- *Corresponding Author:
- Li Zheng
Department Of Obstetrics and Gynecology, Physical Examination Center, Chongqing Hospital of Traditional Chinese Medicine, Jiangbei, Chongqing 400000, China
E-mail: 15086929311@163.com
| This article was originally published in a special issue, “Clinical Advancements in Life Sciences and Pharmaceutical Research” |
| Indian J Pharm Sci 2024:86(5) Spl Issue “175-182” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To conduct a meta-analysis and to explore the therapeutic effect of Kuntai capsules on patients with polycystic ovary syndrome. The search for relevant studies involved utilizing the Kuntai Jiaonang and Duonang Luanchao Zonghezheng terms in the China National Knowledge Infrastructure and Wanfang databases, and the Kuntai Capsule and polycystic ovary syndrome terms in the Embase, Web of science, PubMed and Cochrane library databases. Utilizing Cochrane scores, two evaluators assessed the quality of the article. The included research articles were analyzed to gather relevant data, and the effectiveness of Kuntai capsules was assessed through a meta-analysis using RevMan 5.4. Following the literature search, selection process and quality evaluation, 16 publications met the criteria for inclusion in the meta-analysis. The meta-analysis findings indicated a notable elevation in the pregnancy rate, ovulation rate and total effective rate among polycystic ovary syndrome patients treated with Kuntai capsules, as opposed to the control group. In relation to luteinizing hormone, estradiol, follicle-stimulating hormone, and T levels, the patients in the observation group exhibited substantially decreased values than those observed in the control group. Indicated by this systematic review and meta-analysis, Kuntai capsules have been found to possess substantial advantages in treating polycystic ovary syndrome patients, establishing their potential as a viable treatment option that can be promoted.
Keywords
Carfilzomib, polycystic ovary syndrome, long-term efficacy, meta-analysis
As the pioneer traditional Chinese medicine endorsed for addressing ovarian dysfunction[1], Kuntai capsules offer notable benefits for enhancing ovarian function in individuals diagnosed with Polycystic Ovary Syndrome (PCOS). Their effectiveness is particularly evident in elevating ovulation and pregnancy rates, promoting the development of mature follicles, thickening the endometrial lining and improving cervical mucus scores[2]. By leveraging traditional Chinese medicine principles, Kuntai capsules effectively enhance endometrial blood flow and regulate the secretion of ovarian and uterine hormones. They achieve this by opening cell veins, promoting blood circulation, eliminating stasis, nourishing blood, and unblocking collateral vessels. Letrozole (LE) is a commonly used medication for PCOS, and its pharmacological action involves inhibiting aromatase to reduce estrogen production. By inhibiting estrogen synthesis in premenopausal women, the levels of gonadotropin hormones (such as Follicle-Stimulating Hormone (FSH) and Luteinizing Hormone (LH)) are elevated. This in turn, stimulates the growth of follicles and triggers the process of ovulation[3-5]. Nevertheless, it has been observed through numerous clinical studies that the declining estrogen levels in the late stage of follicular development can cause a decrease in the effectiveness of LE. This decline is primarily attributed to the short half-life of LE, leading to a potential failure in the expulsion of dominant follicles. The success rate of ovulation induced by LE has been reported to be around 70 % to 84 % in various studies, yet the pregnancy rate remains relatively low at 20 % to 27 %[6]. Extensive, evidence-based medicine verification with a large sample size is necessary to ascertain whether the Kuntai capsules in conjunction with LE can exhibit an integrated effect in the treatment of PCOS. By researching validation in evidence-based medicine, combined with expert clinical experience, consensus among experts can be reached to form guidelines for the combined use of Kuntai capsules and LE by frontline clinicians. Improving clinical efficacy and minimizing clinical risks makes this particularly significant. This article sets out to assess the clinical value of the combined approach involving Kuntai capsules and LE as an intervention for PCOS, aiming to yield novel insights into the synergistic and antagonistic effects of Kuntai capsules within clinical practice. With the objective of providing clinical recommendations, a meta-analysis was undertaken to comprehensively evaluate the therapeutic efficacy of Kuntai capsules in patients diagnosed with PCOS.
