- *Corresponding Author:
- Anshul Chawla
Department of Pharmaceutical Sciences, CT College of Pharmacy, Ludhiana, Punjab 142024, India
E-mail: anshul.chawla321@gmail.com
| Date of Received | 26 October 2021 |
| Date of Revision | 08 September 2022 |
| Date of Acceptance | 02 May 2023 |
| Indian J Pharm Sci 2023;85(3):555-564 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Bacterial conjunctivitis commonly referred to as pink eye, having an inflammation of the conjunctiva either due to infection or an allergic condition. The outer lining of the white part of our eye gets reddish or pink in color. Ophthalmic antibiotics are instilled for curing this condition. Many ophthalmic formulations are available in the market for curing this condition. Most of them contain an antibiotic along with a steroidal moiety. Method development plays a pivotal role in drug testing, manufacturing operations, stability studies and the long term performance of drugs. International Council for Harmonisation provides guidelines for validating analytical methods. With minimal use of time and money scientists all over the world are developing new methods for the same. Several studies have already been done for the method development of such ophthalmic solutions but still, some antibiotics remain untouched. This review article emphasizes detailed information regarding the methods developed for ophthalmic formulations and their validation as well.
Keywords
Antibiotic, conjunctivitis, method development, pink eye, validation
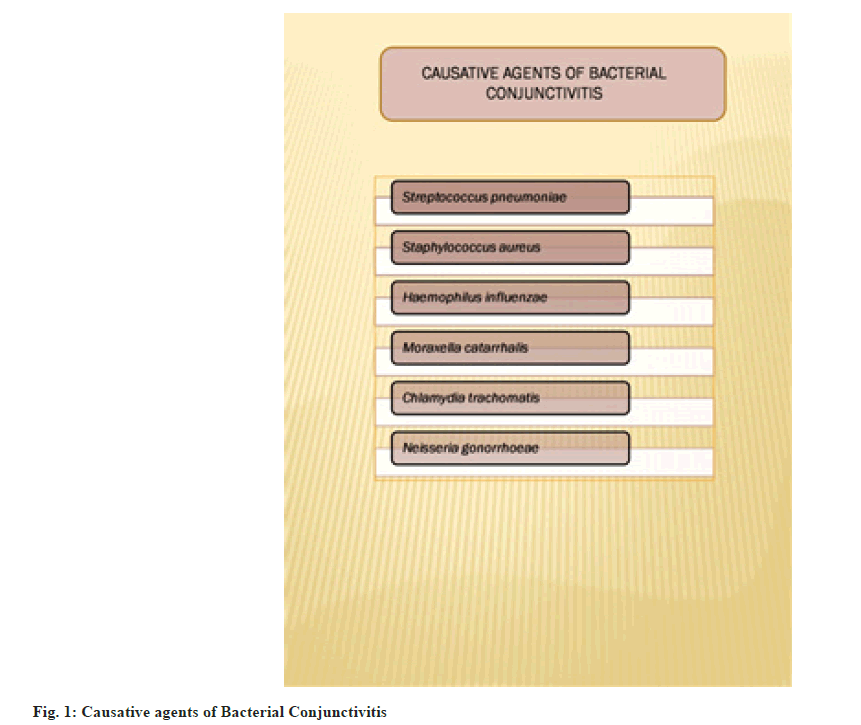
Conjunctivitis is an eye condition in which there’s inflammation of the conjunctiva. It can be due to viral infection, bacterial infection, allergies, eye cancer, immune-mediated or some toxicity. It is an inflammation of the conjunctiva, which is the outer lining of the eyeball. Ocular infections are considered to be minor but they can be "vision-threatening". Bacterial conjunctivitis is a self-restricting disease[1,2]. The most common causative agents for infectious disease are Staphylococcus epidermis (39 % of cases), Staphylococcus aureus (22 % of cases) and Streptococcus pneumonia (6 % of cases) as shown in fig. 1. The most common Gram-negative microorganism found in acute conjunctivitis is Haemophilus influenzae (9 % of cases). In contact lens wearers, the trend is reversed and more Gram-negative strains are found[3,4].
It was found that in neonatal cases causative agents for bacterial conjunctivitis are Chlamydia trachomatis, Neisseria gonorrhoeae. In most cases in children, Moraxella catarrhalis, Staphylococcus epidermis, Streptococcus viridians are the causative agents. It represents one of the most common ocular diseases in childhood, affecting 1 in 10 children each year. It can occur at any age, most frequently in school-going children[5]. Most of the registered medical practitioners prescribe topical antibiotics for minimizing the complication and re-occurring of infection[6]. Risk factors for bacterial conjunctivitis have been mentioned in fig. 2.
Adenoviral conjunctivitis is a major cause of acute infectious conjunctivitis among adults. Bacterial conjunctivitis, is also a major cause for so many cases among children[7]. There are other causes for eye infections like foreign objects in the eye, trauma to the eye, chemical substances entering the eye. Acute bacterial conjunctivitis resolves within 7-10 d but a broad-spectrum antibiotic can decrease its severity, transmission and complications[8,9].
