- Corresponding Author:
- H. M. Soni
Department of Physiology, University of Tennessee Health Science Centre, Memphis, TN−38163, USA
E-mail: dranitalmcp@gmail.com
| Date of Submission | 07 September 2011 |
| Date of Revision | 18 July 2012 |
| Date of Acceptance | 20 July 2012 |
| Indian J Pharm Sci, 2012, 74 (4): 281-291 |
Abstract
The purpose of the present study was to determine the mechanism(s) involved in carbon monoxide-releasing molecule-2, carbon monoxide-releasing molecule-2-induced cardioprotection. We used the transition metal carbonyl compound carbon monoxide-releasing molecule-2 that can act as carbon monoxide donor in cardiac ischaemia-reperfusion injury model using isolated rat heart preparation. Langendorff's perfused rat hearts when treated with carbon monoxide-releasing molecule-2 (50 μM) for 10 min before global ischaemia exhibited significant reduction in postischaemic levels of myocardial injury markers, creatine kinase and lactate dehydrogenase in coronary effluent. Similarly, pretreatment with carbon monoxide-releasing molecule-2 showed significantly improved postischaemic recovery of heart rate, coronary flow rate, cardiodynamic parameters and reduced infarct size as compared to vehicle control hearts. Perfusion with p38 mitogen-activated protein kinase inhibitor, SB203580, a specific inhibitor of α and β isoform, before and concomitantly with carbon monoxide-releasing molecule-2 treatment abolished carbon monoxide-releasing molecule-2-induced cardioprotection. However, p38 mitogen-activated protein kinase alpha inhibitor, SCIO-469, was unable to inhibit the cardioprotective effect of carbon monoxide-releasing molecule-2. Furthermore, protective effect of carbon monoxide-releasing molecule-2 was significantly inhibited by the protein kinase C inhibitor, chelerythrine, when added before and concomitantly with carbon monoxide-releasing molecule-2. It was also observed that, perfusion with phosphatidylinositol 3-kinase inhibitor, wortmannin, before and concomitantly with carbon monoxide-releasing molecule-2 was not able to inhibit carbon monoxide-releasing molecule-2-induced cardioprotection. Interestingly, we observed that wortmannin perfusion before ischaemia and continued till reperfusion significantly inhibited carbon monoxide-releasing molecule-2-mediated cardioprotection. Our findings suggest that the carbon monoxide-releasing molecule-2 treatment may activate the p38 mitogen-activated protein kinase β and protein kinase C pathways before ischaemia and phosphatidylinositol 3-kinase pathway during reperfusion which may be responsible for the carbon monoxide-releasing molecule-2-mediated cardioprotective effect.
Keywords
Carbon monoxide releasing molecule−2, cardioprotection, ischaemia−reperfusion, Langendorff's heart
Carbon monoxide (CO), a diatomic gas has been recognised as a chemical asphyxiate in the blood and considered to be a toxic gas [1]. CO has recently emerged as an endogenous mediator of many physiological and pathological conditions [2]. CO is generated as an endogenous by−product during the catalytic breakdown of heme. Heme oxygenase (HO) is the rate−limiting enzyme which is responsible for heme breakdown, which in turn produces bilirubin, iron (Fe++) and CO. There are two main isoforms of HO, the inducible HO−1 and the constitutive HO−2. Recently, one more isoform has been identified in rat brains termed as HO−3 which is similar to HO−2, but less efficient heme catalyst [3]. It has been reported that selective expression of HO−1 in heart prevents ischaemia−reperfusion−induced (I/R−induced) cardiac dysfunction and apoptosis [4,5]. Exogenously applied CO showed beneficial effects in many physiological conditions which simulate the role of HO−1 [2]. Nitric oxide (NO), a molecule produced by many cells in the body, has several important cardiovascular actions which are well known since decades. NO is primarily produced by vascular endothelial cells. This endothelial−derived NO has several important functions which include relaxation of the vascular smooth muscle (vasodilation), inhibition of platelet aggregation (antithrombotic), and inhibition of leukocyte−endothelial interactions (antiinflammatory). These actions involve NO−stimulated formation of cyclic guanosine monophosphate (cGMP). Nitrodilators are drugs that mimic the actions of endogenous NO by releasing NO or forming NO within tissues. Recently, CO has been recognised as a potential therapeutic agent for many cardiovascular disorders [6]. Administration of CO in gaseous form is not considered to be a good approach as there is no control on the release pattern and concentration which may produce toxicity. Recently, CO−releasing molecules (CORMs) have been developed which provide feasibility for the delivery of CO in a controlled and predictable manner [7]. Tricarbonyldichlororuthenium (II) dimer known as carbon monoxide−releasing molecule−2 (CORM−2) is considered as CO donor and is able to release CO in a controlled manner due to its predictable release kinetics [8]. Motterlini et al. have developed a water−soluble molecule namely, tricarbonyl dichloro (glycinato) ruthenium (II) also referred to as CO−releasing molecule−3 (CORM−3) [9]. CORM−3 has been studied for its cardioprotective potential but mechanism of cardioprotection by CO donor has not been completely understood. Accumulating evidence suggests that p38 mitogen−activated protein kinase (p38 MAPK) activation is essentially involved in the cytoprotective, antiinflammatory, antiapoptotic, and antiproliferative effects of CO [10,11]. There is little information regarding the ability of HO−1 and CO to modulate the protein kinase C (PKC) and phosphatidylinositol 3−kinase (PI3K) pathways.
CO, when used in low concentrations, can exert anti−inflammatory, anti−proliferative, anti−apoptotic and anti−thrombotic activity in various models of cellular injury [12−15]. In the previous work, we demonstrated that CORM−2 (50 μM) protects the rat heart against I/R injury. We have also demonstrated that cardioprotective effect by CORM−2 may be attributed to the activation of KATP channel [16]. In the present study, we investigated the mechanism(s) of CORM−2−mediated cardioprotection using Langendorff’s perfused rat heart model and our aim was to study the role of p38 MAPK, PKC and PI3K in CORM−2−mediated cardioprotection.
Materials and Methods
Tricarbonyldichlororuthenium (II) dimmer (CORM−2), ruthenium (III) chloride hydrate (RuCl3), chelerythrine (PKC inhibitor), wortmannin (PI3K inhibitor), triphenyltetrazolium chloride (TTC) were purchased from Sigma Chemical, St. Louis, Mo., USA. SB−203580 (p38 MAPK inhibitor) was purchased from Calbiochem, La Jolla, USA. SCIO−469 (Specific p38 MAPK alpha inhibitor) was a generous gift from the Chemistry Department, Zydus Research Centre, Ahmedabad, India. Other reagents were obtained from Sigma chemicals, St Louis, MO, USA. In all experiments, 0.01−0.02% dimethyl sulfoxide (DMSO) in Krebs–Henseleit (K–H) buffer was used to ensure that effects were due to CO and not due to DMSO solvent. Also, to dissociate the effects of CO from the donor molecule, RuCl3 which has the same basic structure as CORM−2 but do not liberate CO was used as negative control and termed as inactive carbon monoxide−releasing molecule (iCORM−2).
