- *Corresponding Author:
- N. A. El-gendy
Department of Pharmaceutical Chemistry, The University of Kansas, Lawrence, KS, 66047, USA
E-mail: elgendy@ku.edu
| Date of Submission | 05 October 2011 |
| Date of Revision | 14 August 2012 |
| Date of Acceptance | 24 August 2012 |
| Indian J Pharm Sci 2012, 74 (4): 319-330 |
Abstract
Glipizide is an effective antidiabetic agent, however, it suffers from relatively short biological half-life. To solve this encumbrance, it is a prospective candidate for fabricating glipizide extended release microcapsules. Microencapsulation of glipizde with a coat of alginate alone or in combination with chitosan or carbomer 934P was prepared employing ionotropic gelation process. The prepared microcapsules were evaluated in vitro by microscopical examination, determination of the particle size, yield and microencapsulation efficiency. The filled capsules were assessed for content uniformity and drug release characteristics. Stability study of the optimised formulas was carried out at three different temperatures over 12 weeks. In vivo bioavailability study and hypoglycemic activity of C9 microcapsules were done on albino rabbits. All formulas achieved high yield, microencapsulation efficiency and extended t 1/2 . C9 and C19 microcapsules attained the most optimised results in all tests and complied with the dissolution requirements for extended release dosage forms. These two formulas were selected for stability studies. C9 exhibited longer shelf-life and hence was chosen for in vivo studies. C9 microcapsules showed an improvement in the drug bioavailability and significant hypoglycemic activity compared to immediate release tablets (Minidiab® 5 mg). The optimised microcapsule formulation developed was found to produce extended antidiabetic activity.
Keywords
Extended release, glipizide, microcapsules, oral drug delivery
The primary goal of therapy with many drugs is to achieve a steady?state at the tissue level or in blood that is therapeutically effective and nontoxic for an extended period of time. Therefore, modified release drug delivery systems have been developed in an attempt to realise this goal [1]. Microencapsulation process has been established as a technique to accomplish extended release and drug targeting [2]. Microencapsulation using a variety of polymers and its applications has been previously depicted in standard textbooks and literatures [3?5]. Microcapsule carrier systems made from the naturally occurring biodegradable polymers have attracted considerable attention for several years in extended drug delivery [6,7].
Diabetes mellitus is a group of metabolic diseases characterised by defects in insulin utilisation, either from autoimmune destruction of insulin?producing cells (Type I) or insulin resistance (Type II) [8]. The prevalence of type II diabetes is rising dramatically worldwide [9]. There are more than 171 million people with diabetes and in the US, Canada and Europe, over 80% of diabetes cases are Type II [10]. It was the sixth leading cause of death due to the many complications associated with this disease, such as pulmonary hypertension and ischemia [8,11]. Glipizide is a second?generation sulfonylurea that can acutely lower the blood glucose level in humans by stimulating the release of insulin from the pancreas and is typically prescribed to treat Type II diabetes. Its half?life is relatively short (2?5 h) which necessitates its administration in 2 or 3 doses of 2.5?10 mg/day [12,13]. Therefore, it is a potential candidate for the development of extended release formulations.
The purpose of this work was to microencapsulate glipizide using certain hydrophilic polymers to control the release of this highly water insoluble drug. The polymers used in the microencapsulation process were alginate, alginate–chitosan and alginate–carbomer 934P. Evaluation of the prepared microcapsules was performed by microscopical examination, determination of particle size, yield and microencapsulation efficiency. The in vitro release studies for the determination of glipizide released from the microcapsule formulations were performed and analysed. The effect of the storage at high temperatures, namely, 40, 50 and 60° for a period of 12 weeks on the chemical stability of the selected microcapsules and prediction of the shelf life was also assessed. In addition, the effect of storage at these high temperatures on the release of the drug from the selected formulae was evaluated. The bioavailability and pharmacokinetic parameters of glipizide from the selected microencapsulated formula C9 were conducted in albino rabbits and compared to commercially available to immediate release tablets (Minidiab® 5 mg; Pfizer, Inc., Egypt). Finally, the in vivo assessment of pharmacological activity of the selected formula was done using albino rabbits.
Materials and Methods
Glipizide was kindly supplied by Al?Pheronea Pharmaceutical Company, Cairo, Egypt. Carbomer 934P was received as gift samples from Lubrizol, Belgium. Glipenclamide was purchased from Tocris Bioscience, USA. Sodium alginate (AL) was purchased from BDH Merck Ltd., Poole, England. Streptozotocin, citric acid, chitosan low molecular weight (CH?LMW; 75?85% deacetylated with viscosity 20?200 cps), chitosan high molecular weight (CH?HMW; >75% deacetylated with viscosity 800?2000 cps) and polysorbate 80 were purchased from Sigma?Aldrich, USA. Acetonitrile and orthophosphoric acid (HPLC grade) were purchased from E?Merck, Germany. Trichloroacetic acid was purchased from Fluka, USA. Potassium dihydrogen phosphate and sodium dihydrogen phosphate were purchased from ADWEC, Egypt. Calcium chloride (CaCl2) and glacial acetic acid were purchased from EL?Nasr Pharmaceutical Chemical Company, Abo?Zaabal, Egypt. Double?distilled water was used throughout the study.
Formulation of glipizide microcapsules
Different formulations were formulated employing sodium alginate alone or in combination with various coating polymers as reported in Table 1. An orifice?ionotropic gelation process that has been extensively used to prepare large alginate beads was employed to fabricate the microcapsules [14,15].
