- *Corresponding Author:
- M. J. Rosemary
Medical Devices, Corporate R&D Centre, HLL Lifecare Ltd., Thiruvananthapuram, Kerala 695017, India
E-mail: rosemarymj@lifecarehll.com
| Date of Received | 16 June 2023 |
| Date of Revision | 14 December 2023 |
| Date of Acceptance | 20 August 2024 |
| Indian J Pharm Sci 2024;86(4):1296-1306 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The primary objective of this work is to enhance the bioavailability of ferrous sulphate, which has low oral bioavailability and hence is subjected to frequent drug administration which results side effects. In this study, ferrous sulphate was microencapsulated with carbopol, a mucoadhesive polymer to form carbopol encapsulated ferrous sulphate microspheres and evaluated their surface morphology, mucoadhesiveness, in vitro drug release, swelling characteristics, in vitro cell cytotoxicity, acute systemic dose and oral bioavailability. Carbopol-encapsulated ferrous sulphate microspheres have good encapsulation efficiency, good mucoadhesive properties and superior swelling characteristics and offered a sustained drug release over 24 h of drug administration compared to pure ferrous sulphate. The median lethal dose of the developed formulation was estimated from the in vivo acute oral toxicity studies. It was also found from the bioavailability studies that microencapsulation of ferrous sulphate with carbopol improves systemic oral bioavailability of ferrous sulphate and can be of great potential as a mucoadhesive drug delivery system in the oral therapy of iron deficiency anaemia.
Keywords
Microspheres, mucoadhesion, bioavailability, pharmacokinetic parameters
Despite the emergence of variable routes of drug administration over the past decades, the oral route still continues to be the most preferred route for drug intake. Oral iron therapy in iron-deficient anemic patients is often associated with side effects like poor compliance, limited gastrointestinal absorption and poor tolerance of the drug[1]. The complexities associated with gastrointestinal tract upon administration of oral dosage forms such as the variations in bioavailability, development of gastric irritation etc., were reduced with the development of controlled and sustained release oral drug formulations[2]. Mucoadhesive drug delivery systems can be designed as controlled release formulations to enable prolonged drug retention at the delivery site, thus attaining improved therapeutic outcomes[3]. The process of mucoadhesion is an aftermath of interaction between the mucoadhesive polymer and the mucus layer of the mucosa, which in turn is greatly depended upon the mucus and the polymeric structure of the mucoadhesive polymer[4]. These systems remain in close contact with the mucous membrane for an extended period of time, thus increasing their residence time at the absorption site, providing a controlled release of the drug and thereby increasing the curative consequence[5]. The choice of mucoadhesive polymers further contributes to the cause, serving the better purpose. According to literature, a polymer with strong intermolecular hydrogen bonding, rapid mucosal adhesion, good mucosal penetration and biodegradability can be considered ideal as a mucoadhesive polymer[6]. Carbopol (CP) has been widely used as a biodegradable and biocompatible mucoadhesive polymer and is considered very effective owing to its biosafety, bio adhesiveness and swelling behavior[7,8]. The standard approach for treating iron-deficiency anemia involves treatment with oral iron salts. However, more than 50 % patients complained of gastrointestinal side effects thus leading to reduced compliance with the treatment. Even though intravenous formulations of iron are safe, the need for venous access, risk of infusion and possibility of hypersensitivity reactions often limit their use[9]. Ferrous formulations are widely studied for oral iron supplementation in iron deficient persons; major studies report that slow-release Ferrous sulphate (FeSO4) preparations to be the best choice for oral iron therapy[10]. Conventional iron tablets containing FeSO4 were often reported causing gastric intolerances upon frequent oral uptake, while certain clinical studies reported that controlled-release formulations produce fewer side effects[11]. Taking into consideration the above facts and FeSO4 being more economical as compared to other ferrous formulations, it has been chosen as the drug of choice in our current work.
Few reports are available on the formulation and characterization studies of coated-iron microspheres like graphene-encapsulated iron microspheres[12], poly-vinyl alcohol coated iron microspheres[13] and carbonyl iron microspheres[14]. But these studies are more focused on the development and characterization studies of coated iron particles, such that their stability characteristics and in vivo behavior are given the least importance. The mucoadhesive polymer based FeSO4 microspheres were found to be having good mucoadhesive capacity, swelling capacity and encapsulation efficiency[15,16]. This article aims to study the potential of mucoadhesive CP coated-FeSO4 microspheres for oral iron delivery. Mucoadhesive polymer coated FeSO4 is suitably characterized, evaluated for drug release pattern and in vivo behavior was suitably established through animal models. We hypothesize that microencapsulating FeSO4 with a mucoadhesive polymer like CP would result in improved drug bioavailability in systemic circulation when compared to administering raw FeSO4 orally, thus resulting in improved rate and extended drug absorption.
Materials and Methods
Dried ferrous sulfate was purchased from Himedia Laboratories Pvt. Ltd., India; CP-974P NF was obtained as a gift sample from Arihant Innochem Pvt. Ltd., India; ascorbic acid was procured from Merck, India. All reagents and buffers used for cell culture studies such as Dulbecco’s Modified Eagle Medium (DMEM) were purchased from Lonza, Switzerland; penicillin and streptomycin were purchased from Thermo Fisher Scientific, India; fetal bovine serum was purchased from Gibco, United States of America (USA). Phosphate Buffer Saline (PBS) was purchased from Himedia, India; Trypsin, acridine orange and ethidium bromide were procured from Sigma Aldrich, USA. All other reagents used were of analytical grade.