Male wistar rats weighing 250−300 g were maintained in AAALAC−accredited, climatecontrolled facilities and allowed free access to food and water. All studies were carried out in accordance with the ‘Guide for the Care and Use of Laboratory Animals.’ The animals were housed in quiet rooms with 12:12 h light–dark cycle (07:00 am−07:00 pm). The protocol for use of animals for conducting this study has been reviewed and approved by the Institutional Animal Ethics Committee (IAEC).
Isolated perfused rat heart preparation
Langendorff’s perfused rat heart model was used for the study as described earlier [16]. Briefly, the rats were heparinised, anaesthetised and their hearts were rapidly excised, placed in ice−cold K-H buffer containing (m mol/l): NaCl 118, KCl 3.2, MgSO4 1.2, NaHCO3 25, NaH2PO4 1.2, CaCl2 1.25 and glucose 11 at pH 7.4. Isolated heart was cannulated via aorta and perfused in the Langendorff’s mode at constant perfusion pressure 70 mmHg using Radnoti Langendorff constant pressure non−recirculating system (Radnoti glass technology Inc., CA, USA). The perfusate was equilibrated with 95% O2 and 5% CO2 and maintained at a temperature of 37°C. Global ischaemia was produced for 30 min followed by 120 min reperfusion.
Experimental protocol
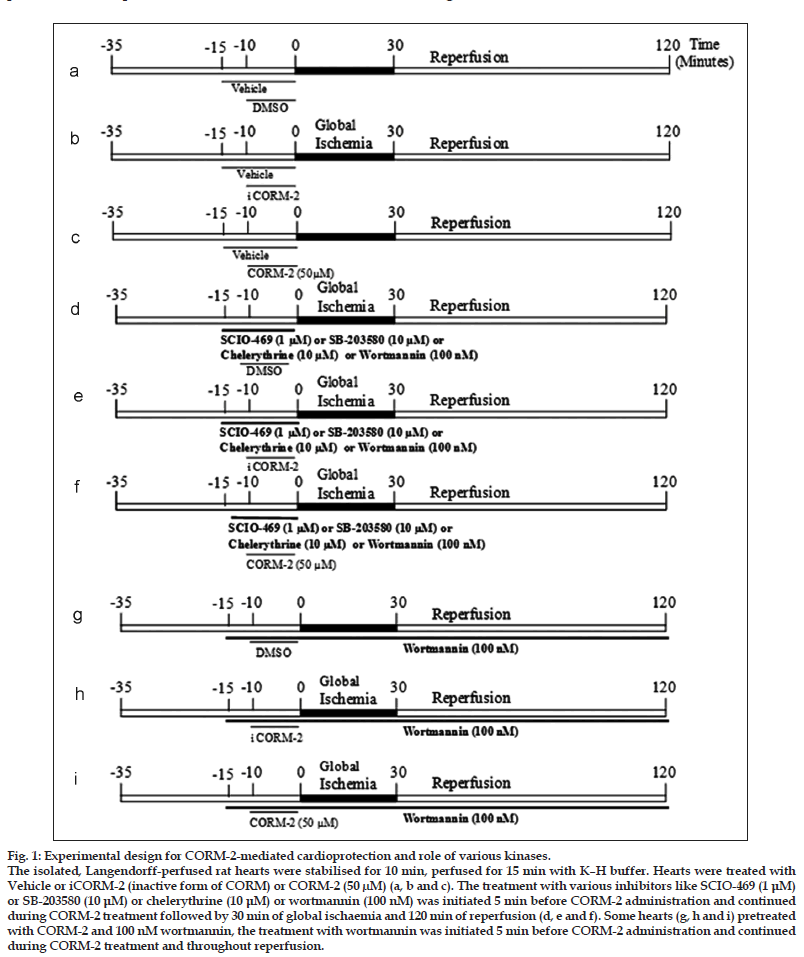
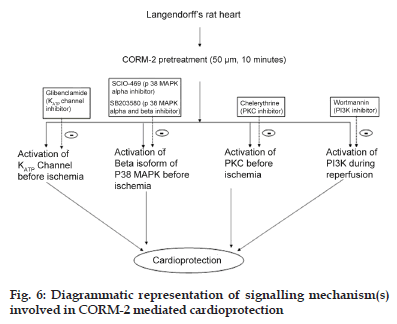
Experiment was designed to determine CORM−2− induced cardioprotection and to observe the role of (a) p38 MAPK, using SCIO−469 as p38 MAPK−alpha inhibitor and SB203580 as p38 MAPK α and β inhibitor, (b) PKC, using chelerythrine as PKC inhibitor and (c) PI3K, using wortmannin as PI3K inhibitor, in CORM−2−mediated cardioprotection. Experimental protocol is illustrated in fig. 1.
Figure 1:Experimental design for CORM-2-mediated cardioprotection and role of various kinases.
The isolated, Langendorff-perfused rat hearts were stabilised for 10 min, perfused for 15 min with K–H buffer. Hearts were treated with
Vehicle or iCORM-2 (inactive form of CORM) or CORM-2 (50 μM) (a, b and c). The treatment with various inhibitors like SCIO-469 (1 μM)
or SB-203580 (10 μM) or chelerythrine (10 μM) or wortmannin (100 nM) was initiated 5 min before CORM-2 administration and continued
during CORM-2 treatment followed by 30 min of global ischaemia and 120 min of reperfusion (d, e and f). Some hearts (g, h and i) pretreated
with CORM-2 and 100 nM wortmannin, the treatment with wortmannin was initiated 5 min before CORM-2 administration and continued
during CORM-2 treatment and throughout reperfusion.
Determination of myocardial injury markers
Levels of lactate dehydrogenase (LDH) and creatine kinase (CK) in coronary effluent were measured before global ischaemia (BGI) and immediately (within 1 min) after reperfusion by commercially available kits (Randox Laboratories Ltd, London, UK) using Rx Daytona analyser (Randox Laboratories, Boston, MA, USA).
Determination of heart rate and coronary flow
For the measurement of ECG two silver electrodes were attached to the aorta and apex of the heart and ECG tracings were recorded using an ECG machine (BPL MK801, Bangalore, India) for monitoring the heart rate (HR). Coronary flow (CF) was measured by collection of coronary effluent BGI and 120 min after reperfusion (120 Rep) in a graduated measuring cylinder.
Assessment of cardiodynamic parameters
For the determination of cardiodynamic parameters, a fluid−filled latex balloon connected to a pressure transducer (Biopac−MP 100; Biopac, Santa Barbara, CA, USA) was inserted in to the left ventricle of the isolated rat heart. Balloon was inflated to achieve left ventricular end−diastolic pressure (LVEDP) of approximately 10 mmHg. AcqKnowledge data acquisition software was used for data collection and to process the left ventricular developed pressure (LVDP), dp/dt max (indices of left ventricular contraction) and dp/dt min (indices of left ventricular relaxation).
