveness, widespread cultural acceptability and lack of regulatory oversight.
Preventing kidney stones is possible with several different medications. Among them are the diuretic thiazide and the alkaline citrate having said that, they are not well-tolerated and have limited effectiveness[5]. Kidney stone surgical procedures are having their own set of drawbacks. Hence, investigating novel pharmacological treatments is worthwhile, research has shown that medicinal plants that have antioxidant, antispasmodic and diuretic properties can also inhibit crystallization, nucleation and aggregation[6]. When it comes to urolithiasis, they work wonders. Research has shown that consuming more plant-based foods can enhance the volume and pH of urine, in addition to increasing the levels of stone-inhibiting minerals such as magnesium, potassium, citrate and phytate[7]. Uric acid and CaOx excess are linked to these inhibitors. Lychee, or Litchi chinensis (L. chinensis) as it is officially known, is an edible fruit that has several health benefits and this article will discuss those benefits.
Sapindaceae family includes the medicinal plant L. chinensis, which has several useful chemicals including phenolics, triterpenes, flavonoids and sterols[8]. There are many different kinds of phytochemical substances found in L. chinensis. These include phenolic acids, proanthocyanidins, anthocyanins, coumarins, lignans, chromanes, sesquiterpenes, fatty acids, sterols and triterpenes[9]. The anti-hyperlipidemic, anti-platelet, anti-tussive, analgesic, antipyretic, hemostatic, diuretic and antiviral effects of these substances are only a few of the many positive health effects that have been demonstrated[10]. Research on the efficacy of L. chinensis in avoiding stone development in rats suffering from ethylene glycol-induced lithiasis was carried out in this study.
Materials and Methods
Fruit materials:
Prof. Sathya Narayana Raju, University Department of Botany and Microbiology, verified the authenticity of the ripe fruits of L. chinensis before they were harvested from the cultivation field in Guntur, Andhra Pradesh, India. The fruits are ground into a powder after being dried in the shade. The experimental research utilized the powder, which was suspended in 2 % gum acacia.
Experimental animals:
Male albino Wistar rats utilized in this investigation were procured from Mahaveer Laboratories in Hyderabad and weighed 180-200 g. In their normal housing, the animals were kept at a temperature of 23±1°, relative humidity of 50±10 % and on a 12:12 light/dark cycle. A typical laboratory meal consisting of 70 % carbohydrates, 25 % proteins and 5 % lipids (Hindustan Lever, Bangalore) was provided to the rats ad libitum, along with unlimited access to water. The rats were given 2 w to acclimatize after being randomly assigned before the trial. The protocols for housing and managing the animals were laid out by that Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA). Approval number 011/IAEC/NCPA/PhD 21-22 indicates that our college has the necessary approvals from CPCSEA to perform animal studies and the study also has the prior approval of our Institutional Animal Ethics Committee (IAEC).
Experimental procedure:
The experimental groups were as follows; group 1 indicates normal control (vehicle); group 2 indicates disease control (0.75 % ethylene glycol); group 3 indicates 0.75 % ethylene glycol+standard (750 mg/kg of cyston); group 4 indicates0.75 % ethylene glycol+L. chinensis (200 mg/kg) and group 5 indicates 0.75 % ethylene glycol+L. chinensis (400 mg/kg).
To produce calculi, groups 2-5 will be given drinking water containing 0.75 % ethylene glycol from 1st d until 28th d. Male Wistar rats developed hypercalciuria and hyperoxaluria after being chronically administered 0.75 % ethylene glycol. After heart puncture, blood samples were taken from the rats. Centrifugation was used for 15 min at 2500 rpm to separate the collected blood into plasma and serum. The serum samples were kept at -20° until they could be used for biochemical parameter measurement. Once the 28 d treatment period had passed, the rats were put to sleep and their pancreas was carefully removed before being cleaned with ice-cold saline. Next, 2 ml of ice-cold phosphate buffer (20 mm, pH 7.4) was added to the pancreas, and the mixture was homogenized using a Teflon pestle in a glass homogenizer. Analysis was performed on the supernatant obtained by centrifuging the homogenized mixture.
Biochemical analysis:
Upon completion of the 28 d research period, serum biochemical markers including urea, uric acid, creatinine, higher Blood Urea Nitrogen (BUN), calcium, oxalate and Lactate Dehydrogenase (LDH) were predicted. Before collecting blood samples, the rats were fasted for one night. Coral Diagnostics Ltd., of India provides commercially accessible kits that were used for the estimation by their recommended methods.