Materials and Methods
Study selection:
In accordance with the 2010 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and the A MeaSurement Tool to Assess systematic Reviews (AMSTAR) guidelines focused on methodological quality assessment in systematic reviews[7], this study performed meticulous literature searches. Embase, Web of Science, PubMed, Cochrane Library, Wanfang database, and China National Knowledge Infrastructure (CNKI) were queried extensively, encompassing publications until 30th June 2023. The literature search strategy involved using terms such as Kuntai capsules and PCOS. Subsequently, a thorough examination of the reference lists of all relevant articles was carried out to locate additional literature pertinent to the study. In accordance with the eligibility criteria, two reviewers autonomously screened and selected studies. Thereafter, trial data pertaining to pre-defined endpoints were extracted.
Inclusion and exclusion criteria:
Inclusion criteria: Being Randomized Controlled Trials (RCTs) that compared the administration of Kuntai capsules in the intervention group against no administration of Kuntai capsules or alternative non-Kuntai capsule treatments in the control group; containing the essential data required for statistical analysis or encompassing one or more of the following clinical outcomes; pregnancy rate, ovulation rate, overall effective rate, LH, FSH, Testosterone (T) and Estradiol (E2); emerging from either the same author or research center, with two or more publications available, including the most recent, larger-scale, or high-quality publications. In cases where multiple studies from the same center provided data on entirely different patient cohorts, those studies were analyzed.
Exclusion criteria: They were case reports, review articles, conference reports, letters, animal experiments, comments, or clinical trial registrations and they lacked the requisite statistical data needed for analysis.
Assessment of methodological quality:
The Cochrane risk of bias assessment tool, which consists of various items such as random sequence generation, incomplete outcome data, allocation concealment, blinding, selective reporting and other biases, was used by two assessors to evaluate the quality of RCT studies.
Statistical analysis:
Utilizing the Review Manager 5.4 (Cochrane Collaboration, Oxford, United Kingdom (UK)), a meta-analysis was performed. Continuous variables were evaluated using Weighted Mean Differences (WMD) and 95 % Confidence Intervals (CI), while dichotomous variables were assessed using Odds Ratios (OR) and corresponding 95 % CI. Heterogeneity was evaluated using I2 statistics, and heterogeneity levels were categorized as low (<25 %), moderate (25 % to 50 %), and high (>50 %). If the heterogeneity test yielded significant results (I2>50 % or p<0.05), a random-effects model was employed; otherwise, a fixed-effects model was used. Statistical significance was determined with a threshold of p<0.05.
Results and Discussion
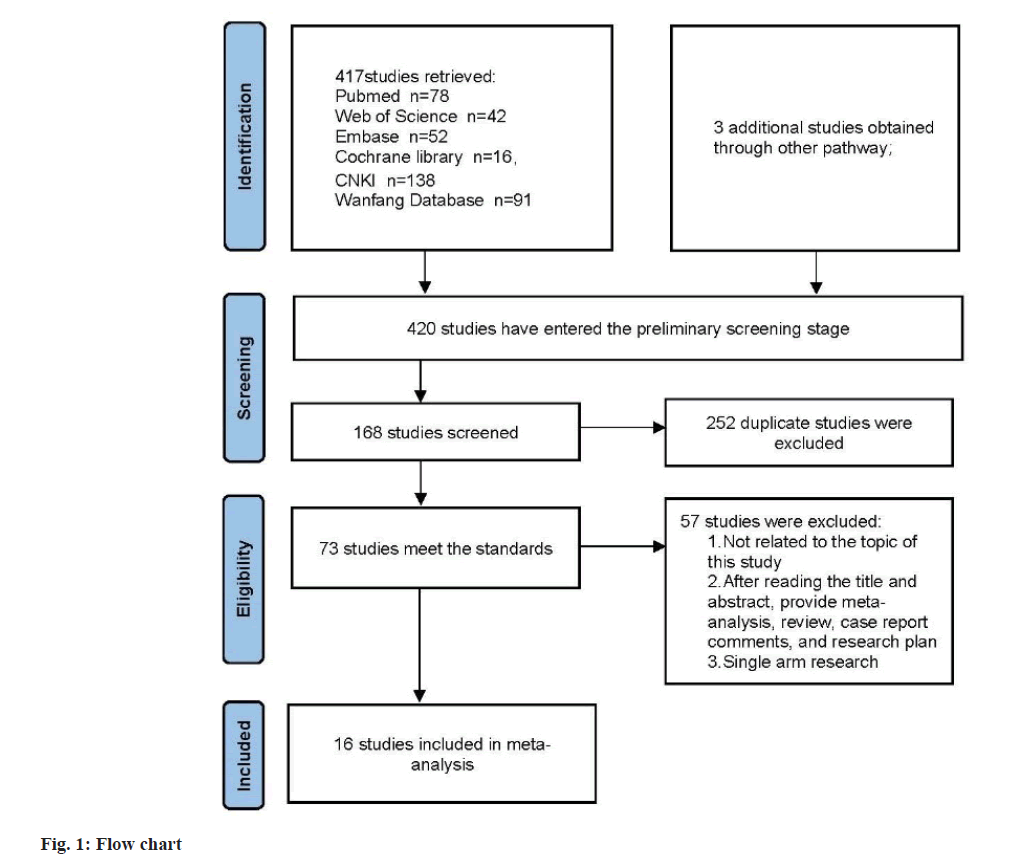
Based on the inclusion criteria flowchart (fig. 1) and quality assessment table (fig. 2), sixteen studies were selected for the meta-analysis. The general characteristics is presented in Table 1, with references ranging from[8-23]. The study comprised 1647 patients, among whom 825 PCOS patients were allocated to the observation group and treated with Kuntai capsules, while 822 patients received non- Kuntai capsule treatment and served as the control group. Notably, all of these studies were conducted within China.