Assessment of infarct size
The frozen heart samples were cut into 2−3 mm sections and incubated for 10 min in 1% 2,3,5−triphenyltetrazolium chloride (TTC) in phosphate buffer, pH 7.4 at 37°, followed by a fixation with 10% formal saline for 30 min. Sections were scanned using an HP scanner (HP ScanJet ADF, Colorado, USA). The normal myocardium was stained brick red whereas the infarcted portion remained unstained. Infarct size was measured by Image/J software version 1.37v and expressed as percentage of total area.
Statistical analysis
Results were expressed as mean±SEM. Data were analysed by one−way ANOVA followed by Tukey's multiple comparison tests. All analysis was done using GraphPad Prism software version 4.0. P<0.05 was considered to be statistically significant.
Results
In the present study, we evaluated the role of p38MAPK in CORM−2 mediated cardioprotection. SCIO−469 was used to inhibit p38MAPK−alpha isoform in isolated rat heart. SB203580 was also used to inhibit p38MAPK (inhibits p38 MAPK α and β). Preischaemic exposure of SCIO−469 (1 μM) concomitantly with CORM−2 produced postischaemic recovery in CK, LDH, HR, CF, LVEDP, LVDP, dp/dt max and dp/dt min (Table 1), which was similar to CORM−2 alone.
| Parameter | Time | Groups | ||||||
|---|---|---|---|---|---|---|---|---|
| point | Vehicle | Vehicle | Vehicle | SCIO-469 | SCIO-469 | SCIO-469 | ||
| control | control+iCORM-2 | control+CORM-2 | (1 µM)+vehicle | (1 µM)+iCORM-2 | (1 µM)+CORM-2 | |||
| (50 µM) | (50 µM) | |||||||
| CK (IU/L) | BGI | 17.8±2.4 | 17.5±2.9 | 15.8±2.4 | 18.5±2.0 | 17.5±2.5 | 18.5±2.5 | |
| Imm Rep | 118.3±10.6 | 110.3±6.9 | 21.5±2.5a | 82.3±6.0 | 88.2±6.8 | 22.5±3.5a | ||
| LDH (IU/L) | BGI | 31.7±4.4 | 28.8±3.5 | 34.0±8.1 | 33.3±3.6 | 34.3±3.5 | 65.6±6.1 | |
| Imm Rep | 535.3±59.7 | 557.5±51.6 | 139.5±17.5a | 426.0±41.2 | 422.3±38.9 | 125.6±16.2a | ||
| Heart rate (beats/min) | BGI | 215.8±10.5 | 210.8±11.7 | 214.2±9.8 | 214.2±8.5 | 215.0±8.0 | 210.5±8.5 | |
| 120 Rep | 110.7±5.8 | 110.8±7.7 | 169.5±8.9a | 118.5±7.5 | 120.8±7.6 | 172.3±10.2a | ||
| Coronary flow (ml/min) | BGI | 7.1±0.5 | 7.1±0.6 | 7.1±0.4 | 7.0±0.3 | 7.0±0.2 | 7.2±0.3 | |
| 120 Rep | 2.0±0.2 | 2.0±0.1 | 4.1±0.3a | 2.3±0.1 | 2.4±0.2 | 4.2±0.4a | ||
| LVEDP (% recovery) | 120 Rep | 30.3±3.1 | 30.6±3.2 | 78.5±7.0a | 40.4±2.9 | 40.8±3.0 | 80.1±7.3a | |
| LVDP (% recovery) | 120 Rep | 33.6±3.5 | 32.9±4.1 | 78.0±7.2a | 42.5±3.7 | 43.1±3.5 | 81.2±6.5a | |
| dp/dt max (% recovery) | 120 Rep | 26.8±2.7 | 26.4±3.2 | 54.3±4.3a | 33.5±3.4 | 32.6±3.1 | 55.3±4.2a | |
| dp/dt min (% recovery) | 120 Rep | 23.6±2.4 | 24.0±2.7 | 53.1±4.9a | 30.8±2.6 | 31.1±2.8 | 56.1±4.5a | |
determined before global ischaemia and 120 Rep-values determined 120 min after reperfusion. BGI-Before global ischaemia, Imm Rep-Immediately after
reperfusion, CK=Creatine kinase, LDH=Levels of lactate dehydrogenase, LVEDP=Left vetricular end-diastolic pressure, LVDP=Left ventricular developed pressure.
Table 1: Effects of pharmacological inhibitor of p38 mapk α on cardiac injury parameters in isolated rat heart
| Parameter | Time point | Groups | ||
|---|---|---|---|---|
| SB-203580 | SB-203580 | SB-203580 | ||
| (10 µM)+vehicle | (10 µM)+iCORM-2 | (10 µM)+CORM-2 (50 µM) | ||
| CK (IU/L) | BGI | 20.7±2.4 | 18.3±1.8 | 18.0±1.8 |
| Imm Rep | 103.8±6.5 | 99.5±14.2 | 81.5±7.3b | |
| LDH (IU/L) | BGI | 33.7±3.5 | 30.8±3.1 | 35.8±2.8 |
| Imm Rep | 429.5±41.6 | 428.2±45.7 | 312.0±25.8b | |
| Heart Rate (beats/min) | BGI | 209.2±8.7 | 207.5±8.0 | 210.8±7.2 |
| 120 Rep | 118.7±6.5 | 114.2±5.2 | 125.0±5.7b | |
| Coronary flow (ml/min) | BGI | 7.1±0.2 | 7.1±0.3 | 7.1±0.2 |
| 120 Rep | 2.2±0.2 | 2.3±0.2 | 2.4±0.3b | |
| LVEDP (% recovery) | 120 Rep | 32.9±3.8 | 32.8±3.6 | 35.0±3.2b |
| LVDP (% recovery) | 120 Rep | 38.1±4.2 | 36.4±3.6 | 39.5±4.1b |
| dp/dt max (% recovery) | 120 Rep | 29.2±3.7 | 29.7±3.1 | 32.0±2.8b |
| dp/dt min (% recovery) | 120 Rep | 26.5±3.0 | 27.9±3.2 | 31.8±2.9b |
Table 2:Effects of pharmacological inhibitor of p38 mapk α and β on cardiac injury parameters in isolated rat heart
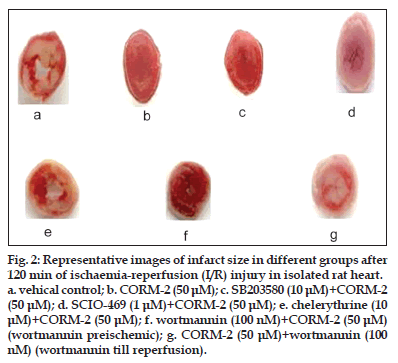
Figure 2:Representative images of infarct size in different groups after 120 min of ischaemia-reperfusion (I/R) injury in isolated rat heart.
a. vehical control; b. CORM-2 (50 μM); c. SB203580 (10 μM)+CORM-2 (50 μM); d. SCIO-469 (1 μM)+CORM-2 (50 μM); e. chelerythrine (10 μM)+CORM-2 (50 μM); f. wortmannin (100 nM)+CORM-2 (50 μM) (wortmannin preischemic); g. CORM-2 (50 μM)+wortmannin (100 nM) (wortmannin till reperfusion).