FeSO4-CP compatibility studies:
Binary mixture compatibility testing, whereby binary (1:1 or customized) mixtures of drug and the excipient with or without the addition of water were stored under stressed conditions and analyzed using a technique such as differential scanning calorimetry for evaluating its thermal stability[17] and Fourier Transform Infrared (FTIR) for assessing FeSO4-CP compatibility.
Preparation of CP-coated FeSO4 microspheres:
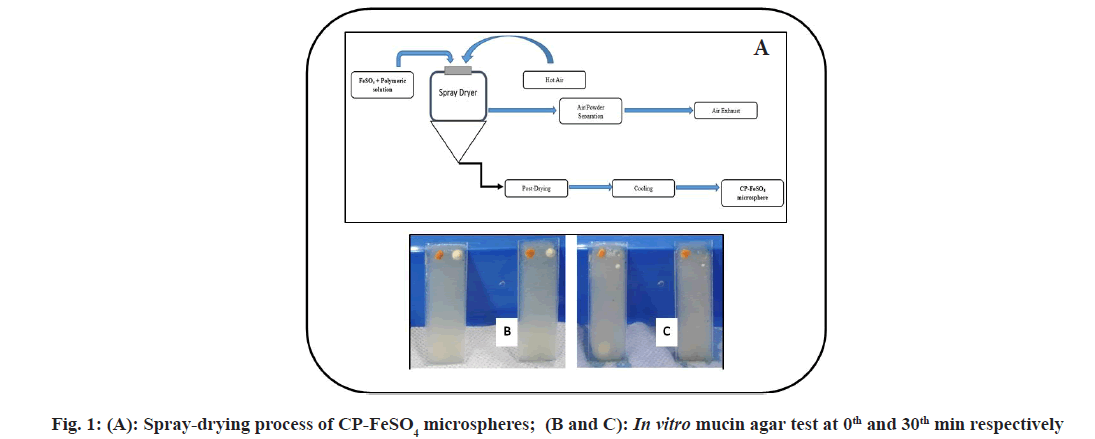
CP coated FeSO4 microspheres were prepared by spray drying technique[14] using “Jay Instruments Spray Mate” laboratory spray dryer. CP (2 % w/v) was dissolved in distilled water to form a homogenous polymer solution at room temperature. FeSO4 (1 % w/v) along with ascorbic acid (15:1) was dissolved in water at room temperature and mixed thoroughly with the prepared polymer solution and homogenized for 30 min at 10 000 revolutions per minute (rpm). Ascorbic acid was added along with FeSO4 so that the absorption of FeSO4 is enhanced. The samples were spray-dried using “Jay instruments Spray Mate” laboratory spray dryer with a standard 0.5 mm nozzle. During the spray drying process; the feed solutions were sprayed at an inlet temperature of 135°-140° and an outlet air temperature of 70°-80° with a flow rate of 2 ml/min. The dry microspheres were collected from the bottom of the cyclone separator.
Spray-dried iron microspheres were subjected to gamma (γ) sterilization at 25°, 60 % RH using 60Co gamma cell in dark condition by irradiating it at 25 kGy (Microtol Sterilization Services Pvt. Ltd., Bengaluru, India). Scanning Electron Microscopy (SEM) analysis, encapsulation efficiency, mucoadhesion, swelling studies and in vitro release studies were performed on the microspheres to confirm that properties were retained after γ irradiation.
Characterization studies of CP-FeSO4 microspheres:
A major challenge in the development of microsphere formulation remains the effective encapsulation of the drug within the polymeric matrix, such that the drug does not leach out before reaching the targeted site. The procedure for determination of encapsulation efficiency of iron microsphere formulations is as per the reported protocol[15]. The in vitro mucoadhesion potential of the CP-FeSO4 was evaluated using agar-mucin method[18]. Swelling of microspheres influences its mucoadhesion potential as well as the nature of drug release from the polymeric coating. The in vitro swelling behaviour of microspheres was studied using pH 7.4 PBS. For swelling studies, accurately weighed microsphere formulations were placed in centrifuging tubes containing pH 7.4 PBS and centrifuged at 3000 rpm for 10 min. The excess amount of water was removed and the weights of wet samples were measured. The swelling index was then calculated using the following equation.
S=SW-SI/SI
Where, S is swelling index; SW is weight of wet sample and SI is initial weight.
The in vitro drug release studies of the formulation were done in pH 1.2 and pH 7.4 as per the reported protocol[14] in order to understand the mechanism of drug release. The morphology and size of the microspheres were examined before and after sterilization using scanning electron microscope (Zeiss Evo 18 and HITACHI S4200, Japan). FTIR of CP-FeSO4 microspheres, pure FeSO4 and CP 974P NF were recorded using an infrared spectrometer (Frontier Perkin Elmer FTIR, USA) in the frequency range of 400-4000 cm-1.
Stability analysis of CP-FeSO4 microspheres:
The optimized formulation of CP-FeSO4 microspheres was stored at an accelerated condition of 40°±2°/75 % RH±5 % RH for 6 mo and periodically withdrawn in 0th, 3rd and 6th mo according to International Council for Harmonisation Q1A guidelines (stability testing of new drug substances and products) in a stability chamber (Binder GmbH, KBF 720, Germany)[19].
Cell cytotoxicity by MTT assay:
Cell viability assay was performed on fibroblast/ Caco-2 epithelial cell line following the protocol given by Mossman et al.[20], by 3-(4, 5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) assay.
In vivo studies of CP-FeSO4 microspheres:
Systemic oral toxicity studies were conducted on female Wistar rats, weighing about 150-175 g. Prior approval from Institutional Animals Ethics Committee of the Palamur Biosciences Pvt. Ltd., was obtained under the study number PAL/ IAEC/2019/04/01/45 for executing the study. The animals were fasted overnight, prior to dosing with free access to water, but feed was withheld further for a period of 3-4 h after dosing. In order to evaluate the acute toxicity, animals were tested in 4 steps, containing 3 individuals in each step. The effect of the drug was studied by administering a single dose by gavage using a stainless steel balltipped oral intubation needle at the desired dose level. The administered dose volume was 10 ml/ kg body weight.