| Formula no. | Composition | ||||||
|---|---|---|---|---|---|---|---|
| Drug (mg) | Microcapsule coat | Drug: polymer ratio | CaCl2 (M) | H2O (ml) | |||
| Polymer type | Amount (mg) | %w/v | |||||
| C1 | 15 | Sodium alginate | 30 | 1 | 1:2 | 0.2 | 30 |
| C2 | 15 | 30 | 2 | 1:2 | 0.2 | 15 | |
| C3 | 15 | 15 | 1 | 1:1 | 0.2 | 15 | |
| C4 | 15 | 15 | 2 | 1:1 | 0.2 | 7.5 | |
| C5 | 15 | Sodium | 30 | 1 | 1:2 | 0.2 | 30 |
| C6 | 15 | alginate-chitosan | 30 | 2 | 1:2 | 0.2 | 15 |
| C7 | 15 | (LMW) | 15 | 1 | 1:1 | 0.2 | 15 |
| C8 | 15 | 15 | 2 | 1:1 | 0.2 | 7.5 | |
| C9 | 15 | Sodium | 30 | 1 | 1:2 | 0.2 | 30 |
| C10 | 15 | alginate-chitosan | 30 | 2 | 1: 2 | 0.2 | 15 |
| C11 | 15 | (HMW) | 15 | 1 | 1:1 | 0.2 | 15 |
| C12 | 15 | 15 | 2 | 1:1 | 0.2 | 7.5 | |
| C13 | 15 | Sodium | 30 | 1 | 1:2 | 0.2 | 30 |
| C14 | 15 | alginate-carbomer | 30 | 2 | 1:2 | 0.2 | 15 |
| C15 | 15 | 934P (6:4) | 15 | 1 | 1:1 | 0.2 | 15 |
| C16 | 15 | 15 | 2 | 1:1 | 0.2 | 7.5 | |
| C17 | 15 | Sodium | 30 | 1 | 1:2 | 0.2 | 30 |
| C18 | 15 | alginate: carbomer | 30 | 2 | 1: 2 | 0.2 | 15 |
| C19 | 15 | 934P (8:2) | 15 | 1 | 1:1 | 0.2 | 15 |
| C20 | 15 | 15 | 2 | 1:1 | 0.2 | 7.5 | |
Table 1: Composition Of Different Formulas Of Glipizide Microcapsules
Formulation of sodium alginate coated glipizide microcapsules
Alginate?coated microcapsules of glipizide were prepared by dispersing the drug (15 mg) into aqueous solutions of sodium alginate under constant stirring (500 rpm) at 25±0.5° for 10 min. The microcapsules were formed by dropping the dispersion into gently agitated aqueous solutions of the counterion 0.2 M CaCl2 at a rate of 3 ml/min through a syringe with a needle of size no. 18. The ratio of alginate solution and CaCl2 solution was adjusted to be 1:10. The mixtures were then stirred slowly for 10 min to cure alginate microcapsules and to produce spherical rigid microcapsules. The microcapsules were collected by decantation, rinsed with distilled water and air dried for 24 h, followed by drying at 40° for 4 h and stored in desiccator until used.
Formulation of sodium alginate–chitosan coated glipizide microcapsules
Drug?alginate dispersions were prepared as previously mentioned. CaCl2 solutions containing chitosan were prepared by adding 1% v/v glacial acetic acid containing chitosan to CaCl2 solution with mild agitation at ambient temperature. The concentration of chitosan in CaCl2 aqueous solution was 0.3% w/v, and two different molecular weights were employed. Then, the drug?alginate dispersion was added drop?wise to CaCl2 solution. The formed microcapsules were retained in CaCl2 solution for 10 min to complete the curing reaction to produce spherical rigid microcapsules and treated as previously discussed.
Formulation of sodium alginate–carbopol 934P coated glipizide microcapsules
Sodium alginate and carbopol 934P were dissolved in distilled water to form homogeneous polymer solution. The active substance, glipizide was added to the polymer solution and mixed thoroughly with stirrer to form viscous dispersion. The resulting dispersion was then added drop?wise into CaCl2 solution as mentioned before.
Evaluation of the prepared microcapsules
Microscopical examination and particle size measurement of glipizide microcapsules were done using optical microscope. The prepared microcapsules was mounted in few drops of distilled water and examined under an optical microscope (Lecia Image, Germany) and photographed at a magnification of ×100, by means of a fitted camera (JVC, Japan). The particle size of the microspheres was also determined using the calibrated optical microscopy method, where approximately 100 microspheres were counted for particle size.
The yield of glipizide microcapsules was determined using the equation: % Process yield=(Recovered mass/ Mass entered into the experiment)×100
For drug assay and microencapsulation efficiency, ten milligrams of the microcapsules were added to 200 ml phosphate buffer, pH 7.4 containing 0.1% polysorbate 80 in a 250 ml conical flask and left overnight with occasional vigorous shaking. The dispersion was filtered through Whatman filter membrane (0.45 μm) prior to drug analysis spectrophotometrically at 276 nm (UV–VIS spectrophotometer; Jasco, V?530, Japan). The experiment was done twenty times. Then microencapsulation efficiency was calculated using the the following formula: % Microencapsulation efficiency=(Recovered drug mass/Total mass)×100.
Chemical stability by HPLC?UV
To assess the chemical degradation of glipizide microcapsules compared to the drug as received, high pressure liquid chromatography (HPLC?UV) was used. The powders were dissolved in the mobile phase in triplicate, and 20 μl of each sample was injected onto the HPLC column for analysis. To determine the degradation, the percentage area of drug peak was compared against total area of all the peaks in the chromatogram. Any decrease in the percentage area of the drug peak in the microcapsule powder was considered as degradation. Before injecting the samples, a blank (without drug) was always injected onto the HPLC column under the same conditions. The chemical stability of glipizide in the prepared formulations was determined by chromatographic analysis of the microcapsules compared to the drug as received.
HPLC analysis of glipizide
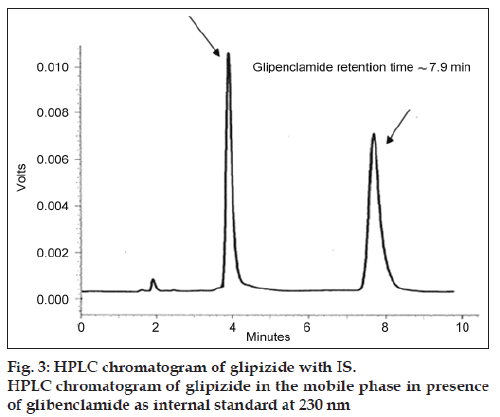
A reverse?phase HPLC method was used for quantifying glipizide samples (n=3). The HPLC system consisted of a solvent delivery pump (Shimadzu L?7110, Hitachi Ltd, Japan), a controller (SCL?10A), and a UV/Vis detector (SPD?10A). The peak areas were integrated using Shimadzu C?R6A chromatopac, Hitachi Ltd, Japan. The drug was separated on C18 column packed with Nucleosil 120 (250×4.6 mm, 5 μ), Teknokroma, Barcelona, Spain. Standards and samples were prepared in MillQ water. Mobile phase consisted of a mixture of acetonitrile: 2 mM phosphate buffer (50:50% v/v), adjusted to pH 3.5 with orthophosphoric acid. The drug was eluted isocratically at a mobile phase flow rate of 1.2 ml/min and monitored with a UV detector operating at 230 nm. Glibenclamide was used as internal standard. The run time for the assay was 10 min, and the retention time for the drug was 3.9±0.2 min and glipenclamide retention time was 7.9±0.3 min [16,17].
Preparation of glipizide capsules
An appropriate amount of prepared microcapsules equivalent to 15 mg of glipizide was filled into hard gelatin capsules size 3.
Content uniformity
Twenty capsules of each formula were individually analysed for initial drug content by dissolved each capsule in 200 ml phosphate buffer pH 7.4 containing 0.1% polysorbate 80, using water bath sonicator for 30 min. The mixture was then filtered through Whatman filter membrane (0.45 μm) prior to drug analysis spectrophotometrically at 276 nm.