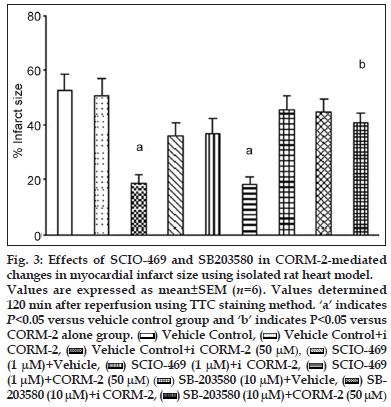
There was also significant reduction in % infarct size with SCIO−469 + CORM−2 group (18.1±3.0, P<0.05) versus vehicle treated group (52.5±5.9), which was comparable to CORM−2 alone (18.5±3.1, P<0.05) (figs. 2 and 3). Although SCIO−469 alone showed trend of postischaemic recovery in all above parameters, it did not reach the statistical significance. We have also performed an experiment to find out the role of p38MAPK by using SB203580, a p38MAPK inhibitor which inhibits both α and β isoform. Surprisingly, we found that preischaemic treatment with SB203580 + CORM−2 abolished the improvement in CK, LDH, HR, CF, cardiodynamic parameters (Table 2) and infarct size (figs. 2 and 3).
Figure 3: Effects of SCIO-469 and SB203580 in CORM-2-mediated changes in myocardial infarct size using isolated rat heart model. Values are expressed as mean±SEM (n=6). Values determined 120 min after reperfusion using TTC staining method. ‘a’ indicates P<0.05 versus vehicle control group and ‘b’ indicates P<0.05 versus CORM-2 alone group.  Vehicle Control,
Vehicle Control,  Vehicle Control+i CORM-2,
Vehicle Control+i CORM-2,  Vehicle Control+i CORM-2 (50 μM), (
Vehicle Control+i CORM-2 (50 μM), ( ) SCIO-469 (1 μM)+Vehicle,
) SCIO-469 (1 μM)+Vehicle,  SCIO-469 (1 μM)+i CORM-2,
SCIO-469 (1 μM)+i CORM-2,  SCIO-469 (1 μM)+CORM-2 (50 μM) (
SCIO-469 (1 μM)+CORM-2 (50 μM) ( ) SB-203580 (10 μM)+Vehicle,
) SB-203580 (10 μM)+Vehicle,  SB- 203580 (10 μM)+i CORM-2, (
SB- 203580 (10 μM)+i CORM-2, ( ) SB-203580 (10 μM)+CORM-2 (50 μM)
) SB-203580 (10 μM)+CORM-2 (50 μM)
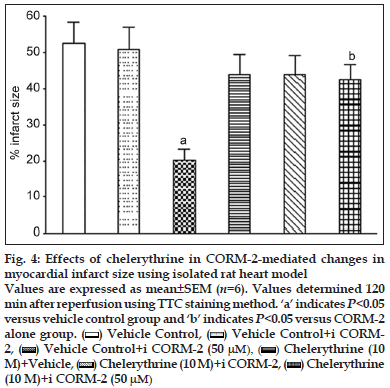
It has been found that the activation of PKC is responsible for cardioprotective activity against I/R injury. We investigated the role of PKC signalling pathway in CORM−2−induced cardioprotective effect using chelerythrine as PKC inhibitor. Our study results indicated that chelerythrine (10 μM) before and concomitantly with CORM−2 treatment significantly prevented decrease in CK (68.8±6.3 IU/l, P<0.001) and LDH levels (325.5±32.1 IU/l, P<0.05) as compared to CORM−2 alone (CK, 21.5 ± 2.2 IU/l; LDH, 139.5±17.5 IU/l) (Table 3). Similarly, there was significant prevention of postischaemic recovery in HR, CF, LVEDP, LVDP, dp/dt max and dp/dt min (Table 3). CORM−2 (50 μM) alone produced significant reduction in % infarct size (18.5±3.1, P<0.001) as compared to vehicle control (52.5±5.9). When chelerythrine was used to inhibit PKC, % infarct size was found to be 42.5±4.2 (figs. 2 and 4).
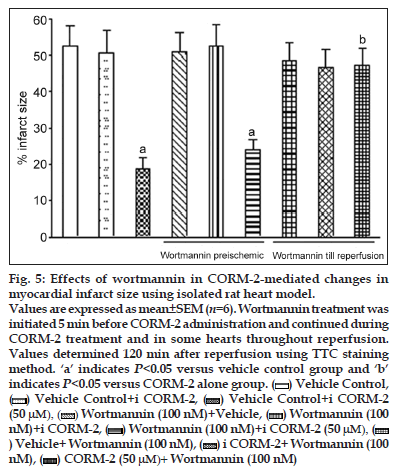
In the heart, activation of the PI3K/Akt pathway has been considered to be cardioprotective during I/R injury. Thus, we hypothesised that the cardioprotective effect of CORM−2 may be attributed to CORM−2−mediated activation of the PI3K pathway in the heart. We initially examined whether addition of PI3K inhibitor, wortmannin (100 nM), before and concomitantly with CORM−2 would block the protection afforded by CORM−2. Langendorff ’s perfused rat hearts were treated with CORM−2 either in the presence or the absence of the PI3K inhibitor, wortmannin. Treatment of the hearts with wortmannin alone showed no significant effect on the recovery of cardiac function, and wortmannin pretreatment (100 nM) also did not inhibit the cardioprotective effect of CORM−2, which was indicated by similar degree of recovery in CK, LDH, HR, CF, LVEDP, LVDP, dp/dt max and dp/dt min (Table 4). There was also a similar pattern of reduction in % infarct size in wortmannin (100 nM) + CORM−2 (50 μM) and CORM−2 treated hearts (figs. 2 and 5). However, addition of 100 nM wortmannin concurrently with CORM−2 as well as during reperfusion would inhibit CORM−2−mediated cardioprotection. Wortmannin alone showed no significant effect on the recovery of CK, LDH, HR, CF and cardiodynamic parameters. Addition of wortmannin concurrently with CORM−2 as well as during reperfusion significantly inhibited CORM−2–induced improvement in postischaemic recovery of CK, LDH, HR, CF, LVEDP, LVDP, dp/dt max and dp/dt min (Table 4). We also found that wortmannin treatment before ischaemia and during reperfusion significantly (18.5±3.1 CORM−2 alone versus 47.2±4.9 CORM−2 + wortmannin, P<0.01) inhibited CORM−2−induced reduction of % infarct size (figs. 2 and 5).