Urine analysis:
Rats had their urine samples taken every 24 h on 28th d. While we collected their data, the animals were housed in separate metabolic cages with unrestricted access to water. The collected urine was treated with a drop of strong hydrochloric acid before being stored at 4°. The urine samples were used to estimate specific parameters, including urine production, pH, calcium, uric acid, phosphate and magnesium.
Tissue analysis:
Anesthesia was given to the rats after they had been treated with Hydroalcoholic extract of L. chinensis Fruit (HALCF) for 28 d. After that, the kidneys and pancreas were removed and rinsed with an ice-cold saltwater solution. In a glass homogenizer, the pancreas and kidneys were further mixed with 2 ml of ice-cold phosphate buffer (20 mm, pH 7.4) and a Teflon pestle. Analysis was performed on the supernatant obtained by centrifuging the homogenized mixture. To determine the total and soluble protein was in the lens homogenate, the Lowry method was used[11]. The photoreduction method known as Nitro Blue Tetrazolium (NBT) was used to measure Superoxide Dismutase (SOD). Step one involved adding 1.2 ml of a 0.052 M sodium pyrophosphate buffer (pH 8.3) to 0.1 ml of homogenate supernatant. Step two involved adding 186 µm phenazonium methosulphate (0.1 M), 300 µm NBT (0.3 ml) and 780 µm NADH (0.2 ml). Before adding 1 ml of glacial acetic acid to terminate the process, the mixture was incubated for 90 s at 30°. Centrifuged at 4000 rpm for 10 min after vigorously stirring and shaking with 4 cc of n-butanol. At 560 nm, the organic layer's absorbance was recorded[12]. The capacity of the sample to hydrolyze H2O2 was used to calculate Catalase (CAT). The incubation mixture was prepared by combining 20 µl of kidney homogenate with 1 M Tris (hydroxymethyl) aminomethane-hydrochloride, 5 mm Ethylenediaminetetraacetic Acid (EDTA) at pH 8.0 and 900 µl of 10 mm H2O2 enzyme substrate. Distilled water was added until the volume reached 30 µl. The capacity to degrade H2O2 was assessed at 230 nm using a spectrophotometer[13]. A solution containing 0.2 ml of kidney homogenate, 0.2 ml of 0.8 mm EDTA, 0.1 ml of sodium azide, 0.1 ml of 4 mm Glutathione (GSH), 0.1 ml of H2O2 solution and 0.4 ml of 0.4 M phosphate buffer (pH 7) was used to evaluate reduced GSH activity. Centrifuge at 2000 rpm for 10 min after adding 0.5 ml of 10 % Trichloroacetic Acid (TCA); incubate at 370° for 10 min; add 0.1 m of 0.04 % Ellman's reagent (DTNB) solution to the supernatant. Keep the tubes at room temperature. At 420 nm, the optical density of the resulting mixture was measured[14]. Following the protocol laid forth by Ohkawa et al. we assessed the concentrations of Malondialdehyde (MDA) in kidney homogenates by mixing 0.1 ml of homogenate with 2 ml of distilled water, then adding 0.1 ml of thiobarbituric acid and 1 ml of TCA[15]. Once the mixture had cooled, butanol was added to it after it had been incubated in a water bath. After centrifugation was used to separate the organic phase, the optical density was measured at 532 nm[15]. When the research period concluded, urine samples were analyzed within 1 d of collection. Urine Creatinine (Cr), uric acid, and urea concentrations were determined using commercially available kits. The volume of urine was also estimated. The following equation was utilized to determine Cr clearance, abbreviated as Ccr.
CCr=(urine Cr/serum Cr)×(1000/mass of the body)
The carbonyl content of the proteins in the homogenates was measured by adding 1 ml of a solution containing 10 mm 2,4-dinitrophenylhydrazine (DNPH) in 2 M HCl to the reaction mixture. For 30 min, the samples were left to incubate at room temperature. After that, 1 ml of cold TCA (10 % w/v) was added to the concoction and then spun at 3000 rpm for 10 min. The protein pellet was dissolved in 1 ml of guanidine hydrochloride (6 M, pH 2.3), after being rinsed three times with 2 ml of ethanol/ethyl acetate (1:1, v/v). A wavelength of 370 nm was used to measure the sample's absorbance[16].