The starting dose level was 300 mg/kg. In step- 1, three female rats (fasted overnight) were administered orally with the test item and evaluated for clinical signs of toxicity and mortality. In step- 2, three more female rats (fasted overnight) were administered orally at 300 mg/kg to confirm the results. Step-3 proceeded with three female rats (fasted overnight) administered orally at 2000 mg/ kg. Any clinical signs of toxicity and mortalities were noted. The dose was confirmed in step- 4 with three more female rats (fasted overnight) administered orally at 2000 mg/kg body weight. All the surviving animals were observed up to 14 d after treatment. The animals were observed for integrated indicators like body weight, feed and water intake, mortality, signs of toxicity and histopathological studies of liver, kidneys, lungs, heart, ovaries, adrenal, spleen, intestine and brain. Acute toxicity values were expressed as median Lethal Dose (LD50) oral values or as Acute Toxicity Estimates (ATE). Based on the obtained LD50 value, the test item was classified according to the Globally Harmonized System (GHS) of classification and labelling of chemicals.
Histopathological evaluation:
After the study period, animals were sacrificed by excess anaesthesia. Histopathological examination of the organs such as liver, pancreas, heart and kidney was performed to gain understanding of the degree of damage to these vital organs upon oral administration of the CP-FeSO4 microsphere formulation. These organ tissues were pressed between filter pads, weighed and then fixed using 10 % neutral formalin solution by use of standard techniques and then stained with haematoxylin and eosin for histopathological examination. The tissue samples were examined and graded under light microscopy with 500X magnification.Bioavailability studies:
In vivo bioavailability studies were conducted on six albino female rabbits, which were equally classified into two groups, each weighing between 2-2.5 kg. The experiments were carried after approval from the Institutional Animal Ethics Committee under the study number SCT/IAEC- 371/July/2020/106. The rabbits were kept in fasting condition for 24 h. Group 1, was treated with FeSO4 powder (as a control), suspended in 5 ml of saline (10 mg/kg FeSO4) and administered orally via gastric intubation for each fasted rabbit. Group 2, was treated orally with CP-FeSO4 microspheres and suspended in 5 ml of saline (10 mg/kg FeSO4) via gastric intubation. Blood samples (2 ml) were collected at different time intervals (1 h, 2 h, 4 h, 12 h, 18 h, 24 h) post-administration of the drug. The blood samples were centrifuged at 2000 rpm to get clear plasma.The plasma analysis of FeSO4 was determined by ferritin spectrophotometric kit (Human ferritin Enzyme-Linked Immunosorbent Aassay (ELISA) kit, Ramco Laboratories, USA). Based on the plasma concentration-time profile obtained, pharmacokinetic evaluation of the CP encapsulated iron microspheres is compared with control FeSO4.
Bioavailability is described as the fraction of an administered drug that reaches the systemic circulation. When the systemic availability of a drug after oral administration is compared with that of an oral standard of the same drug, it is referred to as relative or comparative bioavailability. Relative bioavailability is calculated as: Relative bioavailability={[AUC]Test/DoseTest}/{[AUC]Control/ DoseControl}
Results and Discussion
The physical mixture of FeSO4 and CP polymer remained unchanged, pure and non-interactive state during the compatibility studies indicating that the drug is compatible with the polymer. FTIR analysis of FeSO4 and CP-FeSO4 mixtures showed all the major peaks of both the polymer and FeSO4 in 0th mo and 1st mo of testing, with a slight shift indicating the interactions between CP and FeSO4.
The encapsulation efficiency of the CP-FeSO4 was found to be 99.72 %. As is evident from the figure for agar-mucin assay (fig. 1A-fig. 1C); control drug FeSO4 was displaced >10 cm within 30 min of the test, while CP-FeSO4 mucoadhesive formulation is displaced <1 cm over 3 h of the test and was found sticking to the agar layer upon completion of the in vitro agar-mucin mucoadhesion test. The mucoadhesive property of the CP-FeSO4 microspheres was further evaluated quantitatively by ex vivo mucoadhesive test on isolated sheep intestine and was found to be in the range of 58 %-75 %. The displacement of the raw FeSO4 was found to be almost instantaneous owing to its inability to interact with mucin either electrostatically or due to entanglements. Very low displacement of the CP-FeSO4 mucoadhesive formulation indicates the mucoadhesive potential of the developed formulation. This high mucoadhesive capacity can in turn be attributed to the higher hydration potential of CP polymers, which allows a formulation to establish a quick contact with the mucosal layer[3] upon administration and consolidation of the adhesion using hydrogen bonding or macromolecular inter chain penetration between mucin components and CP polymer. The swelling index of the microspheres was found to be about 1200, thus indicating good swelling properties of the developed formulations. The swelling index of a mucoadhesive formulation is highly related to its bio adhesive strength. Higher swelling index allows for greater interaction of the mucoadhesive formulation with the mucosal layer and therefore greater retention time in the mucosa[21]. The in vitro release and kinetic studies of the formulation were carried out and it showed zero-order with Korsemeyer-Peppas model release kinetics.
