- *Corresponding Author:
- Xiyu Li
Department of Endocrinology, Queen Mary School, Medical School of Nanchang University, Nanchang, Jiangxi Province 330006, China
E-mail: 4217119188@email.ncu.edu.cn
| This article was originally published in a special issue, “Recent Progression in Pharmacological and Health Sciences” |
| Indian J Pharm Sci 2024:86(2) Spl Issue “174-182” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
By conducting a meta-analysis, this study seeks to examine the efficacy of statins on type 2 diabetes mellitus patients with cardiovascular disease. The key words atuofatating, ruishufatating, tangniaobing and xinxueguanjibing were used to search the Zhongguozhiwang and Wanfang databases, and a comprehensive search was conducted in Cochrane library databases, Embase, Web of Science, and PubMed using the keywords atorvastatin, rosuvastatin, diabetes, and cardiovascular disease. Two evaluators used Cochrane scores to evaluate the quality of articles. Collect relevant data from the included research articles and conduct meta-analysis using RevMan 5.4 software to evaluate the effectiveness of statins. After literature search, selection process and quality evaluation, 11 researches were selected in the meta-analysis. The findings revealed a statistically significant superiority in the enhancement of high-density lipoprotein, total cholesterol, apolipoprotein A1, and apolipoprotein B levels among diabetes patients with cardiovascular disease who received rosuvastatin compared to those treated with atorvastatin. The results suggested that the changes in low-density lipoproteins and triglycerides levels did not differ significantly between the two kinds of patients. This meta-analysis and systematic review confirm that rosuvastatin has significant advantages in improving high-density lipoprotein, total cholesterol, apolipoprotein A1 and apolipoprotein B in diabetes patients with cardiovascular disease, and can be used as a first-line treatment option.
Keywords
Atorvastatin, rosuvastatin, diabetes mellitus, cardiovascular disease, meta-analysis
According to several studies, it is projected that by 2025, the global prevalence of type 2 diabetes will reach approximately 380 million individuals [1]. The risk of cardiovascular disease is significantly elevated in individuals with type 2 diabetes, making it the foremost cause of death worldwide. According to current estimates, the annual worldwide mortality rate is approximately 17.9 million individuals [2,3]. By effectively reducing levels of Low-Density Lipoprotein Cholesterol (LDL-C), statins are regarded as the foundation for cardiovascular disease prevention[4]. Research indicates that statins have demonstrated the highest level of effectiveness in reducing the risk of coronary heart disease in Diabetes Mellitus (DM) patients, with a reduction of approximately 1/3rd[5]. LDL-C values are commonly used as guidelines for evaluating the risk of cardiovascular disease in individuals associated with lipoproteins[6,7]. However, numerous studies have highlighted the importance of considering non- High-Density Lipoprotein Cholesterol (HDL-C) substances, including other lipids and cholesterol, as crucial indicators. Hence, it is essential to comprehensively evaluate the control level of various indicators[8]. The efficacy of different statins is different, and atorvastatin is the most basic drug in statins. Rosuvastatin, as a new type of drug with definite efficacy, has attracted much attention. Despite efforts to compare the effectiveness of these two lipid-lowering treatments in Type 2 Diabetes Mellitus (T2DM) patients with cardiovascular disease, studies have yielded divergent findings. In light of this, a meta-analysis was performed to assess the effectiveness of statins in T2DM patients complicated by cardiovascular disease, aiming to provide clinical recommendations.
Materials and Methods
Study selection:
This study follows the 2010 PRISMA guidelines and AMSTAR guidelines (evaluating the methodological quality of systematic reviews)[9]. In order to gather a comprehensive range of studies, we performed a systematic literature search on Wanfang database, Embase, Web of Science, PubMed, Cochrane Library and Chinese National Knowledge Infrastructure (CNKI). The search words are atorvastatin, rosuvastatin, diabetes and cardiovascular disease.
In addition, we also consulted the references of all the related articles to find the relevant additional literature. It is selected independently by two evaluators according to the qualification criteria. Then the experimental data about the predefined end point are extracted.
