- *Corresponding Author:
- Mangal Nagarsenker
Bombay College of Pharmacy, Kalina, Santacruz (East), Mumbai-400 098, India
E-mail: mangal_nag511@yahoo.co.in
| Date of Submission | 16 January 2010 |
| Date of Revision | 3 May 2011 |
| Date of Acceptance | 15 May 2011 |
| Indian J Pharm Sci, 2011, 73 (3): 311-315 |
Abstract
Present study describes microencapsulation of eugenol using gelatin-sodium alginate complex coacervation. The effects of core to coat ratio and drying method on properties of the eugenol microcapsules were investigated. The eugenol microcapsules were evaluated for surface characteristics, micromeritic properties, oil loading and encapsulation efficiency. Eugenol microcapsules possessed good flow properties, thus improved handling. The scanning electron photomicrographs showed globular surface of microcapsules prepared with core: coat ratio1:1. The treatment with dehydrating agent isopropanol lead to shrinking of microcapsule wall with cracks on it. The percent oil loading and encapsulation efficiency increased with increase in core: coat ratio whereas treatment with dehydrating agent resulted in reduction in loading and percent encapsulation efficiency of eugenol microcapsules.
Keywords
Complex coacervation, eugenol, gelatin, microencapsulation, sodium alginate
Microencapsulation of essential oils or flavors has been reported for offering protection and to reduce volatilization as well as degradation. Yet another objective of encapsulating essential oils or flavors was to design solid particulate forms of liquid core material [1,2], and to provide longer shelf life as a raw material which has better handling characteristics and is compatible with dry ingredients. Extended shelf life of microencapsulated flavors is attributed to limited exposure to temperature, moisture, oxidation and light during storage and processing [3-7]. Of the different encapsulation processes, spray drying is the one of the most frequently used technique, despite its limitations of use of relatively high temperature which may lead to coalescence of size of microcapsules and loss of core material [1,8]. Encapsulation by complex coacervation could serve a suitable alternative for microencapsulation [3,9-11]. Encapsulating polymer is required to possess unique emulsifying and film forming properties defining its ability to function as an encapsulant, therefore correct selection of coating material for each application is an important task. Coating material must retain and protect the encapsulated component from volatilization and chemical degradation during manufacture, storage and handling; moreover, it must release them into final product during desirable state of manufacture. Carbohydrate such as starches, maltodextirns, gum acacia and other gums, sodium alginate and proteins such as gelatin, albumin, lactoglobulin and whey proteins are reportedly used as flavor encapsulants [1]. This study presents complex coacervation using gelatin and sodium alginate for microencapsulation of eugenol. It also evaluates influence of core: coat compostion and drying methodology on properties of microcapsules.
Eugenol, clear pale yellow liquid extracted from clove and cinnamon, is used in perfumeries, flavorings, essential oils and medicine as local antiseptic and analgesic. Eugenol has wide application in dentistry for analgesic and antiseptic properties. Its derivatives are used in perfumery and flavoring, and also used in formulating insect attractants and UV absorbers. Microencapsulation of eugenol with gelatin-sodium alginate complex coacervation system is expected to offer protection against oxidization and volatilization and also ease of handling.
Eugenol was obtained as a gift sample from S. H. Kelkar, Mumbai. The gelatin bacteriological grade (Loba Chemie, Mumbai, India) was alkali processed Type B material (180 bloom). The sodium alginate used was of medium viscosity pharmaceutical grade (Loba Chemie). All other regents were of analytical reagent grade.
In all experiments, colloid encapsulating solution of gelatin and sodium alginate in ratio 4:1 was prepared by hydration followed by stirring till solution was effected. Eugenol was emulsified with encapsulating medium. Microcapsules were prepared at 40o by drop wise addition of dilute hydrochloric acid until phase separation was achieved following which mixture was chilled for 30 minutes. Formaldehyde solution, 37% (1 ml/g of gelatin) was added and stirred for 2 h at room temperature. A fixed volume (20 ml/100 ml batch) of previously chilled isopropyl alcohol (5º) as a dehydrating agent was added to the system, which was hardened with formaldehyde. Microcapsules were then separated by decanting the supernatant, filtered under vacuum, washed with water till free from formaldehyde and air-dried. Microcapsules were sieved through 22 mesh sieve and air-dried at room temperature. Influence of variables i.e core:coat composition (1:1 and 2:1) and treatment of dehydrating agent on encapsulation of eugenol were evaluated.