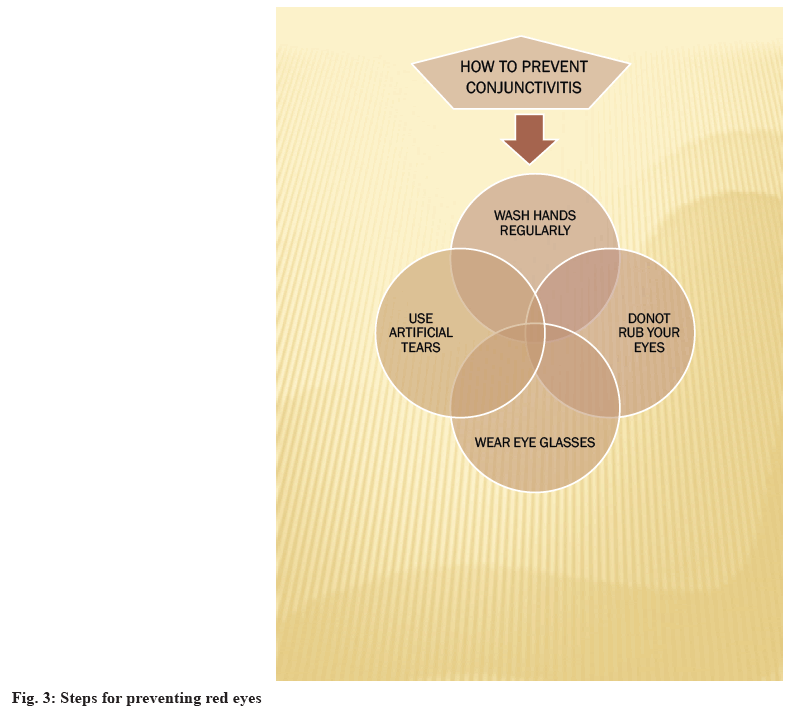
Allergic conjunctivitis is typically treated with antihistamines and mast cell stabilizers, but if symptoms persist, therapy is supplemented with topical steroids[10]. According to the American Academy of Ophthalmology, the most prominent symptom of pink eye is a greenish discharge that lasts all the day. Redness and itching occur along with it. Corticosteroids are used to treat inflammatory conditions of the eye. A combination of antibiotics along with corticosteroids helps in relieving inflammation and infection of the eye[11,12]. Common symptoms of this disease are itchy and watery eyes or red and swollen eyes with mucous discharges. In the case of severe pain in the eyes, one should seek immediate medical advice from a registered medical practitioner[13]. Other measures which can be taken along with like washing hands properly, not rubbing their eyes, not sharing things, eye glasses and handkerchiefs with others and to wear eye glasses to protect your eyes from sunlight, wind and dust particles and to keep clothing and personal belongings clean[14,15]. Steps for prevention of red eyes are shown in fig. 3. In this study, High Performance Liquid Chromatography (HPLC) method development and validation for ophthalmic formulations has been briefed.
Ophthalmic Antibiotic Formulations
Antibiotics such as ciprofloxacin, ofloxacin, gentamicin and other medications that are effective against pink eye infection are found in these ophthalmic preparations. The most often used first-line agents include sodium sulfacetamide, chloramphenicol, gentamicin, tobramycin, azithromycin, neomycin, trimethoprim, ciprofloxacin, gatifloxacin and doxycycline[16,17]. Table 1 enlists all antibiotics used in bacterial conjunctivitis. Immediate diagnosis and treatment are important to avoid vision-threatening outcomes, including corneal scarring or perforation. The choice of treatment is predicated on the use of a single antimicrobial or a combination of antimicrobials that provide a broad range of activity against both gram-positive and gram-negative bacteria[18,19]. The advantages of instilling antibiotic eye drops are to preserve visual acuity, prolonged contact and soothing effect. According to Pujalte et al.[20] microorganisms which are resistant to a systemic antibiotic are effectively treated by the topical route of administration due to the higher local concentrations induced by this route of administration[21].
| Sno | Class of drug | Name of drug | Drug in combinations | Brand names | Mechanism of action | Reference |
|---|---|---|---|---|---|---|
| 1 | Aminoglycosides | Tobramycin | Tobramycin-loteprednol | Zylet, Loto – T | Prevent growth of bacteria and reduces inflammation of eyes | [7] |
| Gentamycin | Gentamycin - prednisolone | Gentapar, Genwell | Inhibits the growth of bacteria | [9] | ||