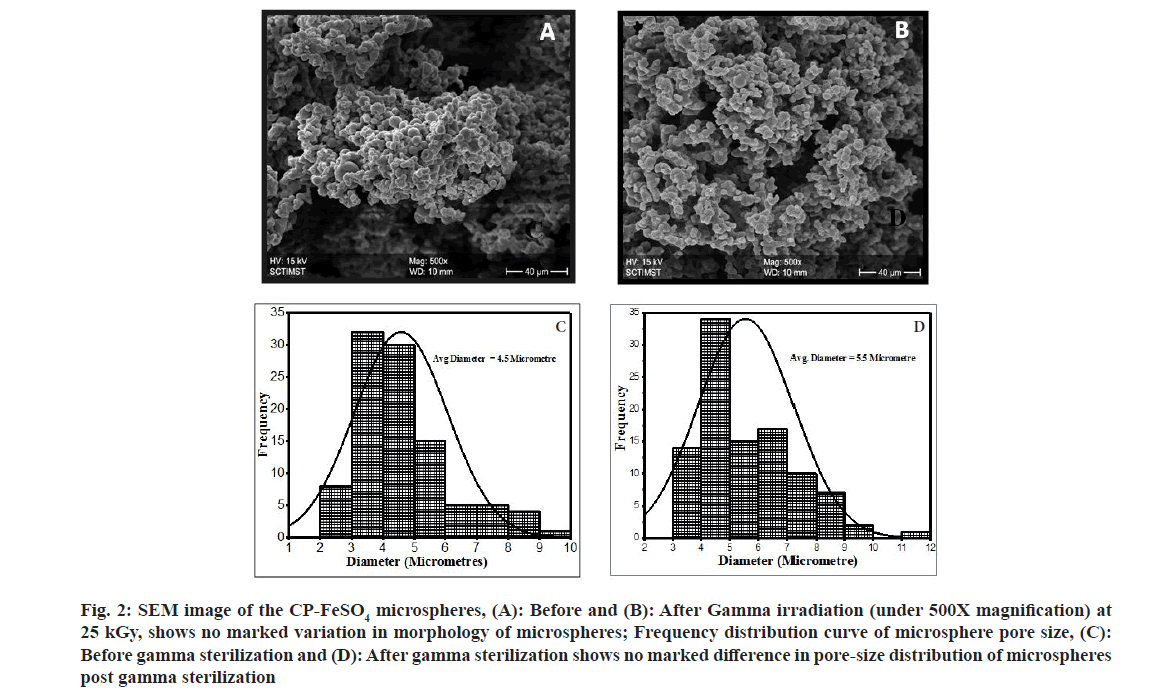
The surface morphology and characteristics of the optimized mucoadhesive microsphere formulation, CP-FeSO4 was investigated using SEM before and after gamma irradiation. The micrographs of the CP-FeSO4 microspheres showed aggregates of spherical particles. The average particle diameter of CP-FeSO4 microspheres as calculated using the Image J software was found to be 4.5 μm with a narrow size distribution in the range of 2.3-9.5 μm. The morphology and size distribution of CP coated iron microspheres post 25 kGy sterilization was also evaluated. The particles were found to be spherical with wrinkled surfaces in aggregates similar to that of pre-sterilized microspheres with an average diameter size of 5.5 μm and narrow size distribution of 3-9.1 μm (fig. 2). The aggregation of microspheres may be due to its accumulation during storage[22]. It can also be observed that the spherical particles were slightly wrinkled, which can be due to excessive accumulation of vapor pressure during the solvent-evaporation process of the spray-drying process[23]. Further, it has been reported that the wrinkled surfaces of microspheres have increased surface-to-volume ratio, thus reducing dosage and frequency of drug delivery in addition to increasing the surface contact between microspheres and cell membranes at the site of absorption[24,25]. Based on the SEM morphology and size analysis before and after gamma sterilization, it can also be concluded that gamma sterilization does not influence the surface morphology and particle size of the microspheres.
Fig. 2: SEM image of the CP-FeSO4 microspheres, (A): Before and (B): After Gamma irradiation (under 500X magnification) at 25 kGy, shows no marked variation in morphology of microspheres; Frequency distribution curve of microsphere pore size, (C): Before gamma sterilization and (D): After gamma sterilization shows no marked difference in pore-size distribution of microspheres post gamma sterilization