Release studies
The release of glipizide from the prepared capsules was performed according to the USP XXIV dissolution tester apparatus 1 (basket method; Hanson Research, SR 8 plus model, USA). Studies are carried out at 37±0.5º in 900 ml 0.1 N HCl for a period of, 2 h followed by the release in phosphate buffer pH 7.4 containing 0.1% polysorbate 80 for 6 h. Rotation speed is 50 rpm. At pre?determined time intervals, aliquots (5 ml) were withdrawn and replaced with fresh medium to maintain constant dissolution volume. Samples were filtered through Whatman filter membrane (0.45 μm), diluted appropriately and analysed spectrophotometrically at 276 nm for the percent glipizide released. All experiments were done in triplicate. The obtained data were subsequently analysed to determine the order of release.
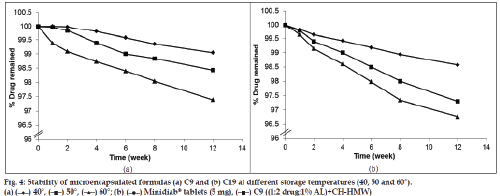
Accelerated stability testing
The accelerated stability testing was performed on the selected formulas (C9 and C19) which gave the most optimum results in all previous tests. The test was carried out by placing the capsules of each selected formula in sealed pouches and stored in thermostatically controlled ovens adjusted at different temperatures, namely, 40, 50 and 60°±0.5 with relative humidity 75% (maintained using a saturated solution of NaCl) for a period of 12 weeks. Three capsules from each formula were taken from the ovens after 1, 2, 4, 6, 8 and 12 weeks. The stored capsules were examined visually for any changes in colour and/or appearance and analysed for the determination of the amount of drug remaining in each formula using HPLC stability-indicating method as previously mentioned [17]. The dosage forms were crushed and dissolved in 100 ml mobile phase. The solution was filtered and the first 20 ml of the solution was rejected, then 10 ml of the filtrate was diluted to 100 ml in a volumetric flask with mobile phase. One millilitre aliquot of the prepared solution was transferred to 10 ml volumetric flask, and the volume was completed with the mobile phase. Twenty microliters of the above solution was injected into the column for quantitation. The unknown concentration of glipizide in each dosage form was calculated as follows:Q=(R/A±B)×Dilution factor, where, Q is the glipizide concentration, R is the peak area ratio (drug/internal standard), A is the slop of calibration curve and B is the Y?intercept.
The stability data were kinetically analysed to determine the order of drug degradation according to zero? and first?order kinetics. The rate constant of the reaction (k) was calculated according to determined order at each of the three temperatures. The logarithmic K values at different temperatures were plotted against the reciprocal of the corresponding temperature according to Arrhenius plot for the determination of the expiration date.
Method validation
Standard samples were prepared to provide final concentrations of glipizide in the range of 2?12 μg/ml. The peak areas of the drug were plotted against the concentrations. The least square linear regression analysis was used to determine the slope, Y?intercept and the correlation coefficient (R) of the standard plot.
The intra? and inter?day precision
The intra?day and inter?day precision was established by analysing samples in the range of 2?12 μg/ml drug solutions in triplicate on the same day, and on three consecutive days, respectively, then, the % relative standard deviation (RSD) of the slope and R were calculated in each case. Two?way analysis of variance (ANOVA) test was performed to determine the significance of the difference between the results (P≤0.05).
Effect of storage at high temperature on the drug release
Aiming to study the effect of storage at high temperature on the release of glipizide from the selected formulas, release study has been conducted on the samples taken from the stored formulas at the three elevated temperature after 2, 4, 6, 8 and 12 weeks as previously mentioned.
Bioavailability studies
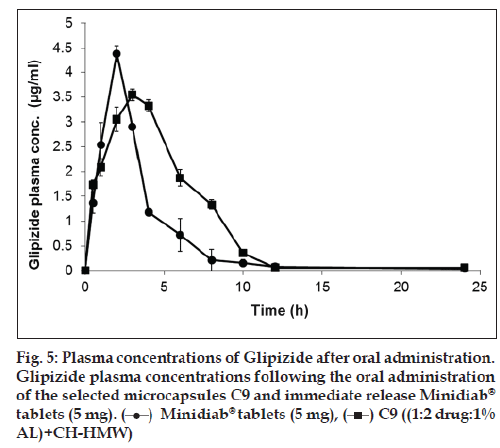
Glipizide microencapsulated formula C9 was selected for the bioavailability study. The criteria for the selection of this formula were based on the results of all previous tests. Also, the relative bioavailability of the drug from this formula was computed to the commercially available Minidiab® tablets (5 mg).
Animals
Twelve male albino rabbits weighing 2?2.5 kg were fasted overnight before dosing. The study was approved by the Animal Ethics Committee of the Faculty of Pharmacy, Cairo University and was conducted in accordance with standard institutional guidelines.
Calculation of animal doses
According to Paget and Barners table which related the animal dose to the daily human dose [13], dose of rabbit (1.5 kg) = daily human dose (15 mg) × 0.07 = 1.05 mg. So, for rabbits weighing 2?2.5 kg; the drug doses were 1.4?1.75 mg. The weight of formula containing this amount of the drug was calculated and given to the animals.
Protocol
The study design was a single?dose, fasting, three?treatment, parallel design, comparing equal doses of the two formulas. An equal number of albino rabbits were randomly assigned to the dosing sequence. Before drug administration, blood samples were collected to be used as a blank. The calculated dose of commercially available Minidiab® tablets were compressed into pellets whereas that of the selected microcapsules were filled into capsule shell size 5 and administered orally to the rabbits by the aid of water. Blood samples were collected at time intervals of 0, 1, 2, 4, 6, 8, 12, 16, and 24 h after drug administration from the marginal vein of the ear into heparinised tubes. The blood samples were immediately centrifuged at 3000 rpm for 15 min and the plasma were collected in special tubes and deep?frozen till required for analysis by HPLC assay. Plain plasma was used for preparing the calibration curve.
HPLC determination of glipizide in the plasma samples
A sample (0.5 ml) of blood plasma was transferred to 10 ml glass tube with a te?on cap and 1 ml internal standard (10 μg/ml) was added. 200 μl of 10% w/v trichloroacetic acid was added and mixed for 5 min by means of a vortex mixer (Thermolyne Maxi Mix II, USA). The resultant mixture was centrifuged at 4000 rpm for 15 min, then filtered through membrane filter of 0.45 μm pore size and injected to the HPLC column. The concentration of drug in each sample was determined as previously mentioned [17].