| Neomycin | Neomycin-dexamethasone-polymyxin B | Maxitrol, Polyzen – D | Inhibts growth of bacteria and reduces swelling of eyes | [9] | ||
| 2 | Macrolides | Azithromycin | Azithromycin - dexamethasone | Azidex, Kapiaz-D | Inhibts the growth of bacteria along with decreasing inflammation of eyes | [9,17] |
| Erythromycin | Erythromycin-mineral oil-white petroleum | Pertigo, Bausch & Lomb | Inhibts growth of bacteria | [16,23] | ||
| 3 | Quinolones | Ciprofloxacin | Ciprofloxacin - dexamethasone | Cipwell, Ciplox | Inhibts growth of bacteria | [23] |
| Moxifloxacin | Moxifloxacin - dexamethasone | Moxicip, Mahaflox | Inhibts growth of bacteria | [23,26] | ||
| Gatifloxacin | Gatifloxacin - dexamethasone | Gatiquin, Zymar | Inhibts growth of bacteria | [23,26] | ||
| Levofloxacin | Levofloxacin - prednisolone | Levokap – P, LV Flox | Inhibts growth of bacteria | [26] | ||
| Lomefloxacin | Lomefloxacin | Lyflox, Lomibaq | Inhibts growth of bacteria | [9,26] | ||
| Norfloxacin | Norfloxacin – benzalkonium chloride | Norflox, Nflox | Inhibts growth of bacteria | [12] | ||
| Ofloxacin | Ofloxacin – benzalkonium chloride | Oflox, Oflacin | Inhibts growth of bacteria | [18] | ||
| 4 | Others | Chloramphenicol | Chloramphenicol - dexamethasone | Clowell-D, Chlorocol | Inhibts growth of bacteria | [12] |
| Fusidic acid | Fusidic acid – mannitol- benzalkonium chloride | Fucidin, Fucithalmic | Inhibts growth of bacteria | [15] | ||
| Rifamycin | Oral | Myrifa 200, Rifwell 300 | Inhibits bacterial infection if any blood | [23] | ||
| 5 | Tetracyclines | Chlortetracycline | Chlortetracycline – benzalkonium chloride | Chlotracare, Chlorcol H | Inhibts growth of bacteria | [23] |
| Tetracycline | Tetracycline – benzalkonium chloride | Kaytet, Terramycin | Inhibts growth of bacteria | [9,23] |
Table 1: The List of Antibiotics Used in Ophthalmic Formulations
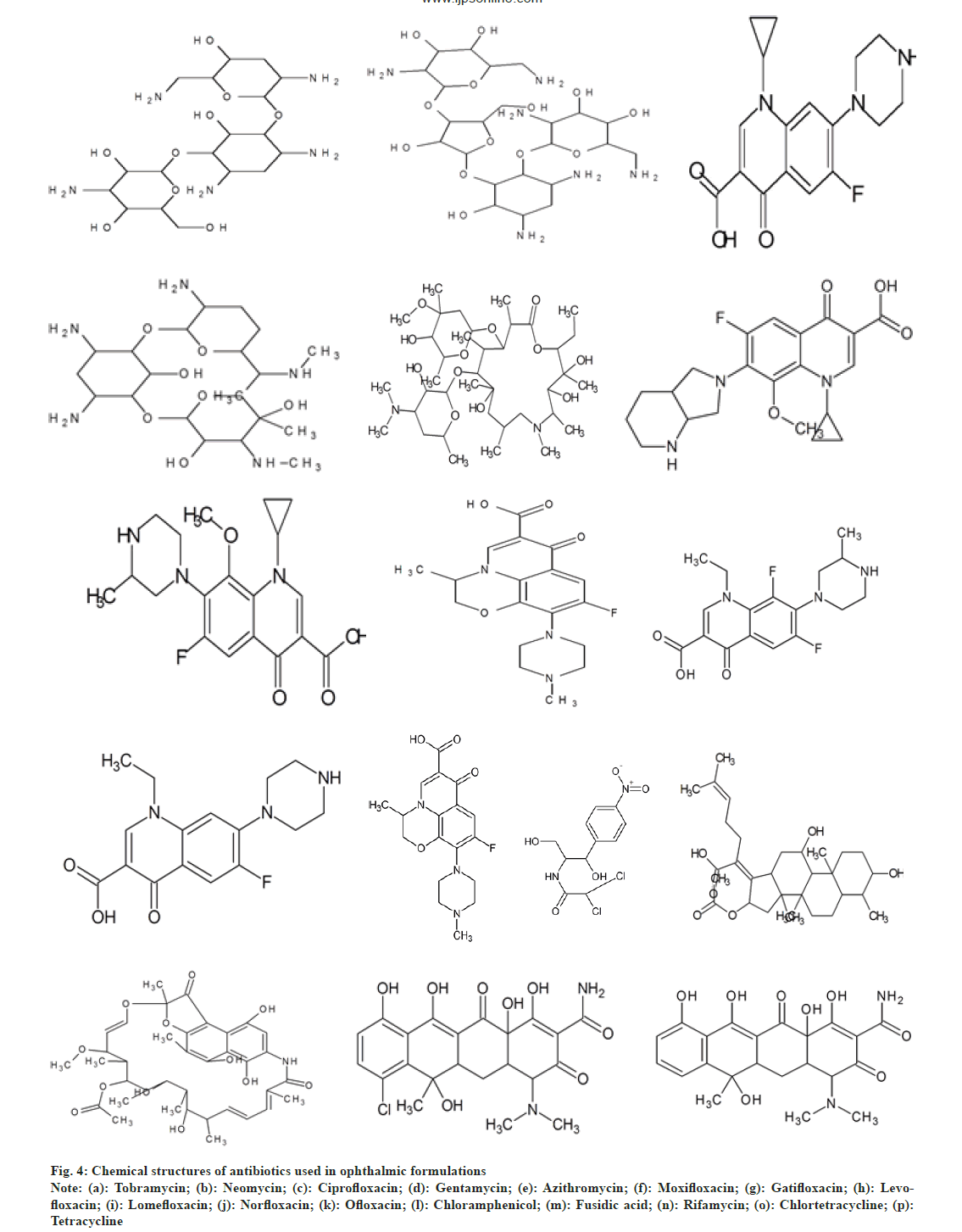
Uncomplicated cases can be treated with a topical antibiotic such as tobramycin, trimethoprim/polymyxin B, a fluoroquinolone or chloramphenicol four times daily for 5-7 d to accelerate recovery and under the observation of a physician every 2-3 d until signs and symptoms are resolved[22]. Aminoglycosides act by inhibiting protein synthesis by binding to both the 16S (in the 30S subunit) and 23S (in the 50S subunit) rRNA molecules of the bacterial ribosome[10,23]. Fluoroquinolones/quinolones prevent bacterial DNA from unwinding and duplicating. Macrolides bind to bacterial ribosomes to inhibit protein synthesis. Chloramphenicol is bacteriostatic in nature[24,25]. It inhibits protein synthesis by deactivating the functioning of peptidyl transferase[26,27]. Tetracyclines are protein synthesis inhibitors that bind to the 30S ribosomal subunit. Rifamycins inhibit DNA-dependent RNA synthesis. Fusidic acid is a bacteriostatic antibiotic[28,29]. Fig. 4 shows chemical structures of these drugs.