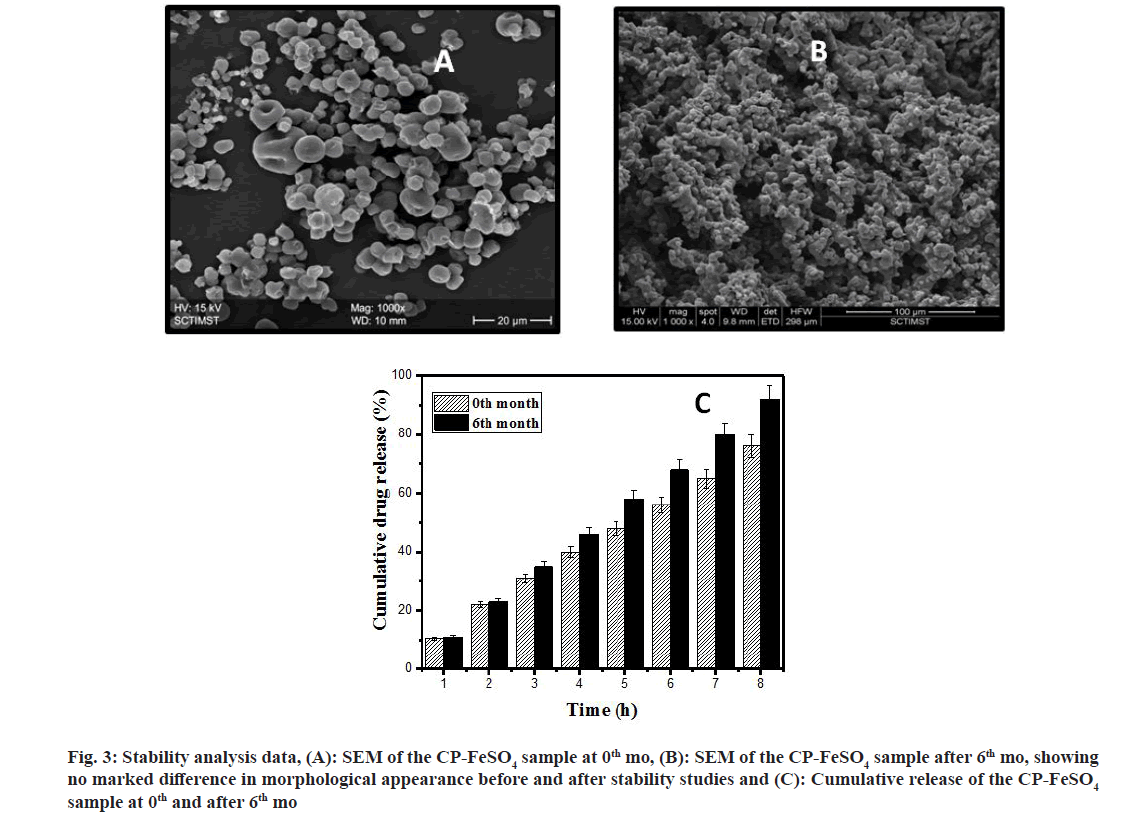
The FTIR studies clearly indicate that FeSO4 has been incorporated into the CP polymer to form CPFeSO4 microspheres. Upon completing accelerated stability studies, the physical mixture of CP-FeSO4 in 6th mo of the study was found to be similar to the 1st mo CP-FeSO4 sample, with no visible change in color, odor and appearance. The morphology of the microspheres was evaluated by SEM before and after the stability studies.
From stability studies data (fig. 3), it is clear that the microspheres before and after the stability study appear similar in terms of morphology. In both the figures the microspheres appear to be spherical with wrinkled surfaces and slightly agglomerated, such that no marked differences were observed with the SEM images before and after stability studies. The cumulative release of iron from CP-FeSO4 microspheres in the 0th and 6th mo of stability studies is also shown in the data for stability studies. From the in vitro drug release data, it can be deduced that the difference in cumulative drug release from the CP-FeSO4 microsphere formulation before and after stability is less than 10 %. Hence, the CP-FeSO4 microsphere formulation can be considered stable under the accelerated conditions of temperature and humidity and is resistant to any chances of drug degradation.
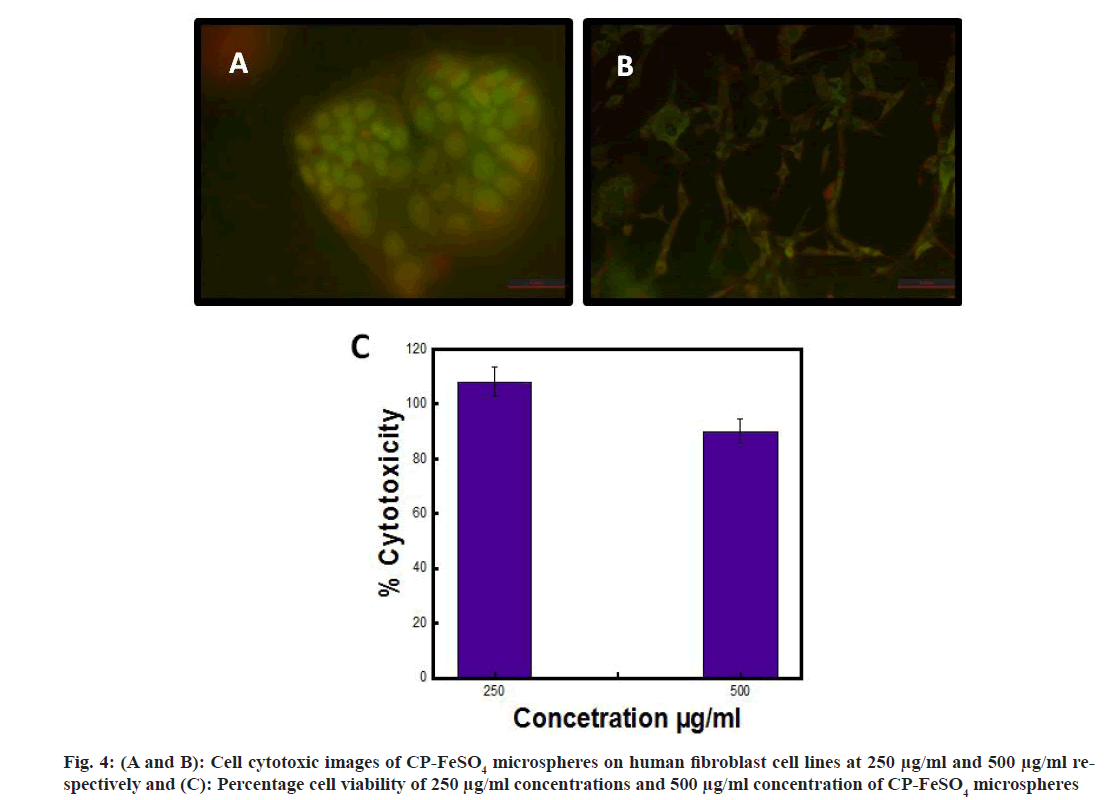
The cell cytotoxic studies of CP-FeSO4 microspheres were assessed by MTT assay. The microspheres showed more than 80 % cell viability at a concentration of 250 μg/ml and 500 μg/ml on Caco-2 cells, while the CP coated FeSO4 microspheres showed a proliferating effect (>100 %) at 250 μg/ml on fibroblast cell lines. CP empty microspheres showed 100 % viability in the cell lines, implying that no significant effect was observed with the control blank microspheres. Cell cytotoxic images of CP-FeSO4 microspheres were as shown in fig. 4A and fig. 4B, while the percentage viability of different concentrations of CP-FeSO4 is shown as fig. 4C.