The morphology and surface topography of microcapsules was studied using Philips XL 30 SEM scanning electron microscope. The size of microcapsules was determined by sieve analysis method. Hausner ratio was calculated based on density and wall thickness [3] of microcapsules was computed using results of particle size and density data. The percent dry yield was estimated by establishing a relation between the total mass of isolated microcapsules and the total mass used for microencapsulation. Eugenol content in microcapsules was estimated by extracting eugenol from microcapsules in methanol and measuring the UV absorbance at 281nm using Shimadzu UV-160A ultraviolet/visible spectrophotometer in 1 cm cuvettes against methanol as blank. A percentage of oil entrapment and oil loading was calculated from the content of eugenol in the microcapsules.
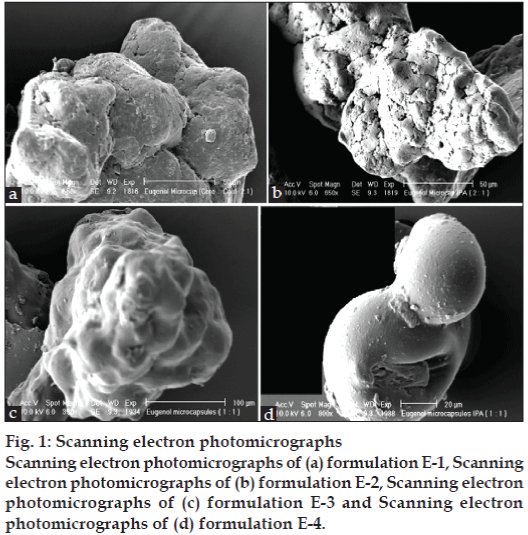
Eugenol, chemically 2-methoxy-4-(2-propeny) phenol was a clear pale yellow liquid. Sample had boiling point of 254°, refractive index of 1.535 and specific gravity 1.0597 g/ccs at 20°. Eugenol was insoluble in water soluble in alkali solution, ethanol and acetone, solution in 95% methanol exhibited UV maxima at 281 nm. Gelatin-sodium alginate microcapsules containing microdroplets of eugenol were prepared by complex coacervation induced by shift in pH [12]. Figs. 1a-d show variation in morphology and surface topography in scanning electron photomicrographs of microcapsules prepared using core to coat ratios of 1:1 and 2:1. Microcapsules prepared with core:coat ratio 2:1 showed rough surface in comparison with smooth globular outline in case of microcapsules prepared with core:coat ratio 1:1. The treatment of dehydrating agent isopropanol resulted in shrinkage of microcapsules as shown in fig. 1b, which was more prominent in microcapsules prepared with core to coat ratio 2:1 which could be attributed to thin wall of microcapsules entrapping high oil content.
As indicated in Table 1 the particle size distribution was markedly affected by the treatment with isopropyl alcohol (IPA) as dehydrating agent. The untreated microcapsules were yellowish in color and larger in size than corresponding IPA treated eugenol microcapsules. IPA treated eugenol microcapsules were more discrete and buff colored. The change in the pattern of particle size distribution with isopropanol treatment could be attributed to rapid withdrawal of moisture form microcapsule wall thus preventing coalescence and merger of microcapsules.