Tumour Necrotic Factor (TNF-Alpha (α)) and Interleukin (IL-6):
The levels of biochemical parameters in liver homogenate were measured by Enzyme-Linked Immuno Sorbent Assay (ELISA) assay using commercially available TNF-α and IL-6 ELISA kits (eBioscience Inc., San Diego, CA, USA), following the manufacturer’s instructions.
Histopathological studies:
Rat pancreas and kidneys were removed and stored for 48 h in 10 % neutral buffered formalin. The next step was to rinse the organs under running water from the tap. To further clean the organs and eliminate extra tissue, an automated tissue processor (Yorco) was utilized. Using a Leica microtome, 3 µm slices were cut from the treated tissue sections, and then they were embedded in paraffin using a Leica embedding station. After staining the slices with hematoxylin and eosin, they were examined for any alterations under a light microscope (Max Erb).
Results and Discussion
In the urinary tract, an imbalance between chemicals that encourage and inhibit the production of stones leads to urinary lithiasis or stones in the urine. Lithogenesis is the mechanism by which calculi in the urinary tract come to be. The majority of stones, according to research, consist of CaOx[17]. Researchers have successfully induced CaOx urolithiasis in rats and other animal models. An example of this is the hyperoxaluria paradigm in rats that is caused by ethylene glycol. In this model, the renal tubules of the rats rapidly develop CaOx crystals[18]. Rapid screening of anti-urolithic medicines is a common application of this technique. Additionally, crystalluria, hyperoxaluria, hypercalciuria, hypocitraturia and hypomagnesemia can be produced by the ethylene glycol model, which is well-liked for generating lithiasis. CaOx deposits harm cells in the pancreas and kidneys[19]. The researchers set out to find out how fruit extracts affected the ethylene glycol model's ability to produce stone development in the kidneys and pancreas. The experiment lasted for 28 d and the rats were given cystone and HALCF administration. As demonstrated in Table 1 and Table 2, the serum levels of urea, creatinine, bilirubin, uric acid, protein, LDH, calcium and oxalate were elevated during ethylene glycol induction, but they returned to normal following HALCF treatment. There is a decrease in urine production in rats with urolithiasis and individuals with renal stones because the Glomerular Filtration Rate (GFR) is reduced. The result is an increase in the blood concentration of nitrogenous waste products, such as urea and creatinine[20]. It has another effect which is a reduction in creatinine clearance. An increase in uric acid levels reduces the inhibitory function of glycosaminoglycans and messes with the solubility of CaOx. It is believed that uric acid is the main component in the development of stones because of the prevalence of uric acid crystals in CaOx stones and the fact that uric acid binding proteins may bind to CaOx and control its crystallization[21]. Within this investigation, the ethylene glycol model group had an elevated concentration of urine uric acid. This rise in uric acid excretion was however, markedly attenuated by cystone therapy and other treatment groups (Table 3). Reduced GFR is the result of urolithiasis, a condition in which stones in the urinary tract restrict urine outflow. Consequently, waste substances including uric acid, creatinine and urea can build up in the blood. It is possible to develop CaOx stones after using ethylene glycol since it lowers urine pH and raises serum, urine and kidney calcium levels. The use of urine pH-neutralizing agents and the reduction of calcium levels by cystone therapy, however can mitigate this effect. Urolithiasis inhibitors include magnesium and citrate. The availability of CaOx for precipitation can be reduced when magnesium and oxalate mix[22]. The same holds for citrate; it can mix with calcium ions to lower supersaturation levels, which in turn reduces crystal formation and aggregation. The levels of magnesium and citrate are much lower in illness groups when compared to normal groups. Table 4 shows that in rats induced with ethylene glycol, treatment with certain fruits significantly decreased levels of crystal promoters like calcium and oxalate and increased levels of crystal inhibitors like magnesium and citrate.