The cell uptake studies of CP-FeSO4 microspheres showed an increase in the mean concentration of ferritin is observed (50-162 ng/mg) in comparison to FeSO4. Upon comparing the mean ferritin concentration of FeSO4 and CP-FeSO4, it can be observed that, CP-FeSO4 microspheres showed better mucosal absorption.
The median Lethal Dose (LD50) or ATE for CP-FeSO4 microspheres was determined as 2000<ATE≤5000 mg/kg body weight. The test item CP coated FeSO4 microspheres thus falls under category 5 or unclassified with LD50 cutoff value of 5000 mg/kg body weight according to GHS classification system. Histopathological examination conducted on animals revealed a few changes with different doses at various steps. The gross pathological examination of animals showed no signs of macroscopic lesions with any of the animals. However, upon histological examination of the lung tissue, perivascular and peribronchial mononuclear blood cell filtration was observed with some animals. The histopathological images of the various organs upon administration of CPFeSO 4 microspheres were as shown in fig. 5. From the histopathological examination of rat tissues, it can be propounded that the rate of occurrence of pathological lesions with different doses at various steps was considered to be spontaneous or incidental in nature as per information available in the literature[26-28].
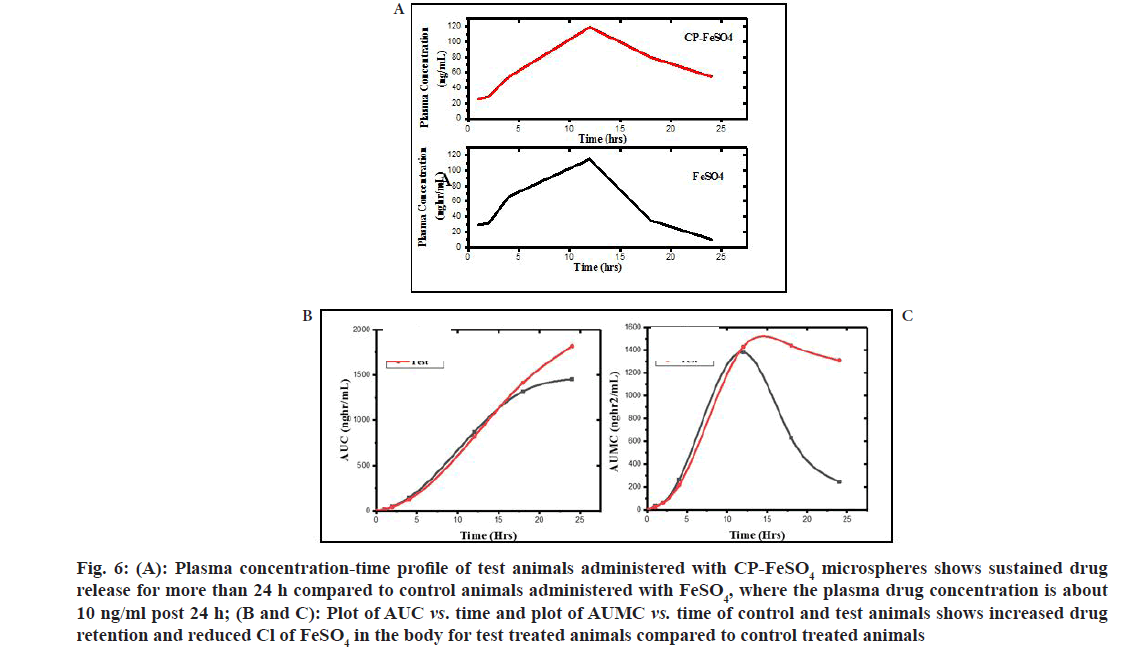
The plasma concentration-time profile of FeSO4 and CP-FeSO4 is as shown in fig. 6A and the area under the curve represents the measurement of total systemic exposure to the drug (fig. 6B and fig. 6C). From the plasma concentration-time profile it is clear that post 24 h, the concentration of iron in the plasma of FeSO4 suspension administered rats is approximately 10 ng/ml, while the concentration of iron in the plasma of CP-FeSO4 microsphere administered rats is 54 ng/ml. Thus, it can be assumed that CP-FeSO4 microspheres offer a sustained drug release over 24 h of drug administration compared to pure FeSO4. The various pharmacokinetic parameters vs. peak plasma Concentration (Cmax), Time for peak plasma concentration (Tmax), Absorption rate constant (Ka), Elimination rate constant (Ke), elimination halflife (t1/2), Volume of distribution (Vd), Clearance (Cl), Area Under the Curve (AUC), Area Under the Moment Curve (AUMC) and Mean Residence Time (MRT) of the two formulations are shown in Table 1.