Pharmacokinetic analysis of the data and determination of relative bioavailability
The individual pharmacokinetic parameters of the drug were calculated by non?compartmental analysis. These parameters included the peak plasma concentration (Cmax) and the time to reach the maximum plasma concentration (Tmax). The areas under the plasma glipizide concentration–time curves AUC(0?48) and AUC(0?∞) were calculated by the linear trapezoidal rule, and extrapolation to infinity, respectively. In addition, the overall elimination rate constant (Ke) and elimination half?life (T1/2) were determined. The percent relative bioavailability of the drug from microcapsule formula C9 in comparison to commercially available immediate release Minidiad® 5 mg was calculated with respect to Cmax, AUC(0?48) and AUC(0?∞).
Statistical studies
Two?way ANOVA was performed to determine the significance of difference between the pharmacokinetic parameters among groups. The level of significance was set at P value of <0.05 using SPSS version 12.0 software computer program.
In vivo evaluation
The evaluation of the pharmacological activity was conducted on C9 microcapsules and compared with the commercially available immediate release Minidiad® 5 mg in normal healthy male albino rabbits weighing 1.5?2 kg. The study was done by measuring serum glucose levels following their oral administration at a dose equivalent to 700 μg/kg of glipizide. The study was approved by the Animal Ethics Committee of the Faculty of Pharmacy, Cairo University. The experiment was performed as per a crossover randomised block design. Three groups of the rabbits (n=6) were used for the study and were fasted (with water) for 12 h prior to the experiment. All rabbits were kept in cages with wide square mesh at the bottom to avoid coprophagy, and maintained in a well?ventilated animal house with 12 h light and dark cycle. Two groups of rabbits were made diabetic by single dose of streptozotocin (45 mg/kg) dissolved in 3 ml of freshly prepared citrate buffer pH 4.5 and administered intraperitoneally following 12 h fasting. The remaining group was kept as control and received equal volume of citrate buffer without streptozotocin.
When diabetes mellitus was well established in the tested rabbits, the pharmacological activity was accessed by measuring blood glucose level at different time intervals. Blood samples (0.3 ml) were collected by puncturing the marginal ear vein of each rabbit at zero time and after 1, 2, 4, 6, 8, 10, 12, 14, 16, 18 and 20 h following oral administration of the drug. The samples were collected into heparinised glass vials and stored at 4° until analysis. The blood glucose level for the control and test samples was estimated by glucometer (glucose oxidase method; OK Biotech Co., Ltd., USA). Statistical analyses of the data were made using ANOVA test to determine the significant difference between the tested formulas.
Results and Discussion
Glipizide microcapsules were prepared by the orifice?ionic gelation process using various polymers alone or in combination to control the release of the drug. Several preliminary optimization studies were performed using different polymer types and concentrations as well as various drug:polymer ratios. Table 1 shows the different formulations of glipizide microcapsules.
Concerning sodium alginate, it was clear that the polymer concentration less than 1% led to the formation of microcapsules that were unable to retain their spherical form during drying process. Above 2%, the prepared sodium alginate solutions were highly viscous and hindered the formation of the drops. It has been reported that alginate concentrations in the range 1.8?2.2% were considered to be appropriate [18]. However, Sezer and Akuge [19] used a maximum alginate concentration of 3.23%. These differences may be due to the variant origins of sodium alginate as received. Drug:alginate ratios of 1:1 and 1:2 were selected depending mainly on the dose of the drug. Ratios less than 1:1 allowed the formation of nonhomogeneous dispersions and the obtained microcapsules were not spheres.
The use of copolymer has been suggested to achieve significant delay in the drug release [20?22]. When considering the negative charge of alginate and its ability to form polyionic complexes with a lower tendency of erosion at high pH values, a cationic polymer can be selected [23]. Chitosan was chosen as a cationic polymer owing to its biodegradable properties and the similarity of its saccharide structures with alginate. This may offer greater interaction between the two polymers and stronger inter?chain reactions [17]. Two types of chitosan were used; low molecular weight with a viscosity of 20?200 cps and high molecular weight with a viscosity of 800?2000 cps.
Different concentrations of acetic acid (1?6% v/v) were used for preparing chitosan solution, but no significant effect of acetic acid concentration was observed on drug entrapment efficiency. This may be as a result of good solubility of chitosan in acetic acid and therefore 1% v/v of acetic acid was selected. Various chitosan concentrations in 0.2 M CaCl2 were selected for preliminary trials. The microcapsules were prepared using 0.3% chitosan in 0.2 M CaCl2 because the maximum sphericity was observed at this level.
The presence of calcium ions with chitosan in solution during the membrane forming step and the incubation step has a significant effect on the ability of a gel bead to bind chitosan. It has been reported that chitosan binds faster and to a higher extent with alginate by increasing CaCl2 concentration up to 0.3 M due to decreasing Debye length of the charges on both alginate and chitosan polymers. This could lead to a network allowing chitosan molecules to diffuse further into the gel before binding. Chitosan is known to be less extended at higher ionic strengths, and consequently achieve a higher diffusion coefficient in a given gel network. Both these effects could result in longer diffusion distances for a given time, and therefore more binding. Moreover, in the presence of Ca2+ ions in chitosan solution, the gelling reaction of alginate will start to compete with the precipitation reaction, leading to the formation of a more porous gel allowing diffusion of chitosan [24].
Microcapsules containing glipizide were also generated employing alginate and CP to form a homogeneous polymer mixture, which was significantly better than an individual polymer for achieving extended release [25,26]. Pellets containing CP usually form a thin gel?barrier in dissolution medium that led to less penetration of medium liquid into the pellets [27?30]. CaCl2 (0.2 M) was found to be the optimal concentration for the fabrication of all microspheres. Increasing CaCl2 concentration led to a decrease in the gelation rate constant due to the relationship between the diffusibility of calcium ions and calcium alginate concentration in the gelling zone. Moreover, there was an increase in the diffusion resistance caused by the formation of a thicker membrane gel [31].
During the preparation of all microcapsules, the curing time was fixed at 10 min. This time was sufficient for full curing of the microcapsules and the effect of increasing the curing time over 10 min was minor. The curing time is not a significant factor for both drug loading efficiency and the time for 50% of the drug to be released. In general, curing time has to be kept minimal to avoid any loss of the encapsulated drug to the external aqueous medium, especially, with drugs showing some water solubility [32].