| Study | Research span | Sample size | Age | BMI/Weight | |||
|---|---|---|---|---|---|---|---|
| OG | CG | OG | CG | OG | CG | ||
| Chen et al.[8] | 2016.08-2018.08 | 40 | 40 | 34.5±3.2 | 32.5±3.6 | NA | NA |
| Chen et al.[9] | 2015.02-2016.03 | 40 | 40 | 36.5±1.7 | 36.5±1.7 | NA | NA |
| Fan et al.[10] | 2016.05-2018.12 | 46 | 46 | 33.96±8.12 | 33.21±7.38 | 57.11±9.89 | 56.24±9.78 |
| Guo et al.[11] | 2017.01-2018.05 | 38 | 38 | 28.42±8.07 | 27.30±9.57 | NA | NA |
| Li et al.[12] | 2016.06-2018.9 | 50 | 50 | 34.56±4.12 | 34.75±3.51 | 23.91±2.15 | 24.03±1.93 |
| Liang et al.[13] | 2014.09-2015.11 | 50 | 50 | 24.67±3.92 | 24.72±3.18 | 23.30±2.26 | 23.28±1.84 |
| Liu et al.[14] | 2016.01-2018.01 | 46 | 46 | 28.75±2.59 | 29.17±2.66 | NA | NA |
| Wang et al.[15] | 2017.01-2018.05 | 48 | 48 | 31.04±3.21 | 31.23±3.09 | NA | NA |
| Wu et al.[16] | 2015.05-2016.05 | 85 | 85 | 26.4±3.2 | 26.3±3.1 | NA | NA |
| Yang et al.[17] | 2015.02-2017.10 | 55 | 55 | 29.59±8.12 | 29.74±7.19 | NA | NA |
| Yang et al.[18] | 2014.12-2015.11 | 35 | 35 | 36.4±2.3 | 36.2±2.1 | NA | NA |
| Yang et al.[19] | 2013.01-2014.12 | 82 | 80 | 26.44±3.67 | 26.53±3.77 | 20.75±2.22 | 20.65±2.10 |
| Yu et al.[20] | 2012.05-2017.05 | 42 | 42 | 29.42±2.34 | 29.11±2.28 | 56.21±8.90 | 56.86±8.44 |
| Zhang et al.[21] | 2018.02-2019.02 | 56 | 55 | 29.26±2.28 | 29.34±2.17 | 55.63±8.16 | 55.59±8.21 |
| Zhang et al.[22] | 2015.02-2017.01 | 52 | 52 | 28.35±3.12 | 28.18±3.88 | NA | NA |
| Zhong et al.[23] | 2015.06-2017.06 | 60 | 60 | NA | NA | NA | NA |
Note: (OG): Observation Group and (CG): Control Group
Table 1: General Characteristics of the Inclusion Study
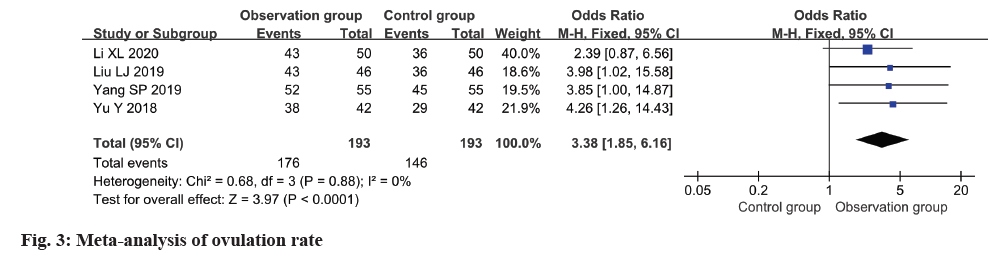
The ovulation rate was reported in four studies, with low heterogeneity observed among these studies (I2=0 %). Employing a fixed-effects model, the analysis revealed a notably higher ovulation rate in the observation group in relation to the control group (OR=3.38, 95 % CI 1.85-6.16, p<0.0001) (fig. 3).