Fig. 6: (A): Plasma concentration-time profile of test animals administered with CP-FeSO4 microspheres shows sustained drug release for more than 24 h compared to control animals administered with FeSO4, where the plasma drug concentration is about 10 ng/ml post 24 h; (B and C): Plot of AUC vs. time and plot of AUMC vs. time of control and test animals shows increased drug retention and reduced Cl of FeSO4 in the body for test treated animals compared to control treated animals
| Pharmacokinetic parameters | Control (powdered ferrous sulphate) | Test (CP coated ferrous sulphate microspheres) |
|---|---|---|
| Tmax (h) | 12 | 12 |
| Cmax (ng/ml) | 115.12 | 119.02 |
| Elimination rate constant, Ke (h-1) | 0.201 | 0.065 |
| Elimination half life, t1/2 (h) | 3.44 | 10.67 |
| Absorption rate constant, Ka | 0.281 | 0.461 |
| [AUC]0-24 (ng h/ml) | 1455.415 | 1818 |
| [AUC]0-∞ (ng h/ml) | 1507.015 | 2659 |
| Volume of distribution, Vd (l/kg) | 0.132 | 0.361 |
| [AUMC]0-24 (ng h2/ml) | 1484.3 | 4480.08 |
| [AUMC]0-∞ (ng h2/ml) | 4102.78 | 37620.61 |
| Mean residence time, MRT (h) | 2.72 | 14.15 |
| Clearance (l/h) | 0.027 | 0.023 |
| Bioavailability | 1.604 |
Table 1: Pharmacokinetic Parameters of Test and Control Formulation
The Ke of FeSO4 suspension was higher compared to CP-FeSO4 indicating that the rate of elimination of FeSO4 from the body was larger for FeSO4 suspension than CP-FeSO4 microspheres, thus offering a prolonged therapeutic action for microspheres. Further to this, the t1/2 of the CPFeSO4 microspheres was found to be 3 times greater than the half-life of the FeSO4 suspension, indicating longer drug retention in the body. The AUC of the CP-FeSO4 microsphere was considerably increased when compared to AUC of FeSO4 suspension indicating a longer systemic exposure of the drug. The absorption rate constant, Ka was used to describe the rate of entry of the drug into the body and it can be observed that the rate of drug absorption is faster for CP-FeSO4 microspheres compared to FeSO4. The rate of drug absorption often depends upon various parameters like disintegration rate from formulations, the solubility of the drug, presence or absence of excipients for oral drug formulations. Vd of CPFeSO 4 was greater compared to CP-FeSO4 and a higher value of Vd indicates more distribution of the drugs to other tissues, while a smaller Vd indicates the lesser distribution of drug to tissue organs[29]. AUMC represents the area under the moment curve and a knowledge of it along with AUC is required for further calculation and analysis of drug characteristics[30]. The AUMC and AUC values are greater for CP-FeSO4 microspheres as compared to FeSO4. The residence time of each of the molecules of the drug inside the body is varied, based on which the mean value of residence time of each of the molecules can be indicated as the MRT and it gives an idea regarding the average residence time of a drug formulation in the body[31,32]. The MRT of CP-FeSO4 was approximately 5 times greater than the MRT of FeSO4 suspension, thus indicating a greater degree of retention of iron in the body in the case of microspheres compared to the FeSO4 oral suspension. Cl represents the volume of blood or plasma from which the drug is completely cleared off per unit time[33]. The Cl value of a drug is related to the efficiency of drug elimination from the body such that, the greater the elimination rate of the drug from the body, the higher the Cl. The Cl values of the control and test formulation indicate a low systemic exposure of the drug in the body when administered with FeSO4 suspension compared to CP-FeSO4 microspheres. The bioavailability of CP-FeSO4 is greater than the raw FeSO4, thus indicating a greater rate and extend of drug absorption into the systemic circulation, which leads to improved efficacy.
Results were expressed as mean±standard deviation for triplicate samples. The statistical significance among the groups was determined by one-way Analysis of Variance (ANOVA). Statistical significance was considered at a level of p<0.05.
In conclusion, microencapsulating FeSO4 with a mucoadhesive polymer like CP improves drug bioavailability in vivo, by enhancing the drug retention time thereby prolonging drug residence in the body. CP-FeSO4 microspheres were found to follow zero-order drug release kinetics with good mucoadhesive properties and swelling characteristics in vitro. Additionally, the drug-polymer compatibility, microsphere biocompatibility, product stability and systemic oral toxicity were also studied and found to be biocompatible, stable and free from oral toxicity, thus resulting in the development of a potential technology for delivering iron to iron deficit patients in a more bioavailable manner.
Acknowledgments:
The financial assistance from Science and Engineering Board (SERB), Government of India, (EMR/2016/002298) is gratefully acknowledged.
Conflict of interests:
The authors declare no conflict of interests.
References
- Macdougall IC. Strategies for iron supplementation: oral versus intravenous. Kidney Int Suppl 1999;55:S61-6.
[Crossref] [Google Scholar] [PubMed]
- Kaushal AM, Garg S. Minimizing bioavailability variations with oral controlled release formulations: What is possible? Am J Drug Deliv 2003;1:103-12.
- Shaikh R, Singh TR, Garland MJ, Woolfson AD, Donnelly RF. Mucoadhesive drug delivery systems. J Pharm Bioallied Sci 2011;3(1):89-100.
[Crossref] [Google Scholar] [PubMed]
- Dhawan S, Singla AK, Sinha VR. Evaluation of mucoadhesive properties of chitosan microspheres prepared by different methods. AAPS Pharm Sci Tech 2004;5:122-8.
[Crossref] [Google Scholar] [PubMed]
- Selvaraj GJ, Balasubramanian A, Ramalingam K. A mini review on mucoadhesion. Int J Pharm Sci Res 2020;11:3521-7.
- Roy S, Pal K, Anis A, Pramanik K, Prabhakar B. Polymers in mucoadhesive drug-delivery systems: A brief note. Des Monomers Polym 2009;12(6):483-95.
- Bera K, Mazumder B, Khanam J. Study of the mucoadhesive potential of carbopol polymer in the preparation of microbeads containing the antidiabetic drug glipizide. AAPS Pharm Sci Tech 2016;17:743-56.