| Groups | Total protein | Creatinine | Uric acid | BUN | Bilirubin |
|---|---|---|---|---|---|
| Control | 7.45±0.37 | 0.79±0.05 | 3.25±0.45 | 20.23±0.24 | 0.77±0.09 |
| Ethylene glycol (0.75 %) | 3.24±0.22@ | 1.20±0.03@ | 8.23±0.22@ | 28.23±0.23@ | 1.21±0.05@ |
| Cystone (750 mg/kg) | 7.13±0.32# | 0.72±0.09# | 3.46±0.33# | 21.31±0.56# | 0.82±0.05# |
| HALCF (200 mg/kg) | 4.95±0.08# | 1.02±0.05$ | 6.92±0.27$ | 24.89±0.85# | 1.02±0.04# |
| HALCF (400 mg/kg) | 5.76±0.21# | 0.89±0.03# | 5.21±0.22# | 23.44±0.48# | 0.82±0.04# |
Note: All values are expressed as Mean±SEM. Statistical comparisons were made by using one way ANOVA followed by Dunnett’s multiple comparison tests using graph pad prism version 8 and found significantly different at p>0.05ns, *p< 0.05,$p< 0.01, #p<0.001 when compared to disease Control and @p<0.001 when disease control is compared with the control group. All parameters were expressed in mg/dl
Table 1: Effect of HALCF on total protein levels in ethylene glycol-induced lithiasis in rats (n=6).
| Groups | Calcium | Oxalate | LDH |
|---|---|---|---|
| Control | 0.79±0.03 | 31.78±1.09 | 231.89±2.12 |
| Ethylene glycol (0.75 %) | 1.23±0.06@ | 43.22±0.98@ | 389.04±3.98@ |
| Cystone (750 mg/kg) | 0.86±0.06# | 34.98±1.23# | 263.23±4.09# |
| HALCF (200 mg/kg) | 0.99±0.04$ | 38.89±0.98# | 329.10±5.33# |
| HALCF (400 mg/kg) | 0.89±0.04# | 36.46±0.58# | 287.04±4.67# |
Note: All values are expressed as Mean±SEM . Statistical comparisons were made by using way ANOVA followed by Dunnett’s multiple comparison test using graph pad prism version 8 and found significantly different at p>0.05ns, *p<0.05, $p< 0.01, #p<0.001 when compared to disease control and @p<0.001 when disease control is compared with the control group calcium and oxalate expressed in mg/dl; LDH: U/L
Table 2: Effect of HALCF on calcium levels in ethylene glycol-induced lithiasis in rats (n=6).
| Groups | Urine volume | Urine pH | Urine creatinine | Urine uric acid | Urine urea |
|---|---|---|---|---|---|
| Control | 14.65±0.23 | 7.19±0.33 | 0.69±0.06 | 4.22±0.17 | 26.43±0.82 |
| Ethylene glycol (0.75 %) | 8.79±0.44@ | 3.52±0.43@ | 1.28±0.07@ | 7.48±0.33@ | 35.41±0.70@ |
| Cystone (750 mg/kg) | 15.89±0.23* | 7.10±0.35* | 0.77±0.05# | 4.49±0.04# | 28.61±0.42# |
| HALCF (200 mg/kg) | 11.12±0.80* | 5.94±0.61* | 1.03±0.06$ | 5.15±0.24$ | 30.41±0.49$ |
| HALCF (400 mg/kg) | 16.21±0.33* | 6.95±0.48* | 0.93±0.04# | 4.84±0.16# | 29.03±0.38# |
Note: All values are expressed as Mean±SEM. Statistical comparisons were made by using one way ANOVA followed by Dunnett’s multiple comparison tests using graph pad prism version 8 and found significantly different at p>0.05ns, *p<0.05, $p<0.01, #p<0.001 when compared to disease control and @p<0.001 when disease control is compared with the control group urine creatinine, uric acid and urea expressed in mg/dl
Table 3: Effect of HALCF on urine volume and urine pH in ethylene glycol-induced lithiasis in rats.
| Groups | Calcium | Oxalate | Magnesium | Citrate |
|---|---|---|---|---|
| Control | 3.20±0.32 | 0.57±0.07 | 0.67±0.07 | 30.23±1.83 |
| Ethylene glycol (0.75 %) | 8.23±0.11@ | 1.60±0.06@ | 0.35±0.07@ | 18.45±1.14@ |
| Cystone (750 mg/kg) | 3.49±0.14# | 0.80±0.12# | 0.59±0.03# | 28.23±1.22# |
| HALCF (200 mg/kg) | 4.88±0.17# | 1.36±0.05$ | 0.50±0.03$ | 21.94±0.74$ |
| HALCF (400 mg/kg) | 4.05±0.17# | 0.91±0.08# | 0.53±0.04# | 26.27±1.45# |
Note: All values are expressed as Mean±SEM. Statistical comparisons were made by using one way ANOVA followed by Dunnett’s multiple comparison tests using graph pad prism version 8 and found significantly different at p>0.05ns, *p<0.05,$p<0.01, #p<0.001when compared to disease control and @p<0.001 when disease control is compared with the control group. All parameters were expressed in mg/dL.