Fig. 4: Chemical structures of antibiotics used in ophthalmic formulations
Note: (a): Tobramycin; (b): Neomycin; (c): Ciprofloxacin; (d): Gentamycin; (e): Azithromycin; (f): Moxifloxacin; (g): Gatifloxacin; (h): Levofloxacin; (i): Lomefloxacin; (j): Norfloxacin; (k): Ofloxacin; (l): Chloramphenicol; (m): Fusidic acid; (n): Rifamycin; (o): Chlortetracycline; (p): Tetracycline
Method Development and Validation For Ophthalmic Formulations
Analytical method development and validation play a vital role in drug discovery, development and production of prescribed drugs[30]. It is the process of proving that the method developed is appropriate for its use in the development, production, and testing of pharmaceutical drugs. As outlined by United States Pharmacopeia (USP), method validation provides an assurance of reliability during regular use. It is generally defined as the process of providing documented evidence that the method does what it is intended to do[31,32]. Validation helps in meeting all desired characteristics of the analytical method. International Council for Harmonisation (ICH) (Q2-R1) guidelines are available for validating an analytical method. It assures a great impact on quality assurance and cost reduction[33,34]. Validation is required in the situations when the process is totally new, while installing and using new equipment, any process or piece of equipment that is subject to changes in usage as a result of altered conditions[29,35] and when process in which the test of the end product is not good, gives an indicator of poor quality.
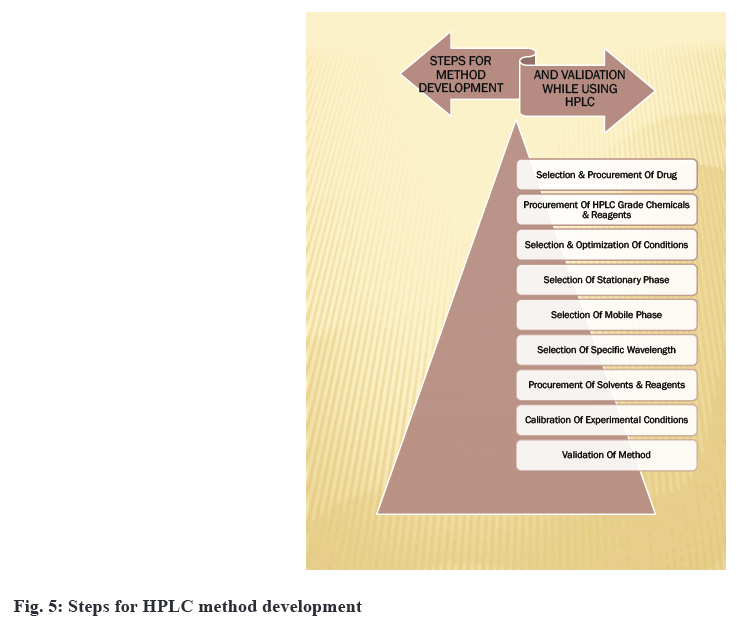
For the analysis of ophthalmic formulations, inwardly developed or significantly modified methods are sometimes used[36,37]. Method development provides critical information about a drug's potency, bioavailability, stability and effects. There is a lack of regulatory guidance on in vitro release testing methods. Method development can also be used to check the compatibility of the active drug with the formulation matrix. Optimization based on a trial and error approach is very time-consuming. Nowadays, software-assisted and automated method development can speed up the process of validation[38]. The development of methods for drug analysis in ophthalmic formulations is required when a new drug combination is developed that is not available in the Pharmacopoeia, and for existing drugs, less reliable methods for their analysis are available based on information provided by a review of literature. When formulation excipients such as benzalkonium chloride, hydroxyethylcellulose and polyvinlyalcohol create interference, no analytical technique has yet been established[39]. So yet, no analytical technique for quantifying analytes in biological fluids has been established. The existing analytical procedures involve the use of costly reagents and solvents. Development of method is also needed for routine analysis of drugs in ophthalmic formulations, to analyze the total content of formulations and to perform an assay of ophthalmic formulations[40]. There are no methods developed for checking the permeability of drugs into the cornea in the case of poorly soluble drugs. The same can also check for impurities and excipients interfering[41]. Disposable systems utilized while manufacturing of ophthalmic formulations also need validation. The method developed is validated for various concentrations of active drugs so that any changes in formulation or concentration do not require additional validation. There are some steps for developing HPLC methods[42,43] for ophthalmic formulation as shown in the fig. 5.
As per guidelines of ICH[44-47], the developed methods will be validated for following parameters like accuracy, precision, specificity, limits of detection, linearity and range and robustness. Accuracy is defined as the closeness between true value and reference value. Specificity means to assess analyte in presence of other components. Precision is the most important parameter which defines closeness between different values obtained. Repeatability means to do the same experimental work with complete precision under same operating conditions. Reproducibility should be achieved while working in different laboratories. The experimental work done should be able to produce results of various tests done.