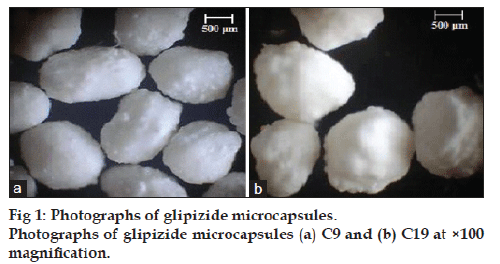
Glipizide microcapsules of almost spherical shape and rough surface were produced for C9 and C19, fig. 1a and b, respectively. On drying, a reduction to about 1/3 of the radius had occurred. Similar results were reported for alginate coated microcapsules of nicardipine hydrochloride and blue dextran [14,33]. The mean particle size of different formulas ranged from 918.42 to 1294.45 μm with SD of less than 40 (Table 2). Drug to polymer ratio markedly affected the microcapsule size, where the size increased with higher amount of the polymer used. These findings were in agreement with Sezer and Akbuge who stated that chitosan treated alginate microcapsules are larger than alginate microcapsules due to extra coating [19]. Furthermore, the larger CP treated alginate microcapsules may be due to higher viscosity of internal phase.
| Formula no. | Microcapsule size (µm) | Yield (%) | Microencapsulation efficiency (%) | Q 8ha |
|---|---|---|---|---|
| C1 | 879 ± 21 | 91 ± 12 | 95 ± 12 | 98 ± 14 |
| C2 | 996 ± 38 | 88 ± 10 | 97 ± 20 | 96 ± 9 |
| C3 | 964 ± 34 | 97 ± 21 | 96 ± 20 | 91 ± 10 |
| C4 | 1309 ± 40 | 91 ± 18 | 95 ± 23 | 97 ± 18 |
| C5 | 845 ± 29 | 84 ± 23 | 88 ± 15 | 96 ± 12 |
| C6 | 1107 ± 12 | 89 ± 24 | 91 ± 13 | 91 ± 21 |
| C7 | 1113 ± 10 | 90 ± 15 | 96 ± 19 | 98 ± 18 |
| C8 | 1058 ± 21 | 95 ± 22 | 85 ± 10 | 80 ± 16 |
| C9 | 822 ± 35 | 96 ± 16 | 98 ± 14 | 99 ± 12 |
| C10 | 1167 ± 35 | 90 ± 30 | 94 ± 12 | 78 ± 20 |
| C11 | 1294 ± 17 | 91 ± 12 | 98 ± 18 | 88 ± 13 |
| C12 | 1185 ± 35 | 87 ± 15 | 97 ± 20 | 84 ± 21 |
| C13 | 847 ± 21 | 92 ± 20 | 88 ± 19 | 48 ± 13 |
| C14 | 1193 ± 30 | 90 ± 14 | 98 ± 25 | 46 ± 15 |
| C15 | 1187 ± 38 | 84 ± 9 | 97 ± 26 | 58 ± 12 |
| C16 | 1137 ± 37 | 92 ± 17 | 98 ± 21 | 52 ± 16 |
| C17 | 821 ± 19 | 96 ± 14 | 93 ± 15 | 69 ± 27 |
| C18 | 1223 ± 18 | 84 ± 18 | 97 ± 17 | 80 ± 5 |
| C19 | 1048 ± 29 | 98 ± 20 | 97 ± 12 | 98 ± 19 |
| C20 | 1095 ± 21 | 85 ± 19 | 96 ± 16 | 76 ± 19 |
Table 2: Mean Size, Product Yield, Drug Loading Efficiency And Release Behavior Of Glipizide Microcapsules (Values=Average ± Sd, N=3)
The process of glipizide microencapsulation was evaluated to determine their yield. The results (Table 2) have shown that the process was efficient providing a high yield (~84?97%) and minimum batch variability. It is worth to note that increasing polymer concentration from 1 to 2%; and drug to polymer ratio from (1:1) to (1:2) depicted a decrease in the microcapsules yield as a result of increasing the viscosity.
To verify the chemical degradation of glipizide in the microcapsule formulations, HPLC?UV chromatograms of the microcapsules were compared to that of the drug, as received. For both microcapsules and drug as received, glipizide had a retention time of ~3.9 min with no significant degradation. It was concluded that glipizide microcapsules had reasonable chemical stability during the formulation process.
All the prepared formulations complied with the pharmacopoeial limits for drug content. The loading efficiency of the drug in the prepared mcrocapsules was found to be between 85 and 95% (Table 1), thus demonstrating minimal loss of drug during formation. Low?coefficient of variation (<2.0%) in the percentage of drug content indicated the uniformity of drug content in each batch of microcapsules.
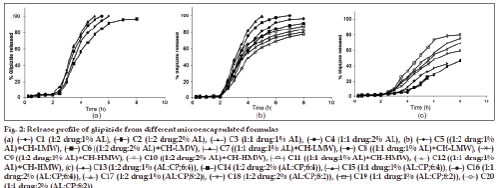
The percentage of glipizide released after 8 h (Q8h) from the prepared microcapsule formulations are shown in Table 1. Glipizide powder as received achieved over 70% release within the first 3 h which is in agreement with previous literatures [12]. The in vitro release of glipizide from sodium alginate coated microcapsules (C1?C4) showed unsuccessful retardation in the drug release (fig. 2a). The extent of drug release from C1 was ~100% within 6 h whereas that of the other three microcapsules achieved ~100% over 8 h. These results may be explained by the fact that sodium alginate is a pH?dependent gelling polymer and is insoluble in water at pH below 3. Consequently, the polymer at the microcapsule’s surface may convert to insoluble alginic acid resulting in an intact, but relatively porous, composite hydrated layer, in which much of the polymer is insoluble and does not contribute to the diffusional barrier.
It has been reported that the non?swelling property of alginate should reduce the matrix permeability and limit the drug release at pH 1.2. Upon changing the pH of the release medium to 7.4, the microcapsules began to swell while retaining their integrity leading to an increase in glipizide passage from this swollen matrix. Over time, the microcapsules became appreciably more swollen and dissolved, almost completely, after 8 h [34]. We may suggest that the release of glipizide from alginate microcapsules at pH 7.4 was due to both the passage through the swollen matrix and the escape from the microcapsules surface which underwent erosion after swelling. Salib et al [35]. assumed that the pronounced control of drug release from alginate microcapsules in acidic medium, in comparison with that in alkaline medium, was due to the conversion of calcium alginate coating into the alginic acid which was less soluble than sodium alginate formed in the alkaline medium.
On the other hand, formulas (C5?C8) were prepared by adding chitosan (LMW) in the coating layer with alginate. The extent of the drug release from these formulations was 96% (8 h), 91% (8 h), ~100% (5 h) and ~100% (7 h), respectively (fig. 2b). These results may signify that this polymer combination was not successful in prolonging the drug release. It may be explained by the low molecular weight grade of chitosan which lead to low viscosity.
When replacing chitosan LMW with HMW in the coating of the microcapsules for C9?C12 formulas, the drug achieved 80, 78, 88 and 84% release, respectively, within 8 h (fig. 2b). It was clear that alginate–chitosan (HMW) coated microcapsules were highly successful in retarding the glipizide release. Formulas C9 and C10 were in agreement with the specified release requirements.