TABLE 4: Effect of HALCF on calcium levels in ethylene glycol-induced lithiasis in rats.
When exposed to CaOx crystals, renal cells release superoxide radicals. Antioxidants and free radical scavengers can help avoid the harm that this can cause to the cells[23]. Free radical scavengers CAT and SOD inhibited oxalate-induced cell death in animal kidney epithelial cell lines in vitro. In the presence of superoxide anions, the mitochondrial GSH can be transformed into Glutathione S-Glutamate (GSSG)[24]. To convert GSSG back to M GSH, the pentose phosphate pathway's NADPH must be supplied to GSH reductase. As shown in Table 5 and Table 6, rats administered ethylene glycol have an increase in levels of SOD, CAT and GSH in their pancreas and kidney homogenates when given fruit extracts. The urolithic rats tissues, as well as the plasma of rats and stone patients, produce an excess of lipid peroxidation products, including hydroperoxides, diene conjugates and thiobarbituric acid-reactive substances. Carbonyl groups attached to proteins are a tried-and-true way to tell when proteins have been oxidized[25]. Stones can be more easily generated at damaged cell sites because they serve as nidus and trap any preformed crystals within the wounded urothelium. In cases of vascular injury and inflammation, nitrite can restore normal functioning of the arginine-nitric oxide synthase-nitric oxide axis. Protein carbonyls and nitrite levels were significantly elevated in the pancreatic and renal tissues of lithaitic control rats. Table 5 and Table 6 show that after treatment with some fruits, their levels returned to normal. Crystals within the kidneys cause inflammation and the death of kidney cells through signaling through the tumor necrosis factor receptor[26]. In numerous immune-mediated renal disorders, multiple triggers have been linked to the onset of glomerular and tubular damage. One study found that patients whose kidneys produce stones had higher levels of IL-6 in their urine. Renal and pancreatic inflammatory mediators were significantly reduced in the treatment groups of rats with ethylene glycol-induced lithiasis, as shown in the tables. According to the histological examinations, both the acinar cell damage (fig. 1) and the glomerulosclerosis (fig. 2) produced by ethylene glycol were significantly ameliorated by the L. chininesis treatment. In conclusion, there is some evidence that the fruit of L. chinensis can protect against ethylene glycol-induced lithiasis. Its preventive effects on the pancreas and kidneys, along with its capacity to prevent crystal formation, might be due to the presence of anthocyanins, which are antioxidants and anti-inflammatory properties of L. chinensis.
| Groups | SOD | CAT | GSH | MDA | Nitrite | PCC | TNF - α | IL6 |
|---|---|---|---|---|---|---|---|---|
| Control | 2.78±0.04 | 1.17±0.12 | 18.23±0.77 | 1.677±0.16 | 26.43±1.50 | 3.22±0.23 | 23.12±1.04 | 24.12±1.26 |
| Ethylene glycol (0.75 %) | 1.43±0.23@ | 0.47±0.04@ | 10.23±0.23@ | 6.12±0.43@ | 54.12±3.23@ | 6.67±0.21@ | 37.24±0.45@ | 36.23±1.12@ |
| Cystone (750 mg/kg) | 2.54±0.23# | 0.99±0.11# | 17.12±0.72# | 2.36±0.26# | 30.56±2.68# | 3.77±0.11# | 24.90±0.58# | 25.65±1.22# |
| HALCF (200 mg/kg) | 2.16±0.17# | 0.62±0.09 | 12.25±0.99$ | 3.16±0.36# | 38.79±2.78# | 4.95±0.08# | 33.50±1.93$ | 32.33±1.54$ |
| HALCF (400 mg/kg) | 2.58±0.08# | 0.94±0.07# | 15.86±0.55# | 2.91±0.30# | 37.95±3.28# | 4.23±0.17# | 31.50±1.34# | 27.50±1.34# |
Note: All values are expressed as Mean±SEM. Statistical comparisons were made by using one way ANOVA followed by Dunnett’s multiple comparison test using graph pad prism version 8 and found significantly different at p>0.05ns, *p<0.05, $p<0.01, #p<0.001 when compared to disease control and @p<0.001 when disease control is compared with control group. SOD: (IU/mg of protein); CAT: (IU/min/mg of protein); GSH: ?mol/mg of protein; MDA: (nmol/mg of tissue); Nitrite: (µg/ml); PCC:nmol/mg of protein; TNF α: pg/mg of protein; IL 6: pg/mg of protein.