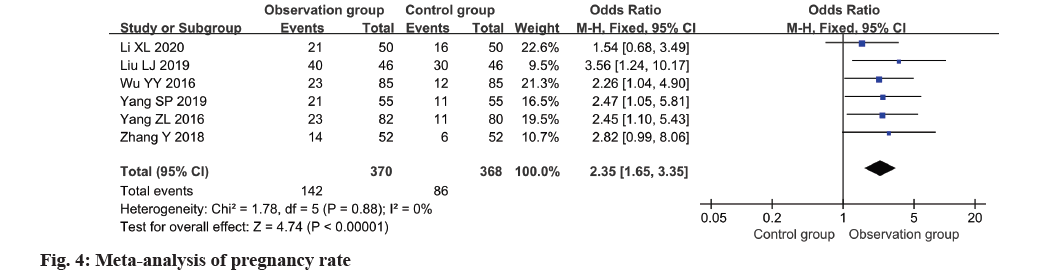
Based on a meta-analysis of six studies, fig. 4 illustrates a clear superiority of the observation group over the control group in terms of pregnancy rate (OR=2.35, 95 % CI 1.65~3.35, p<0.0001).
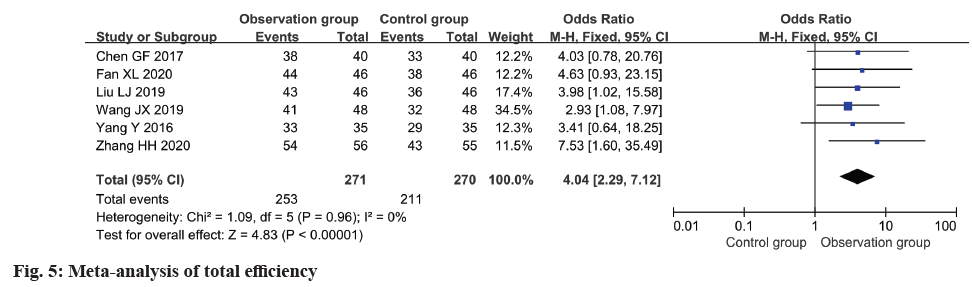
In all six included studies, the total effective rate was reported and subjected to meta-analysis. The analysis demonstrated a notable improvement in the total effective rate within the observation group as opposed to the control group (OR=4.04, 95 % CI 2.29~7.12, p<0.0001) (fig. 5).
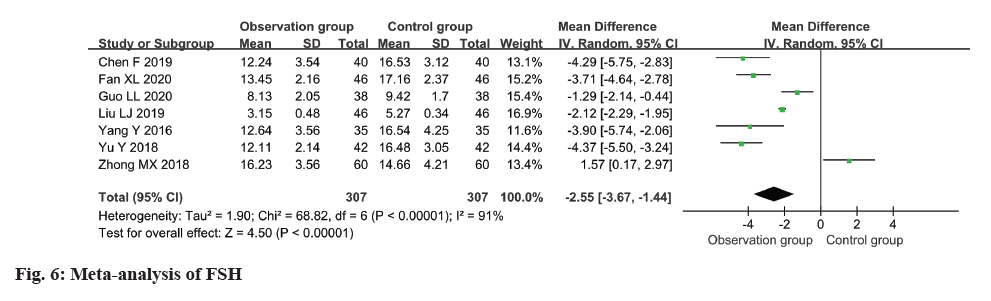
The FSH level was measured and reported in all seven studies, revealing a high degree of heterogeneity (I2: 91 %). The use of a random-effects model revealed a remarkable disparity in the FSH level between the observation and control groups, with individuals in the observation group exhibiting a considerably lower level (MD=-2.55, 95 % CI -3.67 to -1.4) (p<0.0001) (fig. 6).