| Parameter | Time point | Groups | ||
|---|---|---|---|---|
| Chelerythrine | Chelerythrine | Chelerythrine | ||
| (10 µM)+vehicle | (10 µM)+iCORM-2 | (10 µM)+CORM-2 (50 µM) | ||
| CK (IU/L) | BGI | 20.7±2.6 | 16.7±1.9 | 20.7±3.0 |
| Imm Rep | 87.3±6.0 | 97.7±7.1 | 68.8±6.3b | |
| LDH (IU/L) | BGI | 39.0±3.4 | 37.0±6.3 | 36.0±3.5 |
| Imm Rep | 419.7±34.1 | 420.0±46.2 | 325.5±32.1b | |
| Heart Rate (beats/min) | BGI | 214.2±7.0 | 213.3±8.6 | 213.3±9.8 |
| 120 Rep | 112.0±6.1 | 112.0±7.4 | 127.5±7.8b | |
| Coronary flow (ml/min) | BGI | 7.1±0.2 | 7.3±0.1 | 7.1±0.1 |
| 120 Rep | 2.3±0.1 | 2.1±0.2 | 2.4±0.3b | |
| LVEDP (% recovery) | 120 Rep | 32.1±3.3 | 34.1±3.5 | 35.1±3.2b |
| LVDP (% recovery) | 120 Rep | 35.6±4.1 | 35.5±3.9 | 39.3±4.5b |
| dp/dt max (% recovery) | 120 Rep | 31.4±4.2 | 29.6±3.1 | 31.3±3.1b |
| dp/dt min (% recovery) | 120 Rep | 28.2±3.4 | 28.6±3.1 | 30.7±2.8b |
Table 3: Effects of pharmacological inhibitor of pkc on cardiac injury parameters in isolated rat heart
| Parameter | Time | Groups | |||||
|---|---|---|---|---|---|---|---|
| point | Wortmannin | Wortmannin | Wortmannin | Vehicle+ | iCORM-2+ | CORM-2(50 | |
| (100 nM)+ | (100 nM)+ | (100 nM)+CORM-2 | wortmannin | wortmannin | µM)+wortmannin | ||
| vehicle | iCORM-2 | (50 µM) | (100 nM) | (100 nM) | (100 nM) | ||
| (preischaemic) | (preischaemic) | (preischaemic) | (postischaemic) | (postischaemic) | (postischaemic) | ||
| CK (IU/L) | BGI | 16.3±2.2 | 20.0±2.4 | 17.7±2.8 | 16.0±1.9 | 20.3±2.7 | 16.0±2.1 |
| Imm Rep | 104.7±6.0 | 118.8±5.8 | 30.5±2.9a | 95.2±7.2 | 103.3±7.6 | 108.8±7.7b | |
| LDH (IU/L) | BGI | 37.0±5.0 | 36.2±6.5 | 33.3±5.0 | 36.2±4.2 | 37.2±5.5 | 35.8±4.9 |
| Imm Rep | 495.3±42.3 | 470.5±44.7 | 126.3±11.7a | 422.8±45.6 | 407.2±47.2 | 343.8±40.9b | |
| Coronary flow | BGI | 7.2±0.2 | 7.2±0.2 | 7.1±0.3 | 7.3±0.3 | 7.2±0.2 | 7.1±0.2 |
| (ml/min) | 120 Rep | 2.1±0.2 | 2.2±0.2 | 4.6±0.3a | 2.2±0.1 | 2.2±0.2 | 2.3±0.2b |
| LVEDP | 120 Rep | 30.1±3.3 | 30.9±3.7 | 70.9±7.5a | 32.3±3.2 | 35.4±3.6 | 36.3±3.1b |
| (% recovery) | |||||||
| LVDP | 120 Rep | 37.0±3.5 | 34.0±3.4 | 72.1±6.7a | 34.9±2.9 | 33.8±2.9 | 42.0±3.8b |
| (% recovery) | |||||||
| dp/dtmax | 120 Rep | 27.1±3.0 | 25.1±2.7 | 55.5±5.2a | 29.9±3.2 | 29.0±3.1 | 32.6±3.0b |
| (% recovery) | |||||||
| dp/dtmin | 120 Rep | 24.1±2.7 | 24.9±2.9 | 53.6±5.6a | 26.3±2.8 | 28.0±2.7 | 31.2±2.8b |
| (% recovery) | |||||||
Table 4: Effects of pharmacological inhibitor of pi3k on cardiac injury parameters in isolated rat heart.
Figure 4:Effects of chelerythrine in CORM-2-mediated changes in myocardial infarct size using isolated rat heart model.
Values are expressed as mean±SEM (n=6). Values determined 120
min after reperfusion using TTC staining method. ‘a’ indicates P<0.05
versus vehicle control group and ‘b’ indicates P<0.05 versus CORM-2
alone group. Vehicle Control, (
Vehicle Control, ( ) Vehicle Control+i CORM-
) Vehicle Control+i CORM-
2, (  ) Vehicle Control+i CORM-2 (50 μM), (
) Vehicle Control+i CORM-2 (50 μM), ( ) Chelerythrine (10
) Chelerythrine (10
M)+Vehicle, (  ) Chelerythrine (10 M)+i CORM-2, (
) Chelerythrine (10 M)+i CORM-2, ( ) Chelerythrine
) Chelerythrine
(10 M)+i CORM-2 (50 μM)
Discussion
CO is produced endogenously by HO enzyme as a product in the catabolism of heme to CO, biliverdin and iron. A connection between NO and cardiovascular diseases has been demonstrated since decades whereas CO has been emerged as cardioprotective agent in recent years. Considerable evidence supports the protective role for the HO−1/CO system against coronary artery I/R injury. Pharmacological induction of HO−1 significantly reduces infarct size and the incidence of reperfusion arrhythmias following myocardial I/R, whereas cardiac tissue damage is exacerbated by HO inhibitors [17−19]. Similarly, cardiac specific overexpression of HO−1 protects against I/R−induced cardiac dysfunction and apoptosis in isolated perfused heart preparations [4,5]. Yet et al. proposed that in vivo myocardial ischaemia and reperfusion showed infarct size of 14.7% in transgenic mice as compared to 56.5% in wild−type mice [5]. In addition, isolated hearts from heterozygote HO−1 knockout mice demonstrated an increased susceptibility to I/R injury as compared to wild type controls [20].
Figure 5:Effects of wortmannin in CORM-2-mediated changes in myocardial infarct size using isolated rat heart model.