The difference in the drug release profile between alginate and alginate–chitosan (HMW) coated microcapsules may prove the formation of an inter?polymeric complex between alginate and chitosan. The release rate may be a function of the degree of cross?linking between the two polymers and the similarity of the saccharide structures of them. The increase in release rate of glipizide from alginate–chitosan coated microcapsules at pH 1.2 may be related to the solubility of chitosan in acid medium via protonation of the amine groups [36]. The short release time from alginate coated microcapsules at high pH values may be due to the low stability of the chelating junction between sodium alginate and CaCl2 in phosphate buffer above pH 5 whereas the longer release times from alginate–chitosan coated microcapsules were thought to be due to the presence of phosphate ions stabilizing the polycation salt [37].
In a trial, to delay the release rate of glipizide from the alginate coated microcapsules, the use of CP was selected. Microcapsules were prepared in two different ratios leading to a slow release of glipizide. This may principally be due to the fact that CP formed a strong gel structure at pH 4?9 [38?40]. Thus, a remarkable difference of dissolution profile was expected. For alginate–CP coated microcapsules prepared in a ratio of 6:4 (C13?C16), the extent of the drug release was 48, 46, 58 and 52%, respectively, after 8 h (fig. 2c). This may be attributed to the higher ratio of the carbomer. To overcome this significant release retardation, the ratio of sodium alginate:carbopol 934P was altered to be 8:2 (C17?C20) (Table 1 and fig. 2c). The extent of drug release was found to be 69, 60, 80 and 76%, respectively, over 8 h. This may indicate that formulas C19 and C20 complied with the dissolution specifications for controlled release products.
The drug release from carbopol 934P microcapsules may be explained by the fact that the drug was trapped in glassy core in the dry state and forms gelatinous layer upon hydration. The hydrogels were not entangled chains of polymer but discrete microgels made up of many polymer particles. When the hydrogel was fully hydrated, osmotic pressure within the networks broke up the structure essentially by sloughing off discrete pieces of hydrogels. The gel formed upon hydration may act as rate controlling [41].
The drug release from the formulas containing sodium alginate alone or in combination with CP was found to follow zero?order kinetics. In addition, formula C11 containing sodium alginate coated with chitosan high molecular weight followed zero?order kinetics. Although the drug release of the other formulas followed first order kinetics, the t1/2 values of the prepared formulas are in the range of 1.16?8.43 h. The formula C8 showed the lowest value of t1/2 whereas formula C14 exhibited the highest value. A two?way ANOVA was performed to determine the significance of differences in glipizide release kinetics. Significant differences (P<0.05) existed in the release profiles among all capsule formulations.
According to all previous results, C9 and C19 microcapsules were selected for performing accelerated stability testing. None of the stored formulas showed any changes in colour or appearance throughout the storage period of 12 weeks under different temperatures with relative humidity 75%. Furthermore, the capsule shells remained intact and no brittleness was observed till the end of storage period [33]. The typical chromatogram of glipizide obtained following the analysis under the chromatographic conditions previously described is shown in fig. 3. The drug showed sharp and symmetrical peak with good base line resolution and minimum tailing, thus facilitating the accurate measurement of the peak area.
The calibration plot for the peak areas of varying drug concentrations was highly linear (R=0.9998) and this result indicated that there was an excellent correlation between the peak area and the concentration. Intra?day and inter?day variation were determined using concentrations in the range of 4?24 μg/ml. Each concentration was analysed in triplicate and the % RSD of intra?day precision was found to be 0.6 and 0.006 for the slope and R, respectively. The inter?day precision showed % RSD of 0.07 and 0.006 for the slope and R, respectively. From the ANOVA results, there was no significant difference between the results of the intra?day and inter?day variation.
The chemical stability results of glipizide formulations demonstrated that the percentage drug remaining after storage for a period of 12 weeks was found to be 99, 98 and 96% for formula C9 (fig. 4a) and 98, 96 and 95% for formula C19 (fig. 4b) at the three elevated temperatures, respectively. It is worth noting that microcapsule formulations showed very low rate of degradation after storage at the three temperatures for 12 weeks. Regression analysis of stability data indicated that the decomposition of the drug followed first?order kinetics. The degradation rate constant (K25) for each formula was calculated and the expiration dates were determined according to the Garret and Karper equation, which states that: t90%=0.105/K25, where t90% is the time at which the percentage remaining was 90% [42]. The expiration dates were 3.7 and 2.9 years for C9 and C19, respectively. Furthermore, the release of the drug from the stored formulas did not exhibit any significant change after storage at the three elevated temperatures for 12 weeks [43].
The individual pharmacokinetic parameters of the drug were calculated by non?compartmental analysis. It has been reported that non?compartmental (model?independent) analysis is preferred over compartmental analysis in bioequivalence evaluation due to several reasons. The main reason is that non?compartmental analysis is less prone to data manipulation. The primary criticism against compartmental modelling was the model selection was too subjective and data?specific. The same compartmental model might not apply to the same datasets for the same drug given to different individuals or given to the same individuals at different times. Non?compartmental analysis is the most commonly used technique of pharmacokinetic data analysis for studies with frequent sampling. Following the relevant FDA Guidelines, the statistical analysis should be based on the non?compartmental parameters AUCinf and Cmax, derived from the drug concentration–time curve (although plasma is the preferred matrix, sometimes whole blood or free concentrations are used). These parameters are compared by means of an ANOVA in which the variance is partitioned into components due to subjects, periods and treatments [44?47].
In vivo study demonstrated that the microcapsule formula C9 achieved lower Cmax and higher Tmax and ACU values compared to that of the commercially available tablet (Table 3 and fig. 5). Furthermore, the prepared formula exhibited prolonged mean elimination half?life. The t1/2 was found to be 9 h, however; the immediate release Minidiab® tablets attained t1/2 of 4 h. The delayed Tmax, decreased Cmax, unaltered bioavailability, and prolonged t1/2 indicated a slow and prolonged release of the drug from the microcapsules in comparison with the immediate release tablet dosage form. Statistical analysis of pharmacokinetic parameters showed that there was a significant difference (P<0.05) between the values of t1/2, AUC0?48 and AUC0?∞ of the microcapsules C9 when compared to immediate release Minidiab® tablets. The mean percent relative bioavailability of the prepared formula C9 was 93% with respect to Cmax, 199% with respect to AUC(0?48) and 200% with respect to AUC(0?∞).