Inclusion and exclusion criteria:
Inclusion criteria: Clinical studies of atorvastatin and rosuvastatin in managing DM complicated with cardiovascular disease; detailed information on various clinical outcomes, such as LDL cholesterol levels, Triglyceride (TG) levels, Total Cholesterol (TC) levels, HDL cholesterol levels, Apolipoprotein A1 (ApoA1) levels and Apolipoprotein B (ApoB) levels, and two or more studies reported by the same author or research center, including recently published large-scale publications or high-quality publications. In the scenario where more than two studies involve completely distinct patient groups from the same center, we still choose to analyze the data from these studies.
Exclusion criteria: Letters, reviews, conference reports, reviews, case reports and animal experimental studies, and clinical trial registration, etc., and articles that do not have the necessary information for statistical analysis.
Quality assessment of methods:
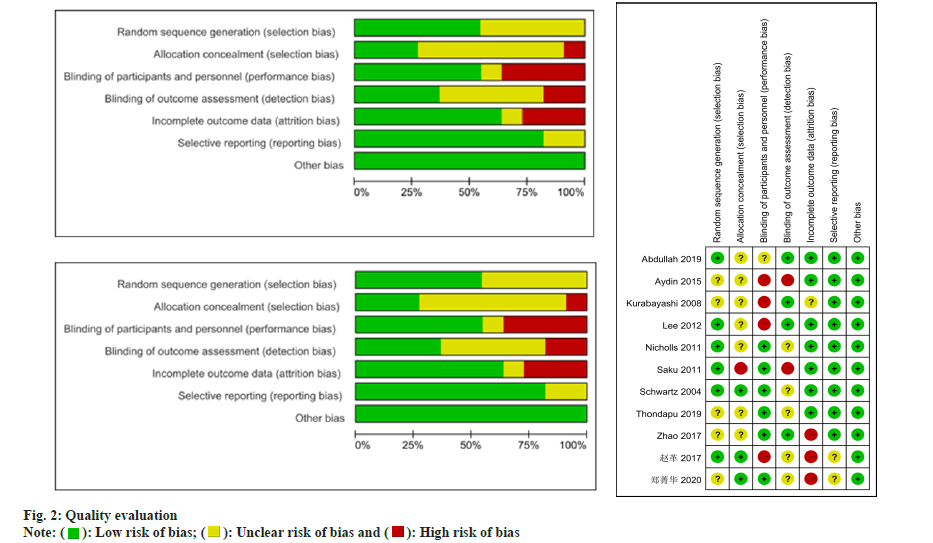
Two reviewers utilized the Cochrane bias risk assessment tool to evaluate the quality of the Randomized Controlled Trials (RCTs) study. When employing this tool, reviewers consider multiple factors, including random sequence generation, blinding methods, allocation concealment, selective publication, incomplete outcome data and other potential biases.
Data extraction:
The data extracted encompassed study characteristics (author, country, publication year, sample size, duration and study design), patient characteristics (age, gender, Body Mass Index (BMI), baseline levels of HDL, LDL, TG, TC, ApoA1 and ApoB), and the impact of medication on LDL, HDL, TG, TC, ApoA1 and ApoB levels. In cases where the study presents a median and range, the mean and Standard Deviation (SD) are estimated utilizing the approach outlined in the method proposed by Hozo et al.[10].
Statistical analysis:
The meta-analysis was conducted utilizing Review Manager 5.4 (Cochrane Collaboration, Oxford, United Kingdom). For continuous variables, the Weighted Mean Difference (WMD) with a 95 % Confidence Interval (CI) was employed, while binary variables were analyzed using the Odds Ratio (OR) with a 95 % CI. The assessment of heterogeneity involved the use of I2 statistics, with low, medium, and high heterogeneity defined as I2<25 %, 25 %≤I2≤50 %, and I2>50 %, respectively. In cases where the heterogeneity test yielded high heterogeneity (I2>50 % or p<0.05), a random effects model was adopted. Conversely, a fixed effects model was employed when the heterogeneity was low or medium. p<0.05 was considered statistically significant.