Values are expressed as mean±SEM (n=6). Wortmannin treatment was initiated 5 min before CORM-2 administration and continued during CORM-2 treatment and in some hearts throughout reperfusion. Values determined 120 min after reperfusion using TTC staining method. ‘a’ indicates P<0.05 versus vehicle control group and ‘b’ indicates P<0.05 versus CORM-2 alone group.  Vehicle Control,
Vehicle Control,  Vehicle Control+i CORM-2,
Vehicle Control+i CORM-2,  Vehicle Control+i CORM-2 (50 μM),
Vehicle Control+i CORM-2 (50 μM),  Wortmannin (100 nM)+Vehicle, (
Wortmannin (100 nM)+Vehicle, ( ) Wortmannin (100 nM)+i CORM-2,
) Wortmannin (100 nM)+i CORM-2,  Wortmannin (100 nM)+i CORM-2 (50 μM),
Wortmannin (100 nM)+i CORM-2 (50 μM),  ) Vehicle+ Wortmannin (100 nM),
) Vehicle+ Wortmannin (100 nM), ) i CORM-2+ Wortmannin (100 nM),
) i CORM-2+ Wortmannin (100 nM),  CORM-2 (50 μM)+ Wortmannin (100 nM)
CORM-2 (50 μM)+ Wortmannin (100 nM)
Recent studies indicated that CO can also confer cytoprotective actions in the heart. Treatment of isolated cardiac cells or hearts with a CO donor preserves cell viability and myocardial performance against hypoxia−reoxygenation damage [9]. Similarly, the administration of a CO donor at the time of reperfusion reduced infarct size in an in vivo murine model of coronary occlusion [21]. Mice receiving a short infusion of CO are protected against MI for up to 72 h, which is equivalent to the protection afforded by ischaemic preconditioning [22]. Inhalation of CO also protects against myocardial I/R injury in rats, safeguards the heart during reperfusion after cardiopulmonary bypass in pigs and attenuates I/R injury following cardiac transplantation [6,23,24]. Tricarbonyldichlororuthenium (II) dimmer known as CORM−2, a lipid−soluble molecule, which delivers CO in a controlled manner and simulates the cytoprotective action of HO−1 derived CO in biological systems. Subsequently, tricarbonyldichloro (glycinato) ruthenium (II) (CORM−3), a water soluble form has been developed and it has demonstrated protection against cardiac I/R injury [25].
It has been emphasised that CO can influence many signalling pathways, especially p38 MAPK. To date, four isoforms of the p38 MAPK family have been identified: p38α, p38β, p38γ and p38δ [26−29]. It was found that p38α and p38β are ubiquitously expressed whereas p38γ and p38δ are differentially expressed depending on tissue type. Activation of p38α is not only dependent on stimulus but also on cell type [30]. Despite all four p38 MAPK group members displaying similar activation profiles, differences have been observed in the kinetics and level of activation of these isoforms [30,31]. Many laboratories showed that expression of HO−1 or exposure of endothelial cells (ECs) to exogenous CO enhances p38 MAPK activation by TNF−α [32]. Specific inhibition of p38 MAPK activation by SB203580 or through overexpression of a p38 MAPK−dominant negative mutant abrogates the antiapoptotic effect of HO−1. On the other hand, evidence exists for the concomitant activation of p38 MAPK and apoptosis induced by a variety of agents, such as nerve growth factor withdrawal and Fas ligation [33]. In Jurkat T cells, Fas/CD95−induced cell death is augmented by exposure to CO, and this occurs in part via inhibition in the activity of MAPK [34,35]. Thus, the role of p38 MAPK in apoptosis is cell type− and stimulus−dependent. Although p38 MAPK signalling can promote cell death in some cell lines, it may enhance survival, cell growth, and differentiation in others [31]. Kim et al. suggested a critical role for the β−isoform of p38 MAPK in mediating the effects of CO on cytoprotection and Hsp70 expression because these effects were abrogated in ECs by SB 203580, a selective inhibitor of α and β isoforms, and in p38β−null fibroblasts [10]. Further, Zhang et al. reported that CO inhibits apoptosis via p38 MAPK−STAT or Akt/PKB−STAT pathways during I/R injury [36]. Owing to the complexity of upstream signalling pathways of p38 MAPK in response to stimuli, it is possible that the intensity or duration of the kinase activation could be dependent on the specific signalling pathways that they utilize. Recently, Kohmoto et al. demonstrated that perioperative exposure of donors and recipients to CO at a low concentration can impart potent antiinflammatory and cytoprotective effects in rat lung I/R injury following extended cold preservation and transplantation [37]. The protective effects of CO seen in this study appear to be, at least in part, mediated through the activation p38 MAPK. We performed the study to assess the role of p38 MAPK in cardioprotective effect of CORM−2 using pharmacological inhibitors of p38 MAPK such as SCIO−469 (p38 MAPK alpha inhibitor) and SB203580 (α and β p38 MAPK inhibitor) in rat model of I/R injury. Pretreatment with SCIO−469 (1 μM) followed by CORM−2 (50 μM) showed similar patterns of recovery in cardiac parameters such as CK, LDH, HR, CF rate, LVEDP, LVDP, dp/dt max, dp/dt min and infarct size as observed with CORM−2 alone. Paradoxically, pretreatment with SB203580 (10 μM) followed by CORM−2 significantly prevented postischaemic recovery in myocardial injury markers CK and LDH. Addition of SB203580 (10 μM) significantly abolished the improvement in HR, CF, LVEDP, LVDP, dp/dt max and dp/dt min as compared to CORM−2 (50 μM) group. Postischaemic rat hearts treated with CORM−2 showed significantly reduced infarct size as compared to vehicle control. On the other hand, when SB203580 (10 μM) was used to inhibit p38 MAPK, infarct size was found to be significantly increased which indicates the significant prevention of cardioprotective effect of CORM−2 once p38 MAPK has been inhibited by SB203580. These results indicated that cardioprotective effect by CORM−2 may not dependent on p38 MAPK alpha activation. But when we used specific inhibitor of p38 MAPK α and β, SB203580, cardioprotection was abolished, indicating that there might be an activation of p38 MAPK β by CORM−2 treatment during I/R injury in isolated rat heart.
Activation of PKC has previously been also shown to be important in cardioprotection during ischaemia [38,39]. Relatively few studies have implied roles for protein kinases A/G/C in HO−1 transcriptional regulation. In primary rat hepatocytes and vascular smooth muscle, treatment with dibutylated cAMP and other agonists of protein kinase A (PKA) activated HO−1 transcription [40]. In contrast, HO−1 expression was induced by the general PKC inhibitor chelerythrine in human aortic ECs. Inhibitor studies have also implied a contributory role for PKCs in the activation of HO−1 by oxidised phospholipids [41]. We performed the study to find out the role of PKC in cardioprotective effect of CORM−2 using the pharmacological inhibitor of PKC chelerythrine in rat model of cardiac I/R injury. Pretreatment with chelerythrine (10 μM) followed by CORM−2 (50 μM) showed significant prevention of postischaemic recovery in myocardial injury markers CK and LDH. Pretreatment with chelerythrine (10 μM) prevented the improvement in HR, CF, LVEDP, LVDP, dp/dt max and dp/dt min as compared to CORM−2 (50 μM) group. Postischaemic rat hearts treated with chelerythrine (10 μM) followed by CORM−2 (50 μM) showed significant increase in infarct size as compared to CORM−2 alone. These results indicated that PKC catalytic inhibitor chelerythrine blocks CORM−2−mediated cardioprotection.