Table 5: Effect of HALCF on pancreatic levels of stress and inflammatory parameters in ethylene glycol-induced lithiasis in rats (n=6).
| Groups | SOD | CAT | GSH | MDA | Nitrite | PCC | TNF - α | IL6 |
|---|---|---|---|---|---|---|---|---|
| Control | 3.12±0.07 | 1.25±0.08 | 21.90±0.45 | 2.67±0.30 | 35.09±2.23 | 4.45±0.04 | 28.12±1.23 | 26.09±1.34 |
| Ethylene glycol (0.75 %) | 1.67±1.23@ | 0.67±0.08@ | 12.89±0.34@ | 8.09±0.23@ | 59.80±1.98@ | 9.34±0.23@ | 40.34±2.13@ | 39.80±1.46@ |
| Cystone (750 mg/kg) | 3.09±0.34# | 1.11±0.06# | 19.87±0.64# | 3.56±0.23# | 37.67±2.09# | 5.09±0.45# | 29.56±0.90# | 27.67±2.09# |
| HALCF (200 mg/kg) | 2.00±0.05# | 0.72±0.05ns | 16.73±0.88# | 5.70±0.42# | 45.96±1.48# | 6.69±0.49# | 35.17±1.83# | 34.83±1.19# |
| HALCF (400 mg/kg) | 2.30±0.03# | 0.99±0.08# | 19.24±0.83# | 4.17±0.43# | 39.16±2.07# | 6.22±0.35# | 29.33±0.88# | 29.33±1.15# |
Note: All values are expressed as Mean±SEM. Statistical comparisons were made by using one way ANOVA followed by Dunnett’s multiple comparison test using graph pad prism version 8 and found significantly different at p>0.05ns, *p<0.05, $p< 0.01, #p<0.001 when compared to Disease Control and @p<0.001 when disease control is compared with control group SOD: (IU/mg of protein); CAT: (IU/min/mg of protein); GSH: ?mol/mg of protein; MDA: (nmol/mg of tissue); Nitrite: (µg/ml); PCC: nmol/mg of protein; TNF α: pg/mg of protein; IL 6: pg/mg of protein
Table 6: Effect of HALCF on renal levels of stress and inflammatory parameters in ethylene glycol-induced lithiasis in rats (n=6).
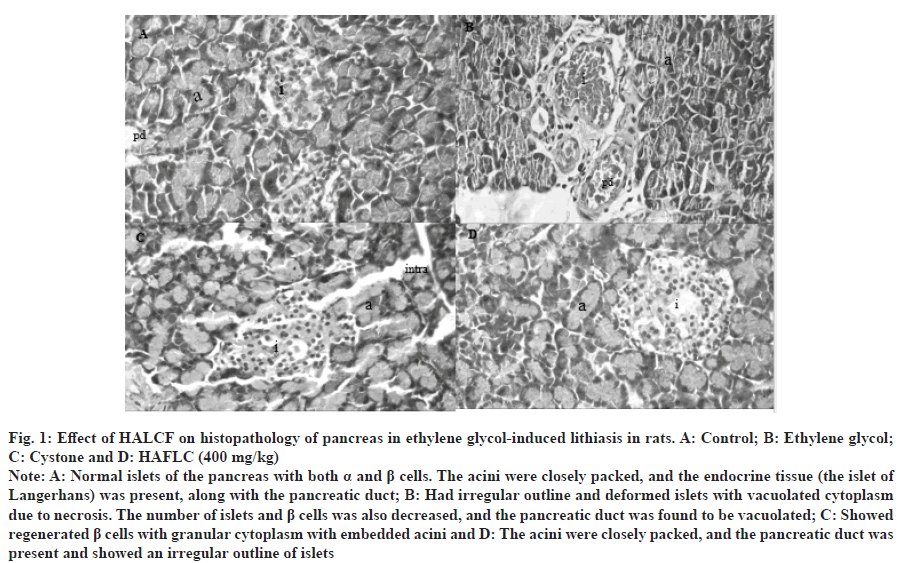
Fig. 1: Effect of HALCF on histopathology of pancreas in ethylene glycol-induced lithiasis in rats. A: Control; B: Ethylene glycol; C: Cystone and D: HAFLC (400 mg/kg).