Results and Discussion
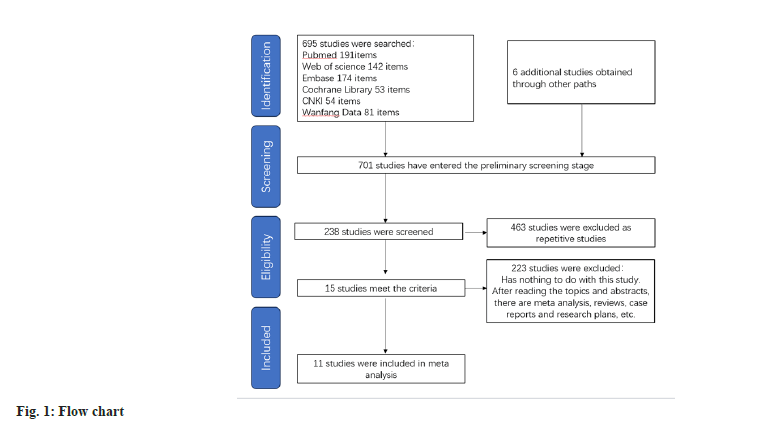
After conducting a literature search based on the predefined inclusion criteria, utilizing a selection flowchart (fig. 1) and a quality evaluation form (fig. 2), 8 researches were selected for inclusion in the study. Table 1 presents an overview of the general characteristics of the involved researches[11-20]. 2998 patients were enrolled in the meta-analysis, including 1503 DM patients with cardiovascular disease. The observation group was administered atorvastatin, while the control group comprised 1495 patients who received rosuvastatin. Three of these studies were from China, three from Japan, two from Turkey, one from the United States, one from South Korea and one from Canada.
| Authors | ||||||
|---|---|---|---|---|---|---|
| Abdullah et al. | Aydin et al. | Kurabayashi et al. | Lee | Nicholls et al. | Saku | |
Country |
Turkey | Turkey | Japan | South Korea | USA | Japan |
Research span |
2015.1-2016.12 | 2006.10-2008.5 | 2006.12-2007.10 | 2004.9-2009.6 | 2008.1-2009.6 | NA |
| Sample size | ||||||
| ATO | 33 | 59 | 207 | 143 | 519 | 99 |
| ROS | 30 | 61 | 208 | 128 | 520 | 100 |
| Age | ||||||
| ATO | 57.67±9.35 | 58±11 | 64.4±10.3 | 57.6±7.6 | 57.9±8.5 | 61.5±9.5 |
| ROS | 58.30±11.98 | 58±11 | 66.7±9.6 | 55.3±9.4 | 57.4±8.6 | 61.7±10.3 |
| Gender (male/female) | ||||||
| ATO | 29-Apr | 45/14 | 78/129 | 117/26 | 386/133 | 34/65 |
| ROS | 26-Apr | 51/10 | 95/113 | 106/22 | 379/141 | 35/65 |
| BMI | ||||||
| ATO | 26.33±2.06 | NA | NA | NA | 29.2±5.5 | 23.8±3.4 |
| ROS | 25.87±1.24 | NA | NA | NA | 28.9±5.0 | 23.9±3.7 |
| LDL-C (mg·dl-1) | ||||||
| ATO | 120.08±27.67 | 144 ± 25 | 109.3±30.6 | 110±31 | 119.9±28.9 | 162±24 |
| ROS | 131.69±24.61 | 141 ± 28 | 102.9±25.1 | 109±31 | 120.0±27.3 | 172±28 |
| HDL-C (mg·dl-1) | ||||||
| ATO | 36.33±9.76 | 38±8 | 60.1±15.3 | 40±13 | 44.7±10.7 | 56.7±13.6 |
| ROS | 37.60±10.72 | 38±9 | 60.9±17.6 | 40±9 | 45.3±11.8 | 57.1±13.4 |
| TC (mg·dl-1) | ||||||
| ATO | 181.64±35.42 | 204±31 | 192.3±34.8 | 183±36 | 193.5±34.2 | NA |
| ROS | 206.33±36.00 | 201±35 | 186.1±28.8 | 186±34 | 193.9±34.1 | NA |
| TG (mg·dl-1) | ||||||
| ATO | NA | 116±72 | 130.9±72.2 | 165±93 | 130 | 142±70 |
| ROS | NA | 109±67 | 128.5±67.4 | 182±121 | 128 | 142±77 |