The lowest amount of sample which can be obtained quantitatively is known as Quantitation limit. The measure of capacity of method to remain unaffected is known as robustness of that method. Table 2 shows various validation parameters according to ICH and USP guidelines. Validation comprises of minimum five steps including planning and performing the tests; statistical evaluation of results; report on the validation parameters; application of all information; full validation processes along with their explanations. Analytical method validation has an important role in having best quality and safety of the final product.
| ICH | USP |
|---|---|
| Specificity | Specificity |
| Linearity | Linearity and range |
| Range | Accuracy |
| Accuracy | Precision |
| Precision | Limit of detection |
| Limit of detection | Limit of quantification |
| Limit of quantification | Ruggedness |
| --- | Robustness |
Table 2: Validation Parameters
Method Developed For Ophthalmic Preparations
Typically, ophthalmic formulations are subjected to forced degradation, stability, and drug determination studies[48-54]. Many drugs used for ophthalmic formulations have undergone such testing. Antibiotics are the core heart of ophthalmology. Following are the highlights of the HPLC methods already developed for the ophthalmic formulations which are used for bacterial conjunctivitis.
HPLC methods:
Khalil et al.[55] developed a Reverse Phase-HPLC (RP-HPLC) technique for chloramphenicol and its hydrolytic derivatives. In this study, eight commercial batches of ophthalmic solutions were used. All of these batches were kept at room temperature. A Waters 600E system controller, a Waters 715 ultra WISP sample processor, a Waters 991 photodiode array detector, and a PDA integrator were utilized. A reverse phase C-18 5u (30 mm×4.6 mm i.d.) column was used. Water, methanol and ammonia make up the mobile phase (58:40:2). Using glacial acetic acid, the pH was adjusted to 7. The flow rate was 1.5 ml/min and the wavelength was 278 nm. Chloramphenicol was acid hydrolyzed to produce the hydrolytic product (2-amino-l-(4-nitrophenyl)-propane-l-diol hydrochloride). There was no influence from preservatives or contaminants. The procedure devised was found to be more precise and accurate.
Muralidharan et al.[56] devised a new RP-HPLC technique for determining ketotifen fumarate. Thermo C18 (250 mm×4.6 mm id) was employed in this isocratic RP-HPLC technique. The mobile phase was made up of methanol and 10 mM ammonium acetate (30:70 % v/v, pH=3.5). The flow rate was 1 ml/min, and the effluent was measured at 298 nm. The average retention time was 5 min. For accuracy, specificity, linearity, robustness and precision, the devised method was validated.
Erk et al.[57] developed an HPLC technique for determining dorzolamide hydrochloride and timolol maleate in eyedrops at the same time. A diode array detector was employed in this approach at two fixed wavelengths, 250 nm for dorzolamide and 300 nm for timolol maleate. The chromatographic separation was performed on an RP-YMC pack ODS A-132 C18 (5 micron, 15 cm×6.0 mm) column with an acetonitrile:phosphate buffer (pH 2.5):methanol (5:85:10 v/v/v) mix with a flow rate of 1.2 ml min-1.
Razzaq et al.[48] developed and validated a new RP-HPLC technique for the simultaneous detection of gatifloxacin and ketorolac tromethamine in combination dose form. For detection at 270 nm, a Shimadzu LC-20A system was used in conjunction with an SPD-M20A Ultra-violet (UV) detector. For this chromatographic separation, a BDS Hypersil C8 column (250 mm×4.6mm) was utilised. At a flow rate of 1.5 ml/min, the mobile phase was composed of methanol:phosphate buffer (55:45 v/v). The technique was linear at concentrations of 30-90 g/ml (for gatifloxacin) and 50-110 g/ml (for ketorolac tromethamine). Both analytes separated well, with adequate tailing and resolution. This created approach may be used on a regular basis for analysis.
Ruckman K et al.[58] assessed ophthalmic formulations containing Tobramycin using RP-HPLC with a UV detector set at 210 nm. It was discovered to be simple, precise, accurate and quick in nature. The error rate was 0.80 percent, while the Relative Standard Deviation (RSD) was less than 2.0 percent. The mobile phase was buffered with 0.05 M diammonium hydrogen phosphate and pH was adjusted to 10.0 using tetramethyl ammonium hydroxide. Purosphere RP-8e column (250 mm×4.6 mm, 5 m) was utilised. At 1.0 ml/min, isocratic elution was performed. Excipients and mobile phase did not cause any interference. This technique may be utilised for routine ophthalmic analysis and quality control. For the measurement of ciprofloxacin in serum, aqueous humour, and ocular drops, a bioanalytical technique for RP-HPLC was devised by Khan A et al. [59] the mobile phase consisted of acetonitrile and 0.25 M phosphoric acid (60:40 v/v) in a Perkin Elmer series 200 HPLC fitted with a UV-visible detector. The wavelength of the UV-visible detector was set to 275 nm. The flow rate was 1 ml per min. The calibration curves linearity was tested from 5-75 ng/ml. The r2 values for mobile phase, serum and aqueous humour were determined to be 0.999, 0.997 and 0.998 respectively. Endogenous compounds did not cause any interference. A simple, selective and isocratic RP-HPLC technique for analysing moxifloxacin hydrochloride in the presence of its degradation products was devised and validated by Dewani et al.[60]. It makes use of a thermo separation product system linked to a quaternary pump system 4000. A UV detector with a wavelength of 294 nm was utilised. For chromatographic separation, a Grace C18 column (250 mm×4.6 mm) was utilized. At a flow rate of 1 ml/min, the mobile phase is composed of 10 mM sodium phosphate buffer and methanol (60:40, v/v) with a pH of 4.4. The regression coefficient was determined to be 0.999. This method's system adaptability, linearity, precision and accuracy were all confirmed[60].