A limited number of studies have examined the role of the PI3K cell survival pathway in the context of HO−1 gene regulation. PI3K, a ubiquitous lipid−modifying enzyme consisting of a p85 regulatory subunit and a p110 catalytic subunit, responds to activation by diverse stimuli including growth factors, cytokines and cytotoxic agents. Inhibitors of p38 MAPK and PI3K blocked the activation of HO−1 by 15d−PGJ2 in human lymphocytes [42]. Stimulation of PI3K/Akt by nerve growth factor and haemin in dopaminergic neuronal cells enhanced HO−1 gene expression in an Nrf2−dependent fashion and provided protection against oxidative stress [43]. PI3K/Akt activation has been associated with the cardioprotective effect of ischaemic preconditioning, which protects the myocardium from injury produced by subsequent sustained ischaemia [44]. The PI3K/Akt and STAT pathways are known to play critical roles in mediating cell proliferation, differentiation and apoptosis. There is little information on the role of PI3K/Akt and STAT pathways in mediating apoptotic signals during I/R injury, and also little is known about the ability of CO to modulate these pathways. Previous data by Zhang et al. showed that CO requires p38 MAPK activation to exert an antiapoptotic effect during I/R injury in Rat pulmonary artery endothelial cell (PAEC) and reported associations amongst MAPKs, PI3K/Akt, and STATs [36]. To best of our knowledge, there are no reports available regarding the role of PI3K in CORM−2−mediated cardioprotection in heart. Thus, we hypothesised that the cardioprotective effect of CORM−2 may be associated with activation of the PI3K/Akt pathway in the heart. We, therefore, initially examined whether addition of PI3K inhibitor before and concomitantly with CORM−2 would block the protection afforded by CORM−2. Inhibition of PI3K activity by wortmannin (100 nM) both concomitantly with CORM−2 treatment (before ischaemia) and continued during reperfusion blocked CORM−2−mediated recovery of cardiac function, whereas inhibition only before ischaemia did not significantly block CORM−2−mediated recovery of cardiac functions such as CK, LDH, HR, CF, cardiodynamics (LVEDP, LVDP, dp/dt max, dp/dt min) and infarct size indicating that PI3K activity is required for CORM−2−mediated cardioprotection during reperfusion. Therefore, we tested the importance of the PI3K pathway in CORM−2−mediated recovery of cardiac function when the hearts were treated with CORM−2 at reperfusion. Based on these data, we conclude that PI3K activity during reperfusion is critical for CORM−2−mediated cardioprotection. Our finding is similar to the findings of Hausenloy et al. who showed that the cardioprotective effects of ischaemic preconditioning can be blocked by addition of the PI3K inhibitor LY294002 at the start of reperfusion [45].
In summary, we demonstrated that Langendorff’s perfused rat hearts treated with CORM−2 exhibited significant cardioprotection. In this study, we sought to further delineate the signal transduction pathways utilised by CORM−2 during I/R injury. We utilised isolated rat heart model to delineate the ability of CORM−2 to modulate various pathways during I/R injury. We demonstrate that activation of β isoform of p38 MAPK is important for CORM−2−mediated cardioprotection. Furthermore, CORM−2−mediated activation of the PKC signalling pathway is required for cardioprotective effect of CORM−2 during I/R injury. Furthermore, using chemical inhibitor of PI3K, we showed that CORM−2 enhances PI3K activation during reperfusion to prevent I/R injury in heart (fig. 6).
Acknowledgment
The auhors thank Dr. Ajay Sharma (Masson Eye Institute, Columbia, MO 65212, USA) for his valuable guidance and support. The authors also thank the management of Zydus Research Centre for all support.
References
- Chance B, Erecinska M, Wagner M. Mitochondrial responses to carbon monoxide toxicity. Ann N Y AcadSci 1970;174:193-204.
- Otterbein LE, Soares MP, Yamashita K, Bach FH. Hemeoxygenase-1: Unleashing the protective properties of heme. Trends Immunol 2003;24:449-55.
- Perrella MA, Yet SF. Role of hemeoxygenase-1 in cardiovascular function. Curr Pharm Des 2003;9:2479-87.
- Vulapalli SR, Chen Z, Chua BH, Wang T, Liang CS. Cardioselective overexpression of HO-1 prevents I/R-induced cardiac dysfunction and apoptosis. Am J Physiol Heart Circ Physiol 2002;283:H688-94.
- Yet SF, Tian R, Layne MD, Wang ZY, Maemura K, Solovyeva M, et al. Cardiac-specific expression of hemeoxygenase-1 protects against ischemia and reperfusion injury in transgenic mice. Circ Res 2001;89:168-73.
- Fujimoto H, Ohno M, Ayabe S, Kobayashi H, Ishizaka N, Kimura H, et al. Carbon monoxide protects against cardiac ischemia–reperfusioninjury in vivo via MAPK and Akt–eNOS pathways. ArteriosclerThrombVascBiol 2004;24:1848-53.
- Motterlini R, Mann BE, Johnson TR, Clark JE, Foresti R, Green CJ. Bioactivity and pharmacological actions of carbon monoxide-releasing molecules. Curr Pharm Des 2003;9:2525-39.
- Józkowicz A, Huk I, Nigisch A, Weigel G, Dietrich W, Motterlini R, et al. Hemeoxygenase and angiogenic activity of endothelialcells: Stimulation by carbon monoxide and inhibition by tin protoporphyrin-IX. Antioxid Redox Signal 2003;5:155-62.
- Clark JE, Naughton P, Shurey S, Green CJ, Johnson TR, Mann BE, et al. Cardioprotective actions by a water-soluble carbon monoxide-releasing molecule. Circ Res 2003;93:e2-8.
- Kim HP, Wang X, Nakao A, Kim SI, Murase N, Choi ME, et al. Caveolin-1 expression by means of p38beta mitogen-activated protein kinase mediates the antiproliferative effect of carbon monoxide. ProcNatlAcadSci USA 2005;102:11319-24.
- Otterbein LE, Choi AM. Hemeoxygenase: Colors of defense against cellular stress. Am J Physiol Lung Cell Mol Physiol 2000;279:L1029-37.
- Ryter SW, Choi AM. Hemeoxygenase-1/carbon monoxide: From metabolism to molecular therapy. Am J Respir Cell MolBiol 2009;41:251-60.
- Shin DY, Chung J, Joe Y, Pae HO, Chang KC, Cho GJ, et al. Pretreatment with CO-releasing molecules suppresses hepcidin expression during inflammation and endoplasmic reticulum stress through inhibition of the STAT3 and CREBH pathways. Blood 2012;119:2523-32.
- Winburn IC, Gunatunga K, McKernan RD, Walker RJ, Sammut IA, Harrison JC. Cell damage following carbon monoxide releasing molecule exposure: Implications for therapeutic applications. Basic Clin PharmacolToxicol 2012;111:31-41.
- Kramkowski K, Leszczynska A, Mogielnicki A, Chlopicki S, Fedorowicz A, Grochal E, et al. Antithrombotic properties of water-soluble carbon monoxide-releasing molecules. ArteriosclerThrombVascBiol 2012;32:2149-57.