| ApoA1 | ||||||
| ATO | NA | 118±23 | NA | NA | 126.2±23.3 | NA |
| ROS | NA | 118±26 | NA | NA | 128.0±25.2 | NA |
| ApoB | ||||||
| ATO | NA | 98±19 | NA | NA | 104.9±21.7 | NA |
| ROS | NA | 99±22 | NA | NA | 105.4±21.2 | NA |
| Schwartz et al. | Thondapu et al. | Zhao et al. | Zhao Ge et al. | Zheng JingHua et al. | ||
| Country | Canada | Japan | China | China | China | |
| Research span | 1999.7-2000.11 | NA | 2008.5-2009.7 | 2015.4-2017.4 | 2017.6-2019.7 | |
| Sample size | ||||||
| ATO | 128 | 19 | 136 | 116 | 44 | |
| ROS | 128 | 24 | 136 | 116 | 44 | |
| Age | ||||||
| ATO | 62±11 | 54.2 | 58.4±9.29 | 53.5±1.5 | 76.37±2.95 | |
| ROS | 62±10 | 57.5 | 59.7±10.57 | 52.2±1.3 | 76.35±2.98 | |
| Gender (male/female) | ||||||
| ATO | 71/57 | 13-Jun | 58/77 | 68/48 | 27/17 | |
| ROS | 81/47 | 14-Oct | 51/85 | 65/51 | 26/18 | |
| BMI | ||||||
| ATO | 29±5 | NA | NA | NA | NA | |
| ROS | 28±4 | NA | NA | NA | NA | |
| LDL-C (mg·dl-1) | ||||||
| ATO | 188±23 | 115±28 | 168.52±26.48 | 153.2±48.4 | 57.2±14 | |
| ROS | 186±20 | 100±21 | 169.68±27.08 | 152±47.6 | 62.4±22.8 | |
| HDL-C (mg·dl-1) | ||||||
| ATO | 47±11 | 50±12 | 49.32±10.2 | 60±30 | 79.2±14.8 | |
| ROS | 47±10 | 51±15 | 51.36±14.08 | 56.4±24.8 | 75.6±10 | |
| TC (mg·dl-1) | ||||||
| ATO | 275±27 | 203±40 | 254±32.4 | 247.2±49.2 | 262±54.8 | |
| ROS | 272±24 | 190±44 | 255.16±31.64 | 252.4±57.6 | 261.2±53.6 | |
| TG (mg·dl-1) | ||||||
| ATO | 202±77 | 183±83 | 181.37±78.9 | 212.96±94.16 | 200.64±36.96 | |
| ROS | 195±72 | 245±214 | 169.05±68.8 | 222.64±100.32 | 198±56.32 | |
| ApoA1 | ||||||
| ATO | 142±23 | NA | 144.5±24.2 | NA | NA | |
| ROS | 143±25 | NA | 146.0±23.5 | NA | NA | |
| ApoB | ||||||
| ATO | 183±22 | NA | 121.2±18.1 | NA | NA | |
| ROS | 176±20 | NA | 120.3±21.0 | NA | NA | |
Table 1: General Characteristics of the Inclusion Study
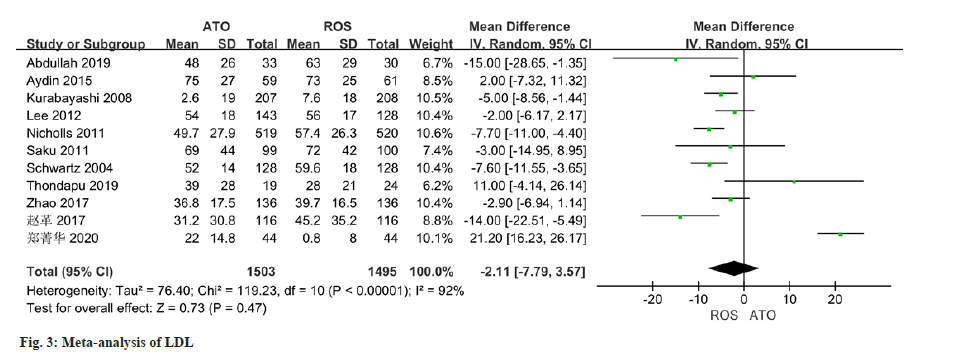
The changes of LDL were reported in all 11 studies, and there was certain heterogeneity (I2-92 %). The application of a random effect model in this study indicated that no statistically significant distinction was observed in the improvement of LDL levels between the atorvastatin-treated observation group and the control group (MD=-2.11, 95 % CI -7.79 to 3.57, p=0.47) (fig. 3).