Note: A: Normal islets of the pancreas with both α and β cells. The acini were closely packed, and the endocrine tissue (the islet of Langerhans) was present, along with the pancreatic duct; B: Had irregular outline and deformed islets with vacuolated cytoplasm due to necrosis. The number of islets and β cells was also decreased, and the pancreatic duct was found to be vacuolated; C: Showed regenerated β cells with granular cytoplasm with embedded acini and D: The acini were closely packed, and the pancreatic duct was present and showed an irregular outline of islets.
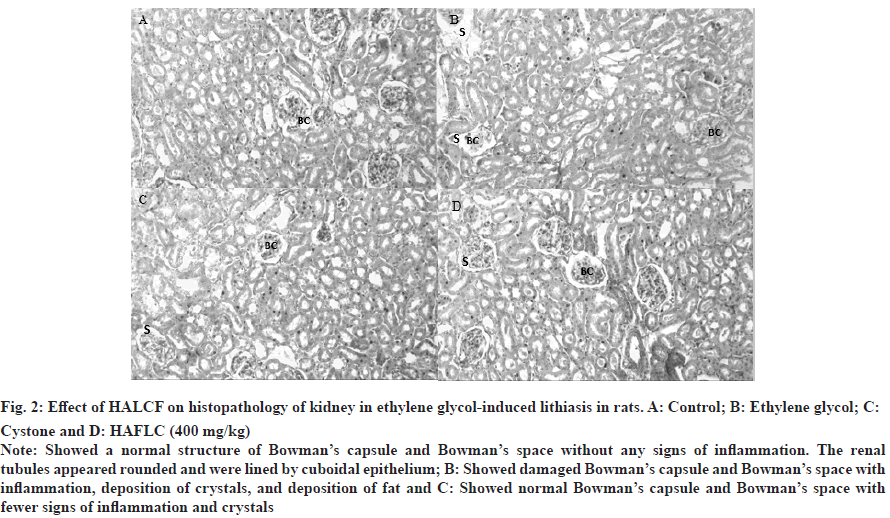
Fig. 2: Effect of HALCF on histopathology of kidney in ethylene glycol-induced lithiasis in rats. A: Control; B: Ethylene glycol; C: Cystone and D: HAFLC (400 mg/kg).
Note: Showed a normal structure of Bowman’s capsule and Bowman’s space without any signs of inflammation. The renal tubules appeared rounded and were lined by cuboidal epithelium; B: Showed damaged Bowman’s capsule and Bowman’s space with inflammation, deposition of crystals, and deposition of fat and C: Showed normal Bowman’s capsule and Bowman’s space with fewer signs of inflammation and crystals.
Conflict of interest:
Authors declare no conflict of interest
References
- Kovesdy CP. Epidemiology of chronic kidney disease: An update 2022. Kidney Int Suppl 2022;12(1):7-11.
[Crossref] [Google Scholar] [PubMed]
- Wang Z, Zhang Y, Zhang J, Deng Q, Liang H. Recent advances on the mechanisms of kidney stone formation. Int J Mol Med 2021;48(2):1-10.
[Crossref] [Google Scholar] [PubMed]
- Cloutier J, Villa L, Traxer O, Daudon M. Kidney stone analysis: Give me your stone, i will tell you who you are! . World J Urol 2015;33:157-69.
[Crossref] [Google Scholar] [PubMed]
- Anand U, Jacobo-Herrera N, Altemimi A, Lakhssassi N. A comprehensive review on medicinal plants as antimicrobial therapeutics: Potential avenues of biocompatible drug discovery. Metabolites 2019;9(11):258.
[Crossref] [Google Scholar] [PubMed]
- Peerapen P, Thongboonkerd V. Kidney stone prevention. Adv Nutr 2023;14(3):555-69.
[Crossref] [Google Scholar] [PubMed]
- Khan MI, Akhtar J, Ahmad M, Siddiqui Z, Fatima G. Role of plant bioactive as diuretics: General considerations and mechanism of diuresis. Curr Hypertens Rev 2023;19(2):79-92.