For the examination of natamycin 5 % w/v eye drops, a RP-HPLC technique was devised by Chaudhari et al.[61]. For chromatographic separation, Perkin Elmer and a series 200 UV-Visible detector were employed. C18 column (100×4.6 mm, 5 m) was used, and sample was injected using a Rheodyne injector valve with a 20 l sample loop. The mobile phase is made up of two solvents, solvent A (methanol) and solvent B (pH 3.5 buffer). 1.36 gm potassium di-hydrogen phosphate and o-phosphoric acid were used to make the buffer (HPLC grade). UV detection was performed at a wavelength of 304 nm (λmax). The retention time was discovered to be 8.96 min. Natamycin was exposed to a forced degradation procedure.
An HPLC technique for Besifloxacin hydrochloride was developed by Costa et al.[62] using an Agilent liquid chromatograph (model Q), 1311A quaternary pump, ALS-G1329 auto sampler, TCC-G1316A column oven, and G1315B photodiode array detector. It was an Agilent Eclipse plus C18 column (150 mm×4.6 mm, id 5 m) that was utilised. The mobile phase is composed of 0.5 % triethylamine solution and acetonitrile (74:26, v/v), with the pH adjusted to 3.0 using 10 % phosphoric acid. The injection volume was 20 l and the flow rate was 1.0 ml/min. UV detection was performed using a photodiode array at a wavelength of 295nm (λmax). This approach was compared to the drug's microbiological test method. There was no statistical difference between the two approaches. Both techniques were quite durable. The linearity, specificity, precision and accuracy of the HPLC technique were all confirmed. For the simultaneous measurement of Moxifloxacin hydrochloride and dexamethasone, a simple and sensitive RP-HPLC technique was devised by Razzaq et al.[48] . Shimadzu LC-20A system with LC-20AT pump and SPD-M20A diode array detector at 254 nm comprised the HPLC system. The stationary phase was a BDS Hypersil C8 column (250×4.6 mm, id 5 m) with a mobile phase consisting of phosphate buffer (20 mM) containing 0.1 % (v/v) triethylamine, pH 2.8, adjusted with dilute phosphoric acid and methanol (38:5:61.5 v/v) at a flow rate of 1.5 ml/min. This technique was verified for specificity, linearity, accuracy, and precision in accordance with ICH standards.
Conclusion
The foundation of ophthalmology is ophthalmic formulations. Analytical investigations have made use of a variety of methods, as well as method development. Almost majority instances of acute bacterial conjunctivitis are self-limiting and will resolve within 10 d with topical antibiotic eye drop treatment. As a result, illness transmission is reduced and disease occurrences are lower in the community. Method development and validation is a crucial stage in analyzing a pharmaceutical formulation component and conducting stability tests, and it is suggested for all newer medication investigations.
Acknowledgements:
We wish to express our gratitude to S. Charanjeet Singh Channi, Honourable Chancellor and worthy Vice-Chancellor Dr. Harsh Sadawarti for their constant inspiration and support for the research.
Conflict of interest:
The authors report no conflicts of interest.
References
- Azari AA, Barney NP. Conjunctivitis: A systematic review of diagnosis and treatment. JAMA 2013;310(16):1721-30.
[Crossref] [Google Scholar] [PubMed]
- Epling J. Bacterial conjunctivitis. BMJ Clin Evid 2012;2012:0704.
- Friedlaender MH. A review of the causes and treatment of bacterial and allergic conjunctivitis. Clin Ther 1995;17(5):800-10.
[Crossref] [Google Scholar] [PubMed]
- Baum J, Barza M. The evolution of antibiotic therapy for bacterial conjunctivitis and keratitis: 1970–2000. Cornea 2000;19(5):659-72.
[Crossref] [Google Scholar] [PubMed]
- Høvding G. Acute bacterial conjunctivitis. Acta Ophthalmol 2008;86(1):5-17.
- Hutnik C, Mohammad-Shahi MH. Bacterial conjunctivitis. Clin Ophthalmol 2010;4:1451-7.
[Crossref] [Google Scholar] [PubMed]
- Leung AK, Hon KL, Wong AH, Wong AS. Bacterial conjunctivitis in childhood: Etiology, clinical manifestations, diagnosis, and management. Recent Pat Inflamm Allergy Drug Discov 2018;12(2):120-7.
[Crossref] [Google Scholar] [PubMed]
- Limberg MB. A review of bacterial keratitis and bacterial conjunctivitis. Am J Ophthalmol 1991;112(4):2S-9S.
[Google Scholar] [PubMed]
- Sheikh A, Hurwitz B. Topical antibiotics for acute bacterial conjunctivitis: A systematic review. Br J Gen Pract 200;51(467):473-7.
[Google Scholar] [PubMed]
- Tarabishy AB, Jeng BH. Bacterial conjunctivitis: A review for internists. Cleve Clin J Med 2008;75(7):507-12.