| Formulation | Colloid ratio(G:SA) | Core: coat ratio | Treatment with IPA | Mean particle size (m)* | Geometric mean diameter dg (m)* |
|---|---|---|---|---|---|
| E-1 | 4:1 | 2:1 | No IPA treatment | 715.97 ± 2.52# | 825 ± 3.76# |
| E-2 | 4:1 | 2:1 | IPA treatment | 453.49 ± 3.61# | 530 ± 3.5# |
| E-3 | 4:1 | 1:1 | No IPA treatment | 658.53 ± 3.6# | 900 ± 4.89# |
| E-4 | 4:1 | 1:1 | IPA treatment | 425.48 ± 4.75# | 500 ± 2.83# |
*mean of three determinations; # P<0.05-significant difference between E1/ E2 and E3/E4
Table 1: Mean particle size and geometric mean diameter of eugenol microcapsules
Hausner ratio is the important tool for comparison of flow behavior of the particles. It is characteristics of fine particles and has been shown to be measure of the frictional forces existing in moving mass. For free flowing particles the Hausner ratio should be nearly one. The overall lower values of Hausner ratio for eugenol microcapsules as given in Table 2 revealed the presence of lesser dynamic friction in the microcapsules, the lower friction being attributed to microcapsules becoming more regular in shape. The computed wall thickness [4] was reduced from 158.83 to 103.54 mm as the core: coat ratio was changed from 1:1 to 2:1 for untreated microcapsules. The similar trend was observed for microcapsules treated with IPA. Percent volatile oil content in eugenol microcapsules (Table 3) prepared with core: coat ratio 2:1 and 1:1 was found to be 34.7 and 21.6% w/w. The treatment with IPA as dehydrating agent reduced the eugenol content to 18.24 and 8.13% w/w respectively. The difference in the content of eugenol in microcapsules was possibly due to dehydration step preceding drying of microcapsules. Dehydrating action of IPA resulted in shrinkage, cracks that were confirmed by scanning electron photomicrography and probably caused leakage of oil. The eugenol microcapsules dried in a stream of air at lower temperature suffered least damage to their walls. One-way AVOVA test yielded p values, were less than 0.001 for encapsulation efficiency and mean particle size data. This indicates that there was a significant difference in encapsulation efficiency and mean particle size after IPA treatment.
| Formulation | Aerateddensity (g/cc)* | Packed density (g/cc)* | True density (g/cc)* | Percent porosity* | Hausner ratio* | Wall thickness (m)* | |
|---|---|---|---|---|---|---|---|
| E-1 | 0.4545 | (±0.003) | 0.4862 (±0.011) | 1.3985 (±0.028) | 65.23 (±3.29) | 1.0477 (±0.014) | 103.54† (±0.58) |
| E-2 | 0.416 | (±0.01) | 0.4448 (±0.022) | 1.3549 (±0.006) | 67.17 (±2.74) | 1.0197 (±0.003) | 101.85† (±0.36) |
| E-3 | 0.5433 | (±0.012) | 0.6125 (±0.01) | 1.3290 (±0.066) | 53.91 (±4.24) | 1.1274† (±0.0012) | 158.83 (±1.06) |
| E-4 | 0.3571 | (±0.018) | 0.3987 (±0.014) | 1.3626 (±0.013) | 70.74 (±4.72) | 1.1164† (±0.005) | 132.23 (±0.12) |
*mean of three determinations; †P>0.05-no significant difference between the results. All other showed significant difference
Table 2: Micromeritic properties and wall thickness of eugenol microcapsules
| Formulation | Percent dry yield* | Percent Eugenol content* | Percent oil loading* | Percent encapsulation efficiency* |
|---|---|---|---|---|
| E-1 | 30.75 (±2.4) | 34.7 (±1.12) | 34.68 (±1.15) | 15.99 (±0.55)# |
| E-2 | 32.28 (±1.1) | 18.24 (±1.67) | 18.24 (±0.55) | 8.83 (±0.24) # |
| E-3 | 35.66 (±1.5) | 21.6 (±0.67) | 21.6 (±0.32) | 15.41 (±0.43) # |
| E-4 | 38.20 (±1.48) | 8.14 (±0.64) | 8.13 (±0.92) | 6.21 (±0.42†)# |
Table 3: Percent dry yield, percent oil loading and percent encapsulation efficiency of eugenol microcapsules
*mean of three determinations; #P<0.05 significant difference between E1/ E2 and E3/E4
Percent loading (Table 3) increased with increase in core: coat ratio for batches treated with IPA and also for those without treatment with IPA. During washing with the dehydrating agent most of surface oil was removed from the microcapsules thus giving less oil content which was indicated by low percent loading in batches treated with IPA.