| Formulation | Pharmacokinetic parameters | |||||
|---|---|---|---|---|---|---|
| Tmax(h) | Cmax (µg/ml) | AUC(0-48) (µg h/ ml) | AUC(0-∞) (µg h/ ml) | Ke(1/h) | t1/2(h) | |
| C9 | 3 ± 0.2* | 3.5 ± 0.02 | 14 ± 2* | 14 ± 3* | 0.1 ± 0.01 | 6 ± 0.1 |
| Minidiab® | 2 ± 0.1* | 4.4 ± 0.2 | 7 ± 1* | 7 ± 1* | 0.2 ± 0.01 | 4 ± 0.2 |
Table 3: The Mean Pharmacokinetic Parameters
The induction of diabetes mellitus in rabbits was confirmed by elevated levels of blood glucose in the tested rabbits. Streptozotocin is well known for its selective pancreatic islet β?cell cytotoxicity and has been extensively used to induce diabetes mellitus in animals. It interferes with cellular metabolic oxidative mechanisms and induces severe and irreversible hyperglycemia [48,49]. The administration of streptozotocin increased the glucose levels in time?dependent manner compared to control group (123 mg/dl). Following drug treatments, a decline in the blood sugar level was observed in this study (fig. 6). C9 showed a higher hypoglycemic activity compared to Minidiab® tablets. With glipizide in the commercial tablets, a rapid reduction in blood glucose levels was observed within 4 h after oral administration and then, the blood glucose levels recovered to normal within 8 h (fig. 6). The reduction in blood glucose levels was gradual and reached maximum reduction 10 h after the administration of optimised glipizide microcapsules. This reduction in blood glucose levels was sustained for longer periods of time (18 h). Significant hypoglycemic effect was observed between 1 and 4 h after oral administration of Minidiab® tablets, whereas with microcapsules, a significant hypoglycemic effect was maintained for 3?18 h after administration. One can speculate that the glipizide particles of the tablets are voided from the stomach much quicker than the microcapsules, and then they immediately dissolve in the duodenal fluid with higher pH and get absorbed. This may not be the case with the drug loaded microcapsules, where the sustained hypoglycemic effect may be due to longer gastric transit time and slower release of glipizide in the duodenum [6,12]. ANOVA test confirmed the significant difference between formula C9 and immediate release Minidiab® (P>0.05).
Glipizide microcapsules were successfully prepared by ionotropic gelation technique using different polymers as single or in combination. These formulations achieved acceptable particle size, high yield and microencapsulation efficiency (~88%). The drug release from almost all the prepared formulas effectively exhibited an extended release of the drug over a prolonged period of time and depended on composition of the coat. There was no change in the colour or physical appearance of selected microcapsules till the end of the storage period. The expiration date of the prepared formulae C9 and C19 were calculated to be 3.7 and 2.9 years, respectively. During the storage period, the release of the drug from the stored formulas did not show any change. C9 microcapsules demonstrated a great enhancement in the drug bioavailability compared to immediate release tablets (Minidiab® 5 mg). The in vivo study demonstrated significant hypoglycemic activity of the glipizide microcapsules C9. Extended release microcapsules may be considered as promising technique for oral delivery of glipizide.
Acknowledgements
We would like to gratefully acknowledge Al?Pheronea Pharmaceutical Company, Egypt for the provision of glipizide. We also thank Lubrizol, Belgium for supplying gift samples of carbomer 971P.
References
- Pillay V, Dangor CM, Govender T, Moopanar KR, Hurbans N. Drug release modulation from cross-linked calcium alginate microdiscs, 1: Evaluation of the concentration dependency of sodium alginate on drug entrapment capacity, morphology, and dissolution rate. Drug Deliv 1998;5:25-34.
- Chowdary KP, Rao YS. Design and in vitro and in vivo evaluation of mucoadhesive microcapsules of glipizide for oral controlled release: A technical note. AAPS Pharm Sci Tech 2003;4:E39.
- Gutcho MH. Microcapsules and Microencapsulation Techniques. Park Ridge, NJ: Noyes Data Corporation; 1976. p. 230-236.
- Kondo A. Microcapsule Processing and Technology. In: J. Wade Van Valkenburg, editor. New York: Marcel Dekker; 1979. p. 18.
- Krishna RR, Murthy TE. Preparation and evaluation of mucoadhesive microcapsules of glipizide formulated with gum kondagogu: In vitro and in vivo. Acta Pharm Sci 2010;52:335-44.
- Patel JK, Patel RP, Amin AF, Patel MM. Formulation and evaluation of mucoadhesiveglipizide microspheres. AAPS PharmSciTech 2005;6:E49-55.
- Sievert B, Siewert M. Dissolution test for extended release products. In: Dressman JB, Lennernäs H, editors. Oral Drug Absorption: Prediction and Assessment. New York: Marcel Dekker, Inc.; 2000, p. 183-95.
- Bailey MM, Gorman EM, Munson EJ, Berkland C. Pure insulin nanoparticle agglomerates for pulmonary delivery. Langmuir 2008;24:13614-20.
- Pelavin PI, Abramson E, Pon S, Vogiatzi MG. Extended-release glipizide overdose presenting with delayed hypoglycemia and treated with subcutaneous octreotide. J Pediatr Endocrinol Metab 2009;22:171-5.
- Feig DS, Briggs GG, Kraemer JM, Ambrose PJ, Moskovitz DN, Nageotte M, et al. Transfer of glyburide and glipizide into breast milk. Diabetes Care 2005;28:1851-5.
- National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2005.
- Shivakumar HN, Patel PB, Desai BG, Ashok P, Arulmozhi S. Design and statistical optimization of glipizide loaded lipospheres using response surface methodology. Acta Pharm 2007;57:269-85.
- Paget GE, Barnes JM. Toxicity tests. In: Laurence DR, Bacharach AL, editors. Evaluation of drug activities pharmacometrics. 34th ed., vol. I. New York: Academic Press; 1964. p. 134–166.
- Kim CK, Lee EJ. The controlled release of blue dextran from alginate beads.Int J Pharm 1992;79:11-9.
- Hari PR, Chandy T, Sharma CP. Chitosan/calcium alginate microcapsules for intestinal delivery of nitrofurantoin. J Microencapsul 1996;13:319-29.
- Yao J, Shi YQ, Li ZR, Jin SH. Development of a RP-HPLC method for screening potentially counterfeit antidiabetic drugs. J Chromatogr B Analyt Technol Biomed Life Sci 2007;853:254-9.
- Gumieniczek A, Berecka A, Komsta ?. Stability-indicating validated HPLC method for simultaneous determination of oral antidiabetic drugs from thiazolidinedione and sulfonylurea groups in combined dosage forms. J AOAC Int 2010;93:1086-92.
- Haug A, Smidsrød O. Fractionation of alginates by precipitation with calcium and magnesium ions. Acta ChemScand 1965;19:1221-6.
- Sezer A, Akbu?a J. Release characteristics of chitosan treated alginate beads: II. Sustained release of a low molecular drug from chitosan treated alginate beads. J Microencapsul 1999;16:687-96.