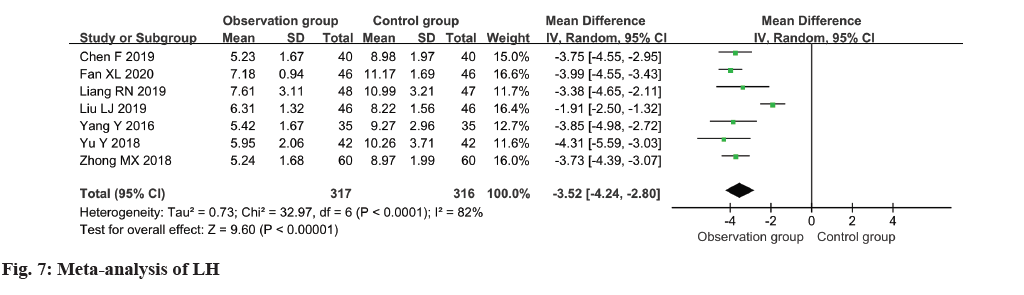
In the analysis, a collection of seven studies evaluated the LH levels among patients, revealing moderate heterogeneity (I2=82 %). The pooled results indicated a notable reduction in LH levels within the observation group in comparison to the control group (MD=-3.52, 95 % CI -4.24~-2.80, p<0.00001) (fig. 7).
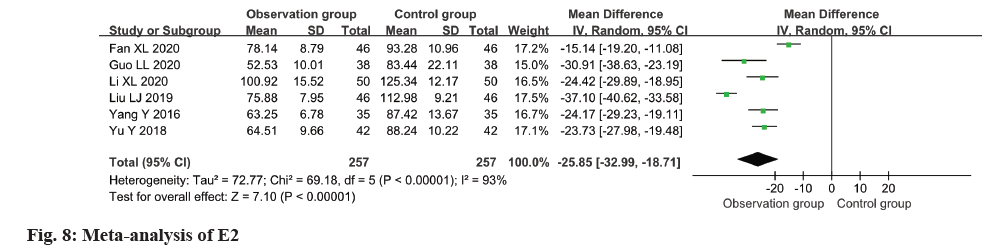
Relevant data regarding the level of E2 in patients were extracted from six studies for this metaanalysis. The findings indicated a remarkable decline in E2 levels within the observation group as opposed to the control group (MD=-25.85, 95 % CI -32.99 to -18.71, p<0.0001) (fig. 8).
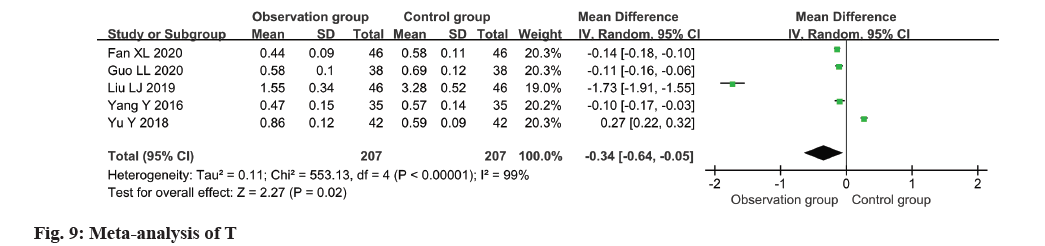
The T level of patients was assessed in all five studies, revealing substantial heterogeneity (I2=99 %). The application of a random-effects model yielded significant findings, demonstrating a decline in the T level within the observation group as opposed to the control group (MD=-0.34, 95 % CI -0.64~-0.05, p=0.02) (fig. 9).
Recent advancements in clinical research have witnessed a surge in the number of publications examining the efficacy of Kuntai capsules as a treatment for PCOS, indicative of the growing significance of Kuntai capsules within the realm of clinical research. The efficacy of Kuntai capsules in conjunction with LE in treating PCOS was systematically evaluated in a previous meta-analysis[24]. However, caution must be exercised due to potential biases resulting from the case number and study quality. This research primarily emphasizes the evaluation of the combined efficacy of these two medications in enhancing ovarian function, encompassing aspects such as number of mature follicles, pregnancy rate, ovulation rate, cervical mucus score and endometrial thickness. Nevertheless, it lacks an extensive analysis of the effects on hormone level regulation.