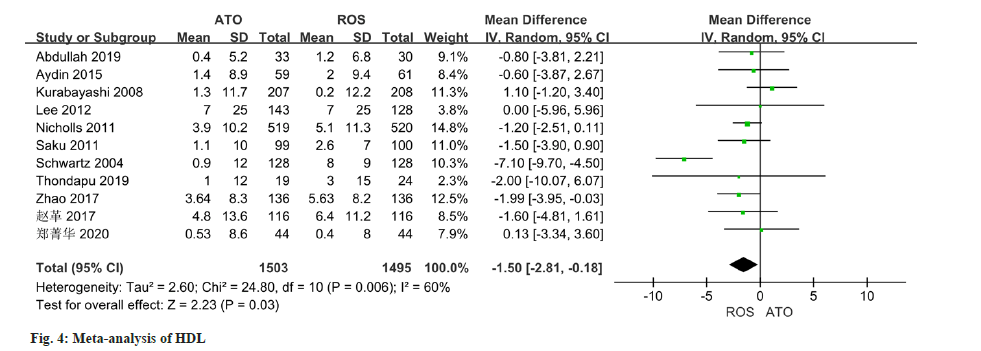
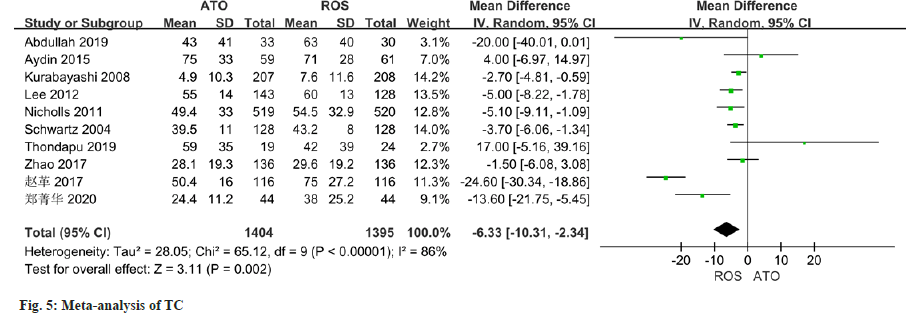
As per the meta-analysis incorporating 11 studies, the results indicated a notable disparity in the improvement of HDL levels between patients treated with atorvastatin and those treated with rosuvastatin (MD=-1.50, 95 % CI -2.81 to -0.18, p=0.03) (fig. 4). All of the 10 studies reported changes in TC, and there was some heterogeneity in the study (I2=86 %, p<0.0001). Employing a random effect model, results of the meta-analysis indicated a significant disparity in the improvement of TC levels between patients managed with atorvastatin and those with rosuvastatin (MD=-6.33, 95 % CI -10.31~-2.34, p=0.002) (fig. 5).