[Crossref] [Google Scholar] [PubMed]
- Nirumand MC, Hajialyani M, Rahimi R, Farzaei MH, Zingue S, Nabavi SM, et al. Dietary plants for the prevention and management of kidney stones: Preclinical and clinical evidence and molecular mechanisms. Int J Mol Sci 2018;19(3):765.
[Crossref] [Google Scholar] [PubMed]
- Emanuele S, Lauricella M, Calvaruso G, D’Anneo A, Giuliano M. Litchi chinensis as a functional food and a source of antitumor compounds: An overview and a description of biochemical pathways. Nutrients 2017;9(9):992.
[Crossref] [Google Scholar] [PubMed]
- Kilari EK, Putta S. Biological and phytopharmacological descriptions of Litchi chinensis. Pharmacogn Rev 2016;10(19):60.
[Crossref] [Google Scholar] [PubMed]
- Yao P, Gao Y, Simal-Gandara J, Farag MA, Chen W, Yao D, et al. Litchi (Litchi chinensis Sonn.): A comprehensive review of phytochemistry, medicinal properties, and product development. Food Funct 2021;12(20):9527-48.
[Crossref] [Google Scholar] [PubMed]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J biol Chem 1951;193(1):265-75.
[Crossref] [Google Scholar] [PubMed]
- Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 1972;247(10):3170-5.
[Crossref] [Google Scholar] [PubMed]
- Bergmeyer HU. Methods of enzymatic analysis. Elsevier 2012.
- Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem 1968;25:192-205.
[Crossref] [Google Scholar] [PubMed]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979;95(2):351-8.
[Crossref] [Google Scholar] [PubMed]
- Kalousová M, Havrdová E, Mrázová K, Spacek P, Braun M, Uhrová J, et al. Advanced glycoxidation end products in patients with multiple sclerosis. Prague Med Rep 2005;106(2):167-74.
[Google Scholar] [PubMed]
- Alelign T, Petros B. Kidney stone disease: An update on current concepts. Adv Urol 2018; (1):3068365.
[Crossref] [Google Scholar] [PubMed]
- Choudhary SS, Panigrahi PN, Dhara SK, Sahoo M, Dan A, Thakur N, et al. Cucumis callosus (Rottl.) Cogn. fruit extract ameliorates calcium oxalate urolithiasis in ethylene glycol induced hyperoxaluric rat model. Heliyon 2023;9(3).
[Crossref] [Google Scholar] [PubMed]
- Makasana A, Ranpariya V, Desai D, Mendpara J, Parekh V. Evaluation for the anti-urolithiatic activity of Launaea procumbens against ethylene glycol-induced renal calculi in rats. Toxicol Rep 2014;1:46-52.
[Crossref] [Google Scholar] [PubMed]
- Patel VB, Acharya N. Effect of Macrotyloma uniflorum in ethylene glycol induced urolithiasis in rats. Heliyon 2020;6(6).
[Crossref] [Google Scholar] [PubMed]
- Riley JM, Kim H, Averch TD, Kim HJ. Effect of magnesium on calcium and oxalate ion binding. J Endourol 2013;27(12):1487-92.
[Crossref] [Google Scholar] [PubMed]
- Khan SR. Reactive oxygen species, inflammation and calcium oxalate nephrolithiasis. Transl Androl Urol 2014;3(3):256.
[Crossref] [Google Scholar] [PubMed]
- Sreekumar PG, Ferrington DA, Kannan R. Glutathione metabolism and the novel role of mitochondrial GSH in retinal degeneration. Antioxidants 2021;10(5):661.
[Crossref] [Google Scholar] [PubMed]
- Peng J, Jones GL, Watson K. Stress proteins as biomarkers of oxidative stress: Effects of antioxidant supplements. Free radical biology and medicine. 2000;28(11):1598-606.
[Crossref] [Google Scholar] [PubMed]
- Mulay SR, Eberhard JN, Desai J, Marschner JA, Kumar SV, Weidenbusch M, et al. Hyperoxaluria requires TNF receptors to initiate crystal adhesion and kidney stone disease. J Am Soc Nephrol 2017;28(3):761-8.
[Crossref] [Google Scholar] [PubMed]
- Rose-John S. Interleukin-6 signalling in health and disease. F1000Res 2020;9(6):1013.
[Crossref] [Google Scholar] [PubMed]