[Crossref] [Google Scholar] [PubMed]
- Watson S, Cabrera-Aguas M, Khoo P. Common eye infections. Aust Prescr 2018;41(3):67.
- Wood M. Conjunctivitis: Diagnosis and management. Community Eye Health 1999;12(30):19-20.
- Yeu E, Hauswirth S. A review of the differential diagnosis of acute infectious conjunctivitis: Implications for treatment and management. Clin Ophthalmol 2020;14:805-13.
[Crossref] [Google Scholar] [PubMed]
- Sheikh A, Hurwitz B, van Schayck CP, McLean S, Nurmatov U. Antibiotics vs. placebo for acute bacterial conjunctivitis. Cochrane Database Syst Rev 2012;9:CD001211.
[Crossref] [Google Scholar] [PubMed]
- Alfonso E, Crider J. Ophthalmic infections and their anti-infective challenges. Survey Ophthalmol 2005;50(6):S1-6.
[Crossref] [Google Scholar] [PubMed]
- Bremond-Gignac D, Chiambaretta F, Milazzo S. A European perspective on topical ophthalmic antibiotics: Current and evolving options. Ophthalmol Eye Dis 2011;3:29-43.
[Crossref] [Google Scholar] [PubMed]
- Bremond-Gignac D, Mariani-Kurkdjian P, Beresniak A, El Fekih L, Bhagat Y, Pouliquen P, et al. Efficacy and safety of azithromycin 1.5 % eye drops for purulent bacterial conjunctivitis in pediatric patients. Pediatr Infect Dis J 2010;29(3):222-6.
[Crossref] [Google Scholar] [PubMed]
- Chung CW, Cohen EJ. Clinical Evidence: Eye disorders: Bacterial conjunctivitis. West J Med 2000;173(3):202.
- Kathrada F. A look into conjunctivitis. South Afr Fam Prac 2019;61(4):6-10.
- Pujalte GG. Primary care dermatology, an issue of primary care: Clinics in office practice. Elsevier Health Sciences; 2016.
- Shields T, Sloane PD. A comparison of eye problems in primary care and ophthalmology practices. Fam Med 1991;23(7):544-6.
[Google Scholar] [PubMed]
- Wickström K. Acute bacterial conjunctivitis–benefits vs. risks with antibiotic treatment. Acta Ophthalmol 2008;86(1):2-4.
[Crossref] [Google Scholar] [PubMed]
- Sheikh A, Hurwitz B. Topical antibiotics for acute bacterial conjunctivitis: Cochrane systematic review and meta-analysis update. Br J Gen Prac 2005;55(521):962-4.
[Google Scholar] [PubMed]
- Bhat A, Jhanji V. Bacterial conjunctivitis. Infect Cornea Conjunct 2021:1-6.
- Morrow GL, Abbott RL. Conjunctivitis. Am Fam Physician 1998;57(4):735-46.
[Google Scholar] [PubMed]
- Holland EJ, Fingeret M, Mah FS. Use of topical steroids in conjunctivitis: A review of the evidence. Cornea 2019;38(8):1062-7.
[Crossref] [Google Scholar] [PubMed]
- Keen M, Thompson M. Treatment of acute conjunctivitis in the United States and evidence of antibiotic overuse: Isolated issue or a systematic problem? Ophthalmology 2017;124(8):1096-8.
[Crossref] [Google Scholar] [PubMed]
- Cronau H, Kankanala RR, Mauger T. Diagnosis and management of red eye in primary care. Am Fam Physician 2010;81(2):137-44.
[Google Scholar] [PubMed]
- Gupta V, Jain AD, Gill NS, Guptan K. Development and validation of HPLC method-a review. Int Res J Pharm Appl Sci 2012;2(4):17-25.
- Singh R. HPLC method development and validation-an overview. J Pharm Educ Res 2013;4(1).
- Swartz M. HPLC detectors: A brief review. J Liq Chromatogr Relat Technol 2010;33(9-12):1130-50.
- Comité de Direcção IC. ICH Topic Q2 (R1) validation of analytical procedures: Text and methodology. In international conference on harmonization. 1994;17.
- Ghanjaoui ME, Mandil A, Ait Sidi Mou A, Slimani R. High performance liquid chromatography quality control. Int J Adv Chem 2020;8:160-9.
- Desai PS, Chavan RV, Amane NB, Shete SD, Dhole AR. High performance liquid chromatography-a validation view. Asian J Pharm Anal 2019;9(4):232-6.
- Chandrul Kaushal K, Srivastava B. A process of method development: A chromatographic approach. J Chem Pharm Res 2010;2(2):519-45.
- Ahuja S, Dong M, editors. Handbook of pharmaceutical analysis by HPLC. Elsevier; 2005.
- Ravisankar P, Navya CN, Pravallika D, Sri DN. A review on step-by-step analytical method validation. IOSR J Pharm 2015;5(10):7-19. [Crossref]
- Guillarme D, Dong MW. Newer developments in HPLC impacting pharmaceutical analysis: A brief review. Am Pharm Rev 2013;16(4):36-43.
- Galea C, Mangelings D, Vander Heyden Y. Characterization and classification of stationary phases in HPLC and SFC–a review. Anal Chim Acta 2015;886:1-5.
[Crossref] [Google Scholar] [PubMed]
- Yandamuri N, Srinivas Nagabattula KR, Kurra SS, Batthula S, Nainesha Allada LP, Bandam P. Comparative study of new trends in HPLC: A review. Int J Pharm Sci Rev Res 2013;23(2):52-7.