Characterized by impaired reproductive capacity and aberrant glucose regulation, PCOS is an endocrine disorder syndrome[25]. The dysregulation of the hypothalamic-pituitary-ovarian axis and the secretion of hypothalamic gonadotropin-releasing hormone are central to the pathophysiological manifestations of this condition. This leads to an elevation in the secretion of PRL, LH and FSH by the pituitary gland, as well as an increase in ovarian testosterone and estrogen secretion. Consequently, follicular development is hindered, resulting in follicular arrest and failure to be released from the ovary. This process leads to the occurrence of absence of menstruation and the inability to conceive. Additionally, in PCOS, the absence of cyclic progesterone secretion results in chronic stimulation of the endometrium by estrogen. This in turn, hampers proper endometrial development and creates an unfavorable environment for the implantation of an embryo.
In this study, 16 RCTs published in Chinese were involved. The findings from the meta-analysis unveiled that the addition of Kuntai capsules to the observation group led to increased rates of overall effectiveness, ovulation and pregnancy as opposed to the control group. Furthermore, a notable reduction in the levels of FSH, LH, E2 and T was observed in the observation group in relation to the control group. Kuntai capsules, as a new formulation, exert a specific impact on the improvement of the ovarian microenvironment, enhancing ovarian function, promoting ovulation, increasing pregnancy rate, and exerting a phytoestrogen-like action that can effectively regulate the levels of endogenous hormones, improve blood flow, and enhance the receptive endometrium, thus providing a higher likelihood of embryo implantation. Effectively regulating hormone levels, restoring ovulation function, and enhancing endometrial receptivity are the fundamental aspects in managing PCOS. For individuals who desire to conceive, induction of ovulation is commonly used in modern medicine. According to traditional Chinese medicine, the development of PCOS is intricately linked to blood stasis, spleen deficiency, liver stagnation, phlegm and kidney deficiency. The composition of Kuntai capsules draws inspiration from Shang Han Za Bing Lun of Zhang Zhongjing and incorporates Huang Qin[26], Sheng Di Huang, Shao Yao, Lu Jiao Jiao, and Fu Ling, six well-known Chinese herbal medicines. Sheng Di Huang helps nourish the marrow, nourish yin, and tonify the kidneys and blood. Huang Lian and Shao Yao have the function of clearing heat, relieving spasm, soothing the liver, sand eliminating dampness. A powerful synergy occurs when Ejiao and Sheng Di Huang are combined, resulting in nourishment of both yin and blood. Furthermore, the presence of Huang Qi as an auxiliary ingredient helps in clearing heat and reducing fire, while the inclusion of Fu Ling contributes to spleen invigoration and mental tranquility. Through the synergistic effect of multiple drugs, the combination therapy not only tackles the root cause and symptoms simultaneously but also nourishes Yin, eliminates heat, promotes calmness, facilitates heart-kidney communication, and balances yin and yang[27]. By combining the pure traditional Chinese medicine formulation of Kuntai capsules with LE, hormone levels can be effectively regulated, ovarian function improved, ovulation rate increased, cervical mucus characteristics optimized for sperm, cervical and endometrial thickness enhanced, conducive environment created for embryo implantation, and the fertility rate in PCOS patients elevated[28].
The objective of this research was to integrate evidence-based medicine findings with clinical expert experience in order to develop and publish guidelines for the utilization of traditional Chinese medicine in frontline clinical practice, thereby establishing an expert consensus. The impact of this study on the reduction of clinical risks and enhancement of clinical efficacy cannot be understated. Nevertheless, it is essential to take into account the limitations inherent in this study. Aiming to establish a more dependable and stringent evidence-based foundation for the concurrent use of these two medications in clinical settings, it is essential to conduct large-scale, high-quality, multicenter clinical trials. Additionally, extending the duration of follow-up is crucial in collecting more comprehensive data on pregnancy outcomes. Thus, a thorough and ongoing analysis and assessment of the joint treatment involving Kuntai capsules and LE for PCOS patients is deemed necessary.
Conflict of interests:
The authors declared no conflict of interests.
References
- Deng Y. Investigation, clinical and basic research on the treatment of polycystic ovary syndrome with integrated traditional Chinese and western medicine. Beijing Union Med Coll 2020;219.