- Lukowski G, Muller R, Muller B, Dittgen M. Acrylic acid copolymer nanoparticles for drug delivery: I. Characterization of the surface properties relevant for in vivo organ distribution.Int J Pharm 1992;84:23-31.
- El-Gibaly I, Safwat SM, Ahmed MO. Microencapsulation of ketoprofen using w/o/w complex emulsion technique. J Microencapsul 1996;13:67-87.
- Giunchedi P, Torre ML, Maggi L, Conti B, Conte U. Cellulose acetate trimellitateethylcellulose blends for non-steroidal antiinflammatory drug (NSAID) microspheres. J Microencapsul 1996;13:89-98.
- Acartürk F, Takka S. Calcium alginate microparticles for oral administration: II. Effect of formulation factors on drug release and drug entrapment efficiency.J Microencapsul 1999;16:291-301.
- Gåserød O, Smidsrød O, Skjåk-Bræk G. Microcapsules of alginate-chitosan–I: A quantitative study of the interaction between alginate and chitosan. Biomaterials 1998;19:1815-25.
- Chang RK, Price J, Whitworth CW. Control of drug release rate by use of mixtures of polycaprolactone and cellulose acetate butyrate polymers. Drug DevInd Pharm 1987;13:1119-35.
- Cha Y, Pitt C. A one-week subdermal delivery system for l-methadone based on biodegradable microcapsules. J Control Release 1988;7:69-78.
- Bruce LD, Petereit HU, Beckert T, McGinity JW. Properties of enteric coated sodium valproate pellets.Int J Pharm 2003;264:85-96.
- Chopra R, Alderborn G, Podczeck F, Newton JM. The influence ofpellet shape and surface properties on the drug release from uncoated and coated pellets.Int J Pharm 2002;239:171-8.
- Sadeghi F, Ford JL, Rajabi-Siahboomi A. The influence of drug type on the release profiles from Surelease-coated pellets.Int J Pharm 2003;254:123-35.
- Sousa JJ, Sousa A, Moura MJ, Podczeck F, Newton JM. The influence of core materials and film coating on the drug release from coated pellets.Int J Pharm 2002;233:111-22.
- Blandino A, Macias M, Cantero D. Formation of calcium alginate gel capsules: Influence of sodium alginate and CaCl2 concentration on gelation kinetics. J Biosci Bioeng 1999;88:686-9.
- Ruiz R, Sakr A, Sprockel OL. A study on the manufacture and in vitro dissolution of terbutalinesulfate microcapsules and their tablets. Drug DevInd Pharm 1990;16:1829-42.
- Bansal G, Singh M, Jindal KC, Singh S. LC and LC-MS study on establishment of degradation pathway of glipizide under forced decomposition conditions. J Chromatogr Sci 2008;46:510-7.
- Fernandez-Hervas M, Holgado M, Fini A, Fell J. In vitro evaluation of alginate beads of a diclofenac salt.Int J Pharm 1998;163:23-34.
- Salib N, El-Menshawy M, Ismail A. Utilization of sodium alginate in drug microencapsulation. Pharm Ind 1978;40:1230-4.
- Tsai T, San YP, Ho HO, Wu JS, Sheu MT. Film-forming polymer-granulated excipients as the matrix materials for controlled release dosage forms. J Control Release 1998;51:289-99.
- Liu LS, Liu SQ, Ng SY, Froix M, Ohno T, Heller J. Controlled release of interleukin-2 for tumour immunotherapy using alginate/chitosan porous microspheres. J Control Release 1997;43:65-74.
- Singla AK, Chawla M, Singh A. Potential applications of carbomer in oral mucoadhesive controlled drug delivery system: A review. Drug DevInd Pharm 2000;26:913-24.
- Llabot JM, Manzo RH, Allemandi DA. Drug release from carbomer: carbomer sodium salt matrices with potential use as mucoadhesive drug delivery system.Int J Pharm 2004;276:59-66.
- Muramatsu M, Kanada K, Nishida A, Ouchi K, Saito N, Yoshida M, et al. Application of Carbopol®to controlled release preparations I.Carbopol® as a novel coating material.Int J Pharm 2000;199:77-83.
- Jivraj M, Martini LG, Thomson CM. An overview of the different excipients useful for the direct compression of tablets. Pharm SciTechnol Today 2000;3:58-63.
- Anderson G, Scott M. Determination of product shelf life and activation energy for five drugs of abuse.Clin Chem 1991;37:398-402.
- Verma RK, Garg S. Selection of excipients for extended release formulations of glipizide through drug-excipient compatibility testing. J Pharm Biomed Anal 2005;38:633-44.
- Messori A, Longo G, Matucci M, Morfini M, Ferrini PL. Clinical pharmacokinetics of factor VIII in patients with classic haemophilia. Clin Pharmacokinet 1987;13:365-80.
- Gillespie WR. Noncompartmental versus compartmental modelling in clinical pharmacokinetics. Clin Pharmacokinet 1991;20:253-62.
- DiStefano JJ III. Noncompartmental vs. compartmental analysis: Some bases for choice. Am J Physiol 1982;243:R1-6.
- Bulitta JB, Holford NH. Non-Compartmental Analysis. Wiley Encyclopedia of Clinical Trials. New Jersy: John Wiley and Sons, Inc; 2008, p. 1-21.
- Mitra SK, Gopumadhavan S, Muralidhar TS, Seshadri SJ. Effect of D-400, a herbomineral formulation on liver glycogen content and microscopic structure of pancreas and liver in streptozotocin induced diabetes in rats. Indian J Exp Biol 1996;34:964-7.
- Papaccio G, Pisanti FA, Latronico MV, Ammendola E, Galdieri M. Multiple low-dose and single high-dose treatments with streptozotocin do not generate nitric oxide. J Cell Biochem 2000;77:82-91.

 AL)+CH-HMW)
AL)+CH-HMW)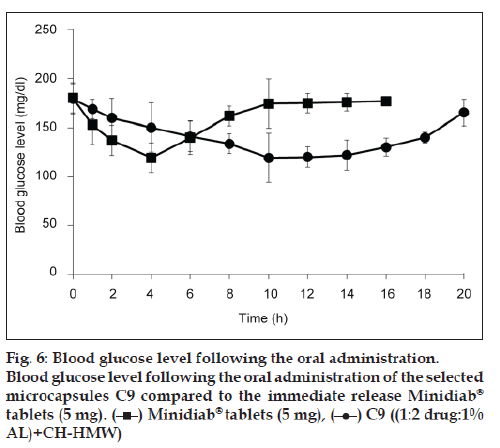
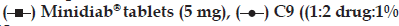 AL)+CH-HMW)
AL)+CH-HMW)