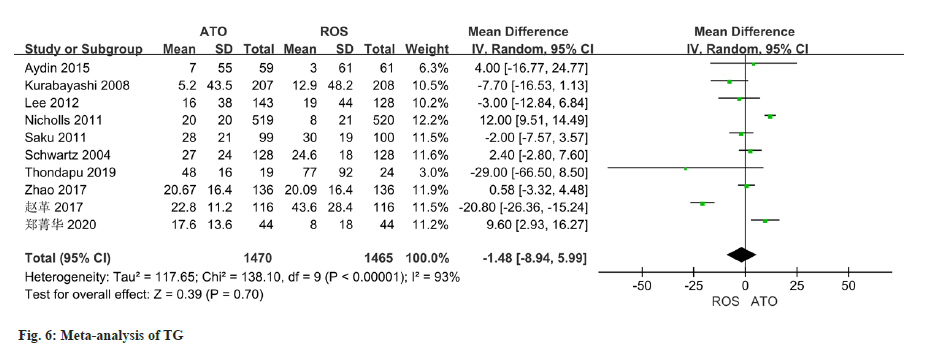
The changes of TG were reported in all 10 studies, and there was significant heterogeneity (I2=93 %). The random effect model used in the analysis demonstrated no notable variation in TG changes between the two groups (OR=-1.48, 95 % CI -8.94~5.99) (fig. 6).
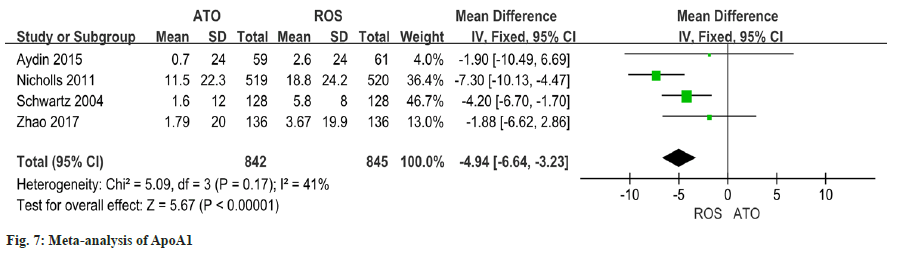
The heterogeneity of the results of the four studies on the changes of ApoA1 is good (I2=41 % quotient 0.17). Findings of this study revealed a significant discrepancy in the improvement of ApoA1 between patients who received atorvastatin and those who received rosuvastatin (OR=-4.94 95 % CI -6.64~- 3.23, p<0.00001) (fig.7).
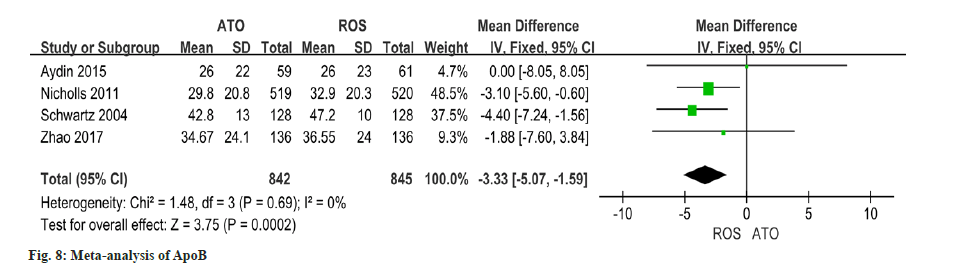
Utilizing data from four studies on changes in ApoB, this study indicated that the improvement of ApoA1 in patients receiving atorvastatin was significantly lower than that in patients receiving rosuvastatin (OR=-3.33, 95 % CI -5.07~-1.59, p=0.0002) (fig. 8).
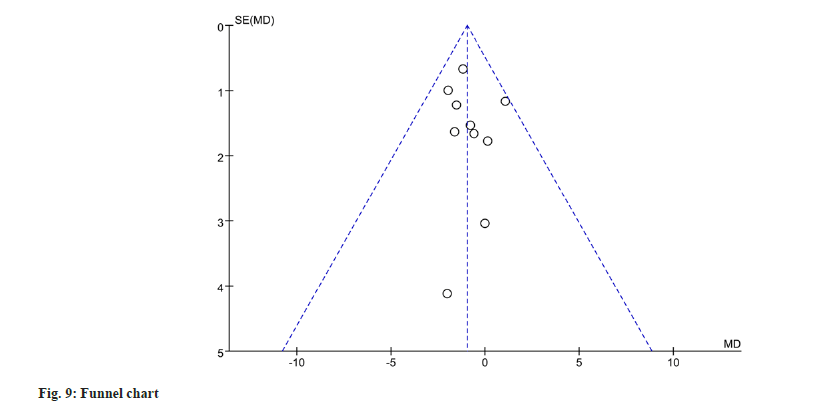
To evaluate publication bias for gastrointestinal adverse events, a funnel plot analysis was performed. The funnel plot analysis revealed no significant evidence of publication bias (fig. 9).