[Crossref] [Google Scholar] [PubMed]
- Ruiz-Rubio L, Alonso ML, Pérez-Álvarez L, Alonso RM, Vilas JL, Khutoryanskiy VV. Formulation of Carbopol®/Poly (2-ethyl-2-oxazoline)s mucoadhesive tablets for buccal delivery of hydrocortisone. Polymers 2018;10(2):175.
[Crossref] [Google Scholar] [PubMed]
- Gómez-Ramírez S, Brilli E, Tarantino G, Munoz M. Sucrosomial® iron: A new generation iron for improving oral supplementation. Pharmaceuticals 2018;11(4):97.
[Crossref] [Google Scholar] [PubMed]
- Santiago P. Ferrous versus ferric oral iron formulations for the treatment of iron deficiency: A clinical overview. Sci World J 2012;2012(1):846824.
[Crossref] [Google Scholar] [PubMed]
- Morrison BO. Tolerance to oral hematinic therapy, controlled release versus conventional ferrous sulphate. J Am Geriatr Soc 1966;14:757-759.
[Crossref] [Google Scholar] [PubMed]
- Guo P, Zhu G, Song H, Chen X, Zhang S. Graphene-encapsulated iron microspheres on the graphene nanosheets. Phy Chem Chem Phys 2011;13(39):17818-24.
- Ramanujan RV, Chong WT. The synthesis and characterization of polymer coated iron oxide microspheres. J Mater Sci Mater Med 2004;15(8):901-8.
- Fang FF, Liu YD, Choi HJ, Seo Y. Core–shell structured carbonyl iron microspheres prepared via dual-step functionality coatings and their magnetorheological response. ACS Appl Mater Interfaces 2011;3(9):3487-95.
[Crossref] [Google Scholar] [PubMed]
- BS Rahul, Rosemary MJ. Mucoadhesive microspheres of ferrous sulphate–A novel approach for oral iron delivery in treating anemia. Colloids Surf B Biointerfaces 2020;195:111247.
- Rosemary MJ, Diksha P. Targeted, bioavailable and safe delivery of mucoadhesive polymer coated iron particles, IN Patent, Patent No. 412841.
- Sims JL, Carreira JA, Carrier DJ, Crabtree SR, Easton L, Hancock SA, et al. A new approach to accelerated drug-excipient compatibility testing. Pharm Dev Technol 2003;8(2):119-26.
[Crossref] [Google Scholar] [PubMed]
- Trenkel M, Scherließ R. Nasal powder formulations: In vitro characterisation of the impact of powders on nasal residence time and sensory effects. Pharmaceutics 2021;13(3):385.
[Crossref] [Google Scholar] [PubMed]
- Razdan K, Sahajpal NS, Singh K, Singh H, Singh H, Jain SK. Formulation of sustained-release microspheres of cefixime with enhanced oral bioavailability and antibacterial potential. Ther Deliv 2019;10(12):769-82.
[Crossref] [Google Scholar] [PubMed]
- Mossman BT, Gray MJ, Silberman L, Lipson RL. Identification of neoplastic versus normal cells in human cervical cell culture. Obstet Gynecol 1974;43(5):635-9.
[Google Scholar] [PubMed]
- Swain S, Behera A, Dinda SC, Patra CN, Jammula S, Beg S, et al. Formulation design, optimization and pharmacodynamic evaluation of sustained release mucoadhesive microcapsules of venlafaxine HCl. Indian J Pharm Sci 2014;76(4):354-63.
[Crossref] [Google Scholar] [PubMed]
- Mishra M, Mishra B. Mucoadhesive microparticles as potential carriers in inhalation delivery of doxycycline hyclate: A comparative study. Acta Pharma Sinica B 2012;2(5):518-26.
- Zhang WF, Chen XG, Li PW, Liu CS, He QZ. Preparation and characterization of carboxymethyl chitosan and β-cyclodextrin microspheres by spray drying. Drying Technol 2007;26(1):108-15.
- Yu HL, Feng ZQ, Zhang JJ, Wang YH, Ding DJ, Gao YY, et al. The evaluation of proanthocyanidins/chitosan/lecithin microspheres as sustained drug delivery system. Biomed Res Int 2018;2018(1):9073420.
[Crossref] [Google Scholar] [PubMed]
- Ogunjimi AT, Fiegel J, Brogden NK. Design and characterization of spray-dried chitosan-naltrexone microspheres for microneedle-assisted transdermal delivery. Pharmaceutics 2020;12(6):496.
[Crossref] [Google Scholar] [PubMed]
- Boorman GA, Eustis SL , Elwell MR, Montgomery CA, Mackenzie WF. Pathology of the Fischer Rat: Reference and Atlas. Academic press, Inc. 1990.
- Peter G. Histopathology of preclinical Toxicity Studies. 3rd edn. Academic press/ Elsevier, 360 park Avenue South, New York, USA 2007: 10010-710.
- Sahota PS, Popp JA, Hardisty JF, Gopinath C, editors. Toxicologic pathology: Nonclinical safety assessment. CRC press; 2013.
[Google Scholar] [PubMed]
- Mansoor A, Mahabadi N. Volume of distribution. InStatPearls; 2023. StatPearls Publishing.
[Google Scholar] [PubMed]
- Saha N. Clinical pharmacokinetics and drug interactions. Pharm Med Transl Clin Res 2018;6:81-106.
- Schrag M, Regal K. Chapter 4-Pharmacokinetics and toxicokinetics, in a comprehensive guide to toxicology in nonclinical drug development, AS Faqi, Editor.
- Johanson G. 1.09 Toxicokinetics and modeling. Comprehensive Toxicol 2010;1:153-77.
- Hinderliter P, Saghir SA. Pharmacokinetics, Encyclopedia of Toxicology. 3rd edn, 2014;10:762-4.