Encapsulation efficiency is more dependent on the percentage oil content than percent coacervation yield [5]. Low values of percent encapsulation efficiency of eugenol microcapsules is in the line with Ribeiro et al. [13], report which states that the greatest single source of loss of the encapsulated oil was loss of some of water-soluble components of oil in aqueous phase during aqueous phase separation coacervation process. At high oil load, microcapsules produced were oily, indicating leaching due to high oil content coupled with thinner barrier in form of wall of microcapsules. There was no significant increase in percent encapsulation efficiency with increase in core: coat ratio irrespective of IPA treatment. In the core coat ratio 1:1 the amount of eugenol and coat material was same and colloid concentration was sufficient to emulsify and encapsulate eugenol. At higher core coat ratio, probably emulsification of oily phase suffered resulting in reduced encapsulation efficiency.
Eugenol was microcapsulated prepared using gelatin sodium alginate complex coacervate system and was converted in dry free flowing powder form. Isopropanol treatment resulted in snap dehydration of microcapsule wall resulting in cracks on the surface of microcapsule. Ratio of eugenol to polymers influenced physical properties of microcapsules as well as encapsulation efficiency.
Acknowledgements
The authors thank S. H. Kelkar Co., Mumbai for providing the gift sample of eugenol.
References
- Krishan S, Kshirsagar AC, Singhal RS. The use of gum arabic and modified starch in the microencapsulation of a food flavoring agent. CarbohydrPolym 2005;62:309-15.
- Nixon JR, Nouh A. The effect of microcapsule size on the oxidative decomposition of core material. J Pharm Pharmacol 1978;30:533-7.
- Thimma RT, Tammishetti S. Study of complex coacervation of gelatin with sodium carboxymethylguar gum: Microencapsulation of clove oil and sulphamethoxazole. J Microencapsul 2003;20:203-10.
- Madan PL. Clofibrate Microcapsules II: Effect of wall thickness on release Characteristics. J Pharm Sci 1981;70:430-3.
- Chan LW, Lim LT, Heng PW. Microencapsulation of oils using sodium alginate. J Microencapsul 2000;17:757-66.
- Lin CC, Lin SY, Hwang LS. Microencapsulation of squid oil with hydrophilic macromolecules for oxidative and thermal stabilization. J Food Sci 1995;60:36-9.
- Pedroza-Islas R, Macías-Bravo S, Vernon-Carter EJ. Oil thermo-oxidative stability and surface oil determination of biopolymer microcapsules. Revasta Mexicana De IngenieríaQuímica 2002;1: 37-44.
- Soottitantawat A, Takayama K, Okamura K, Muranaka D, Yoshii H, Furuta T, et al.Microencapsualtion of l-menthol by spray drying and its release characteristics. Innovative Food Sci Emerging Technol 2005;6:163-70.
- Junyaprasert VB, Mitrevej A, Sinchaipanid N, Boonme P, Wurster DE. Effect of process variables on the microencapsulation of vitamin A palmitate by gelatin-acacia complex coacervation. Drug DevInd Pharm 2001;27:561-6.
- Xing F, Cheng G, Yang B, Ma L. Microencapsulation of capsaicin by the complex coacervation of gelatin, acacia and tannins. J ApplPolymSci 2003;91:2669-75.
- Keipert S, Melegari P. Preparation and characterization of oil containing microparticles. Drug DevInd Pharm 1993;19:603-21.
- Shinde UA, Nagarsenker MS. Characterization of gelatin-sodium alginate complex coacervation system. Indian J Pharm Sci 2009;71: 313-7.
- Ribeiro A, Arnaud P, Frazao S, Venancio F, Chaumeil JC. Development of vegetable extracts by microencapsulation. J. Microencapsul 1997;14:735-42.