Diabetes as a metabolic disease, its influence on the level of metabolites is not only limited to sugars, but also has a significant effect on the deterioration of proteins and lipids in the body, often accompanied by lipid metabolic disorders, which leads to a series of cardiovascular attacks. When diabetes and cardiovascular disease occur together, the related symptoms of cardiovascular disease are often covered up. The condition of such patients is not easy to be detected in the early stage, which often leads to a poor prognosis[20]. Therefore, it is necessary to monitor, prevent and treat the lipid levels of patients with DM who have comorbid cardiovascular disease.
In this research, a meta-analysis of the different results of blood lipid changes was conducted, and the lipid changes were used as an indicator of comparative outcomes. In general, rosuvastatin and atorvastatin are traditional high-efficiency statins. It shows a considerable effect in regulating blood lipids, confirming the reasons for the extensive use of these two statins in clinical application[21]. The results of this study demonstrate a significant and favorable impact of rosuvastatin on HDL, TC, ApoA1, and ApoB levels when compared to atorvastatin. This gives us a hint that rosuvastatin is more adaptable in patients with low HDL requiring increased HDL, or in patients whose TC, ApoA1, and ApoB levels are significantly higher and need to be reduced. From the results, although no notable difference was observed in the effect of reducing LDL and TG, it does not mean that the effect of rosuvastatin and atorvastatin is poor. On the contrary, rosuvastatin and atorvastatin exhibit remarkable potential in reducing LDL-C levels and effectively regulating other blood lipids. They have gained widespread clinical acceptance and are widely prescribed for lipid management and the treatment of cardiovascular diseases due to their robust performance in these areas.
Despite the clear findings from the meta-analysis, it is important to acknowledge certain limitations that should be taken into consideration. First of all, the follow-up time varies from study to study, and this difference will have an impact on the use of lipid average change differences to report results. Secondly, the heterogeneity of the study challenges the significance of the conclusion, which makes us be cautious in interpreting the results of drug efficacy. Finally, the drug doses used in eligible studies are not uniform. An accurate interpretation of the analysis results necessitates an awareness of the limitations at hand. Future studies should aim to provide a more comprehensive assessment and comparison by conducting authoritative large-scale multicenter RCTs.
The findings of this systematic review and metaanalysis leave no doubt about the superior effectiveness of rosuvastatin in enhancing HDL, TC, ApoA1, and ApoB levels in T2DM patients with cardiovascular disease. Given these significant advantages, rosuvastatin should be regarded as a primary therapeutic choice for this specific patient group.
Conflict of interests:
The authors declared no conflict of interests.
References
- Rydén L, Grant PJ. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: The task force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J 2013;34(39):3035-87.
- Zhang X, Xing L, Jia X, Pang X, Xiang Q, Zhao X, et al. Comparative lipid-lowering/increasing efficacy of 7 statins in patients with dyslipidemia, cardiovascular diseases, or diabetes mellitus: Systematic review and network meta-analysis of 50 randomized controlled trials. Cardiovasc Ther 2020;2020:3987065.
[Crossref] [Google Scholar] [PubMed]
- Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. New Engl J Med 2013;369(14):1317-26.
[Crossref] [Google Scholar] [PubMed]
- Herrett E, Williamson E, Brack K, Beaumont D, Perkins A, Thayne A, et al. Statin treatment and muscle symptoms: Series of randomised, placebo controlled n-of-1 trials. BMJ 2021;372:n135.
[Google Scholar] [PubMed]
- Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): Multicentre randomised placebo-controlled trial. Lancet 2004;364(9435):685-96.