- Shah BP, Jain S, Prajapati KK, Mansuri NY. Stability indicating HPLC method development: A review. Int J Pharm Sci Res 2012;3(9):2978.
- Thammana M. A review on high performance liquid chromatography (HPLC). Res Rev J Pharm Anal 2016;5(2):22-8.
- Chauhan A. harti Mittu B, Chauhan P. Analytical method development and validation: A concise review. J Anal Bioanal Tech 2015;6(233):2.
- Kakad SB, Kolhe MH, Dukre TP. A review on pharmaceutical validation. Int J Pharm Quality Assurance 2020;11(03):338-42.
- Shrivastava S, Deshpande P, Daharwal SJ. Key aspects of analytical method development and validation. J Ravishankar Univ 2018;31(1):32-9.
- Barot HN, Dave JB, Patel CN. Development and validation of spectrophotometric method for simultaneous determination of prednisolone acetate and ofloxacin in eye-drop. Int J Pharm Sci Res 2012;3(6):1817.
- Gupta H, Aqil M, Khar RK, Ali A, Sharma A, Chander P. Development and validation of a stability-indicating RP-UPLC method for the quantitative analysis of sparfloxacin. J Chromatogr Sci 2010;48(1):1-6.
[Crossref] [Google Scholar] [PubMed]
- Razzaq SN, Mariam I, Khan IU, Ashfaq M. Development and validation of liquid chromatographic method for gatifloxacin and ketorolac tromethamine in combined dosage form. J Liq Chromatogr Related Technol 2012;35(5):651-61.
- Megoulas NC, Koupparis MA. Development and validation of a novel LC/ELSD method for the quantitation of gentamicin sulfate components in pharmaceuticals. J Pharm Biomed Anal 2004;36(1):73-9.
[Crossref] [Google Scholar] [PubMed]
- Aljuffali IA, Kalam MA, Sultana Y, Imran A, Alshamsan A. Development and validation of stability-indicating high performance liquid chromatography method to analyze gatifloxacin in bulk drug and pharmaceutical preparations. Saudi Pharm J 2015;23(1):85-94.
[Crossref] [Google Scholar] [PubMed]
- Megoulas NC, Koupparis MA. Development and validation of a novel HPLC/ELSD method for the direct determination of tobramycin in pharmaceuticals, plasma, and urine. Anal Bioanal Chem 2005;382(2):290-6.
[Crossref] [Google Scholar] [PubMed]
- Shaikh S, Thusleem OA, Muneera MS, Akmal J, Kondaguli AV, Ruckmani K. A simple and rapid high-performance liquid chromatographic method for the determination of bisoprolol fumarate and hydrochlorothiazide in a tablet dosage form. J Pharm Biomed Anal 2008;48(3):1055-7.
[Crossref] [Google Scholar] [PubMed]
- Patel SK, Smith AA, AmuthaLakshmi S, Gandhi VN, Manavalan R. Analytical method development and validation of Ofloxacin eye drop by HPLC. J Curr Chem Pharm Sci 2011;1(1):59-64.
- Setiawati H, Harmita H, Suryadi H. Development and validation method for simultaneous quantification of neomycin and polymyxin B by HPLC-ELSD and comparison with microbiological method. J Appl Pharm Sci 2020;10(4):129-34.
- Khalil SA, Shah AH, Al-Shareef AH. A reverse phase HPLC method for the determination of chloramphenicol and its hydrolytic product in ophthalmic solutions. Anal Lett 1993;26(6):1163-79.
- Muralidharan S, Han LB, Ming Y, Lau J, Sailishni K, Arumugam S. Simple and accurate estimation of ketotifen fumarate by RP-HPLC. Int J Pharm Chem Biol Sci 2012;2(3):392-6.
- Erk N. Rapid and sensitive HPLC method for the simultaneous determination of dorzolamide hydrochloride and timolol maleate in eye drops with diode-array and UV detection. Pharmazie 2003;58(7):491-3.
[Google Scholar] [PubMed]
- Ruckmani K, Shaikh SZ, Khalil P, Muneera MS. A simple and rapid high-performance liquid chromatographic method for determining tobramycin in pharmaceutical formulations by direct UV detection. Pharm Methods 2011;2(2):117-23.
[Google Scholar] [PubMed]
- Khan A, Iqbal Z, Khan JA, Khan MI, Khan GS, Bilal M, et al. The Development and Validation of HPLC-UV method for Analysis of Ciprofloxacin in serum and aqueous Humour. Arch Pharm Prac 2011;2(3):116.
- Dewani AP, Barik BB, Kanungo SK, Wattyani BR, Chandewar AV. Development and validation of RP-HPLC method for the determination of moxifloxacin in presence of its degradation products. American-Eurasian J Sci Res 2011;6(4):192-200.
- Chaudhari JI, Chhabra G. Development and validation of stability indicating RP-HPLC method for natamycin in bulk and ophthalmic dosage forms. Asian J Pharm Clin Res 2014;7(3):54-9.
- Costa MC, Barden AT, Andrade JM, Oppe TP, Schapoval EE. Quantitative evaluation of besifloxacin ophthalmic suspension by HPLC, application to bioassay method and cytotoxicity studies. Talanta 2014;119:367-74.
[Crossref] [Google Scholar] [PubMed]