- Zhao J, Liu F, Chen X. Study on the effect of proprietary Chinese medicine on in vitro fertilization and embryo transfer in PCOS patients. J Inner Mongolia Med Univ 2018:464.
- Pan Y. Research progress of letrozole in the treatment of infertile patients with polycystic ovary syndrome. Grass Roots Med Forum 2017;21(23):3130-1.
- Legro RS, Brzyski RG, Diamond MP, Coutifaris C, Schlaff WD, Casson P, et al. Letrozole vs. clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med 2014;371(2):119-29.
[Crossref] [Google Scholar] [PubMed]
- Franik S, Eltrop SM, Kremer JA, Kiesel L, Farquhar C. Aromatase inhibitors (letrozole) for subfertile women with polycystic ovary syndrome. Cochrane Database Syst Rev 2018;5(5):CD010287.
[Crossref] [Google Scholar] [PubMed]
- Yu Z, Fu X, Huang Qi. Clinical study on the characteristics of ovulation induced by letrozole and the effect of estradiol level on pregnancy rate. J Reproduct Med 2019;28(5):481-7.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021;29;372.
[Crossref] [Google Scholar] [PubMed]
- Chen F. Analysis of the efficacy of Kuntai capsule combined with letrozole in the treatment of infertility with polycystic ovary syndrome. China Med Eng 2019;27(4):65-7.
- Chen G. Observation on the effect of Kuntai capsule combined with letrozole on ovulation induction in patients with polycystic ovary syndrome. Med Theory Pract 2017;30(17):2590-1.
- Fan X, Xue H. Effect of letrozole combined with Kuntai capsule in patients with polycystic ovary syndrome. Clin Med Res Pract 2020;5(9):121-3.
- Guo L. Observation on the effect of Kuntai capsule and letrozole on ovulation in infertile patients with polycystic ovary syndrome. Chin School Med Offic 2020;34(5):352-400.
- Li X. Kuntai capsule combined with aromatase inhibitor letrozole OI regimen in the treatment of polycystic ovary syndrome infertility. Exp Rational Drug Use China 2020;17(2):58-62.
- Liang R, Liu Z, Li P, Fan P, Xu L, Sun X, et al. Kuntai capsules improve glucolipid metabolism in patients with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. Medicine 2019;98(39):e16788.
[Crossref] [Google Scholar] [PubMed]
- Wang JX. Clinical observation of Kuntai capsule combined with letrozole in the treatment of polycystic ovary syndrome. Clin Pract Integr Tradit Chin Western Med 2019;19(7):31.
- Wang J. Clinical observation of Kuntai capsule combined with letrozole in the treatment of polycystic ovary syndrome. Pract Tradit Chin Western Med 2019;19(7):31-3.
- Wu Y. Effect of Kuntai capsule on ovulation induced by letrozole in patients with intractable polycystic ovary syndrome. Shenzhen J Integr Tradit Chin Western Med 2016;26(15):81-2.
- Yang S, Liu Q, Wang M. Effect of Kuntai capsule combined with letrozole on ovulation induction in patients with polycystic ovary syndrome. Exp Rational Use Drugs China 2019;16(5):71-8.
- Yang Y, Huang F. Effects of Kuntai capsule combined with letrozole tablets on endocrine and ovulation function in patients with polycystic ovary syndrome. Chin Mod Doctor 2016;54(26):1-8.
- Yang Z, Zhou C. Clinical observation of Kuntai capsule and letrozole in ovulation induction in infertile patients with polycystic ovary syndrome. China Mater Child Health 2016;31(5):1010-2.
- Yu Y, Hu X, Luo G. Effect of Kuntai capsule combined with letrozole tablets on endocrine and ovulation function in patients with polycystic ovary syndrome. Med Theory Pract 2018;31(1):89-91.
- Zhang H. Analysis of the efficacy of Kuntai capsule combined with the third-generation aromatase inhibitor in the treatment of patients with polycystic ovary syndrome. Food Med Capital 2020;27(9):99.
- Zhang Y. Effect of Kuntai capsule combined with letrozole on ovulation in infertile patients with PCOS. Pract Clin Combination Tradit Chin Western Med 2018;18(4):44-6.
- Zhong M, Yan H, Chen J. Clinical observation of letrozole combined with Kuntai capsule in ovulation induction in infertile patients with PCOS. Northern Pharm 2018;15(8):125.
- Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: Etiology, pathogenesis and diagnosis. Nat Rev Endocrinol 2011;7(4):219-31.
[Crossref] [Google Scholar] [PubMed]
- Nandi A, Chen Z, Patel R, Poretsky L. Polycystic ovary syndrome. Endocrinol Metab Clin North Am 2014;43(1):123-47.
- Zhang ZJ. Theory of Febrile Diseases Beijing: Beijing People’s Medical Publishing House; 2005.
- Yu Qi. Suggestions on clinical application of Kuntai capsule. Chin J Pract Gynecol Obstetr 2019;35(10):1120-2.
- Gao H, Xu W, Li Y. Research progress of Kuntai capsule in the treatment of premature ovarian failure. Mod Med Clin 2016;31(8):1309-12.
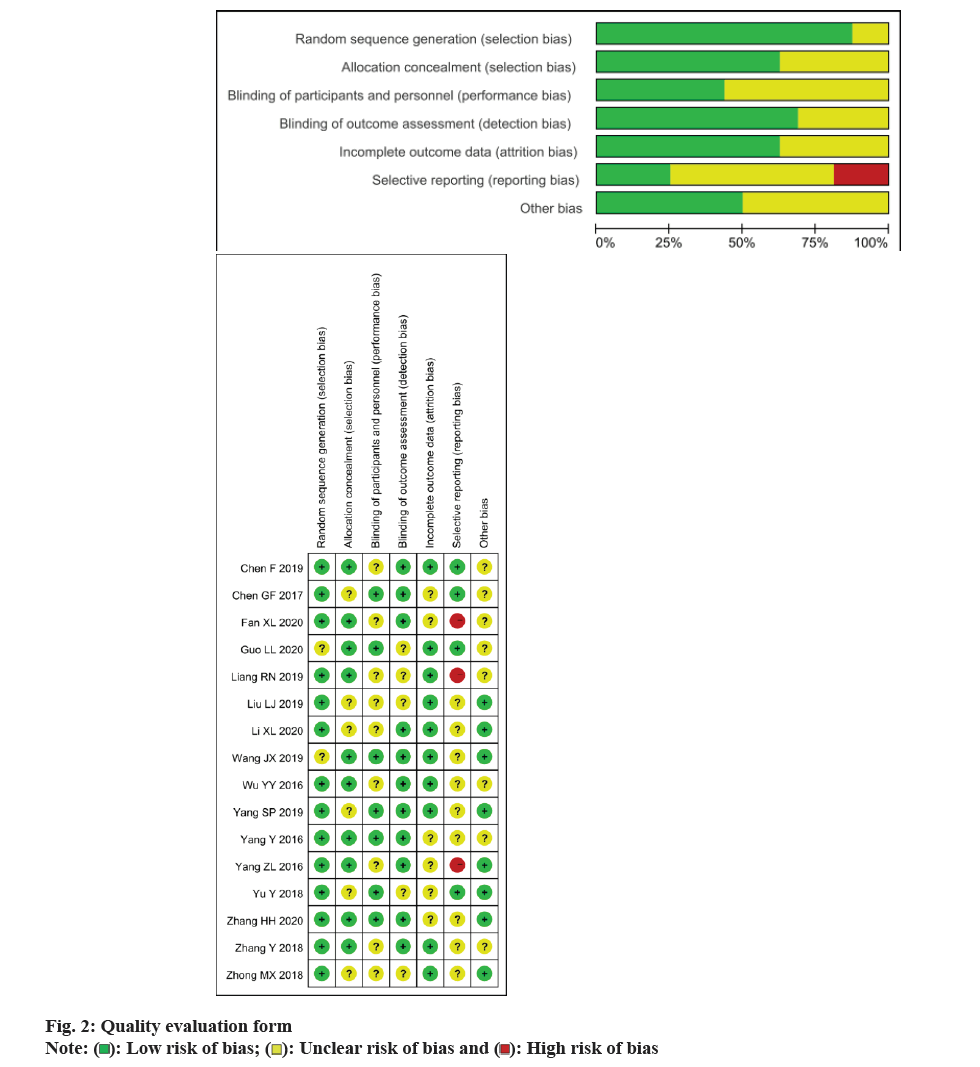
 ): Low risk of bias; (
): Low risk of bias; ( ): Unclear risk of bias and (
): Unclear risk of bias and ( ): High risk of bias
): High risk of bias