[Crossref] [Google Scholar] [PubMed]
- National Institute for Health and Care Excellence. CKS. Lipid modification-CVD prevention 2021.
- Cooney MT, Bruckert E, Cordero A, Corsini A, Giannuzzi P. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J 2016;37:2999-3058.
[Crossref] [Google Scholar] [PubMed]
- Grundy SM, Stone NJ, Bailey AL. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: A report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation 2019;139(25):e1082-143.
[Crossref] [Google Scholar] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg 2021;88:105906.
[Crossref] [Google Scholar] [PubMed]
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5(1):1-3.
- Tunçez A, Altuııkeser BB, Oztiirk B, Ateş MS, Tezcaıı H, Kırık EC, et al. Comparative effects of atorvastatin 80 mg and rosuvastatin 40 mg on the levels of serum endocan, chemerin, and galectin-3 in patients with acute myocardial infarction. Anatolian J Cardiol 2019;22(5):240-9.
[Crossref] [Google Scholar] [PubMed]
- Aydin MU, Aygul N, Altunkeser BB, Unlu A, Taner A. Comparative effects of high-dose atorvastatin vs. moderate-dose rosuvastatin on lipid parameters, oxidized-LDL and inflammatory markers in ST elevation myocardial infarction. Atherosclerosis 2015;239(2):439-43.
[Crossref] [Google Scholar] [PubMed]
- Lee CW, Kang SJ, Ahn JM, Song HG, Lee JY, Kim WJ, et al. Comparison of effects of atorvastatin (20 mg) vs. rosuvastatin (10 mg) therapy on mild coronary atherosclerotic plaques (from the ARTMAP trial). Am J Cardiol 2012;109(12):1700-4.
[Crossref] [Google Scholar] [PubMed]
- Nicholls SJ, Ballantyne CM, Barter PJ, Chapman MJ, Erbel RM, Libby P, et al. Effect of two intensive statin regimens on progression of coronary disease. New Engl J Med 2011;365(22):2078-87.
[Crossref] [Google Scholar] [PubMed]
- Saku K, Zhang B, Noda K. Randomized head-to-head comparison of pitavastatin, atorvastatin, and rosuvastatin for safety and efficacy (quantity and quality of LDL)–The PATROL trial. Circ J 2011;75(6):1493-505.
[Crossref] [Google Scholar] [PubMed]
- Schwartz GG, Bolognese MA, Tremblay BP, Caplan R, Hutchinson H, Raza A, et al. Efficacy and safety of rosuvastatin and atorvastatin in patients with hypercholesterolemia and a high risk of coronary heart disease: A randomized, controlled trial. Am Heart J 2004;148(1):105.
[Crossref] [Google Scholar] [PubMed]
- Thondapu V, Kurihara O, Yonetsu T, Russo M, Kim HO, Lee H, et al. Comparison of rosuvastatin vs. atorvastatin for coronary plaque stabilization. Am J Cardiol 2019;123(10):1565-71.
[Crossref] [Google Scholar] [PubMed]
- Zhao S, Peng D. Efficacy and safety of rosuvastatin vs. atorvastatin in high-risk Chinese patients with hypercholesterolemia: A randomized, double-blind, active-controlled study. Curr Med Res Opin 2018;34(2):227-35.
[Crossref] [Google Scholar] [PubMed]
- Zhao Ge. Efficacy of rosuvastatin in the treatment of unstable angina pectoris complicated with diabetes mellitus. New World Diabetes 2017;20(14):71-2.
- Zheng J. Efficacy and safety of different statins in the treatment of elderly patients with coronary heart disease complicated with type 2 diabetes mellitus. Knowledge Prev Treat Cardiovasc Dis 2020;10(19):38-40.
- Maxwell WD, Ramsey LB, Johnson SG, Moore KG, Shtutman M, Schoonover JH, et al. Impact of pharmacogenetics on efficacy and safety of statin therapy for dyslipidemia. Pharmacotherapy 2017;37(9):1172-90.
[Crossref] [Google Scholar] [PubMed]