- Soni H, Patel P, Rath AC, Jain M, Mehta AA. Cardioprotective effect with carbon monoxide releasing molecule-2 (CORM-2) in isolated perfused rat heart: Role of coronary endothelium and underlying mechanism. VasculPharmacol 2010;53:68-76.
- Clark JE, Foresti R, Sarathchandra P, Kaur H, Green CJ, Motterlini R. Hemeoxygenase-1-derived bilirubin ameliorates postischemic myocardialdysfunction. Am J Physiol Heart Circ Physiol 2000;278:H643-51.
- Hangaishi M, Ishizaka N, Aizawa T, Kurihara Y, Taguchi J, Nagai R, et al. Induction of hemeoxygenase-1 can act protectively againstcardiac ischemia/reperfusion in vivo. Biochem Biophys Res Commun 2000;279:582-8.
- Masini E, Vannacci A, Marzocca C, Pierpaoli S, Giannini L, Fantappié O, et al. Hemeoxygenase-1 and the ischemia-reperfusion injury in the rat heart. ExpBiol Med (Maywood) 2003;228:546-9.
- Yoshida T, Maulik N, Ho YS, Alam J, Das DK. H (mox-1) constitutes an adaptive response to effect antioxidant cardioprotection: A study with transgenic mice heterozygous for targeted disruption of the Hemeoxygenase-1 gene. Circulation 2001;103:1695-701.
- Guo Y, Stein AB, Wu WJ, Tan W, Zhu X, Li QH, et al. Administration of a CO-releasing molecule at the time of reperfusion reduces infarct size in vivo. Am J Physiol Heart Circ Physiol 2004;286:H1649-53.
- Stein AB, Guo Y, Tan W, Wu WJ, Zhu X, Li Q, et al. Administration of a CO-releasing molecule induces late preconditioning against myocardial infarction. J Mol Cell Cardiol 2005;38:127-34.
- Lavitrano M, Smolenski RT, Musumeci A, Maccherini M, Slominska E, Di Florio E, et al. Carbon monoxide improves cardiac energetics and safeguards the heart during reperfusion after cardiopulmonary bypass in pigs. FASEB J 2004;18:1093-5.
- Akamatsu Y, Haga M, Tyagi S, Yamashita K, Graça-Souza AV, Ollinger R, et al. Hemeoxygenase-1-derived carbon monoxide protects hearts fromtransplant associated ischemia reperfusion injury. FASEB J 2004;18:771-2.
- Motterlini R, Clark JE, Foresti R, Sarathchandra P, Mann BE, Green CJ. Carbon monoxide-releasing molecules: Characterization of biochemical and vascular activities. Circ Res 2002;90:E17-24.
- Lechner C, Zahalka MA, Giot JF, Møller NP, Ullrich A. ERK6, a mitogen-activated protein kinase involved in C2C12 myoblast differentiation. ProcNatlAcadSci USA 1996;93:4355-9.
- Li Z, Jiang Y, Ulevitch RJ, Han J. The primary structure of p38 gamma: A new member of p38 group of MAP kinases. Biochem Biophys Res Commun 1996;228:334-40.
- Jiang Y, Gram H, Zhao M, New L, Gu J, Feng L, et al. Characterization of the structure and function of the fourth member of p38 group mitogen-activated protein kinases, p38delta. J BiolChem 1997;272:30122-8.
- Kumar S, McDonnell PC, Gum RJ, Hand AT, Lee JC, Young PR. Novel homologues of CSBP/p38 MAP kinase: Activation, substrate specificity and sensitivity to inhibition by pyridinylimidazoles. Biochem Biophys Res Commun 1997;235:533-8.
- Nebreda AR, Porras A. p38 MAP kinases: Beyond the stress response. Trends BiochemSci 2000;25:257-60.
- Zarubin T, Han J. Activation and signaling of the p38 MAP kinase pathway. Cell Res 2005;15:11-8.
- Ryter SW, Morse D, Choi AM. Carbon monoxide: To boldly go where NO has gone before. Sci STKE 2004;2004:RE6.
- Kummer JL, Rao PK, Heidenreich KA. Apoptosis induced by withdrawal of trophic factors is mediated by p38 mitogen-activated protein kinase. J BiolChem 1997;272:20490-4.
- Song R, Zhou Z, Kim PK, Shapiro RA, Liu F, Ferran C, et al. Carbon monoxide promotes Fas/CD95-induced apoptosis in Jurkat cells. J BiolChem 2004;279:44327-34.
- Pae HO, Oh GS, Choi BM, Chae SC, Kim YM, Chung KR, et al. Carbon monoxide produced by hemeoxygenase-1 suppresses T cell proliferation via inhibition of IL-2 production. J Immunol 2004;172:4744-51.
- Zhang X, Shan P, Alam J, Fu XY, Lee PJ. Carbon monoxide differentially modulates STAT1 and STAT3 and inhibits apoptosis via a phosphatidylinositol 3-kinase/Akt and p38 kinase-dependent STAT3 pathway during anoxia-reoxygenation injury. J BiolChem 2005;280:8714-21.
- Kohmoto J, Nakao A, Stolz DB, Kaizu T, Tsung A, Ikeda A, et al. Carbon monoxide protects rat lung transplants fromischemia-reperfusion injury via a mechanism involving p38 MAPK pathway. Am J Transplant 2007;7:2279-90.
- Ooie T, Takahashi N, Nawata T, Arikawa M, Yamanaka K, Kajimoto M, et al. Ischemia-induced translocation of protein kinase C-ε mediates cardioprotection in the streptozotocin-induced diabetic rat. Circ J 2003;67:955-61.
- Inagaki K, Hahn HS, Dorn GW II, Mochly-Rosen D. Additive protection of the ischemic heart ex vivo by combined treatment with δ-protein kinase C inhibitor and ε-protein kinase C activator. Circulation 2003;108:869-75.
- Immenschuh S, Kietzmann T, Hinke V, Wiederhold M, Katz N, Muller-Eberhard U. The rat hemeoxygenase-1 gene is transcriptionally induced via the protein kinase Asignaling pathway in rat hepatocyte cultures. MolPharmacol 1998;53:483-91.
- Krönke G, Bochkov VN, Huber J, Gruber F, Blüml S, Fürnkranz A, et al. Oxidized phospholipids induce expression of human hemeoxygenase-1 involving activation of cAMP-responsive element-binding protein. J BiolChem 2003;278:51006-14.
- Alvarez-Maqueda M, El Bekay R, Alba G, Monteseirín J, Chacón P, Vega A, et al. 15-deoxy-delta 12,14-prostaglandin J2 induces hemeoxygenase-1 gene expression in a reactive oxygen species-dependent manner in human lymphocytes. J BiolChem 2004;279:21929-37.
- Salinas M, Diaz R, Abraham NG, Ruiz de Galarreta CM, Cuadrado A. Nerve growth factor protects against 6-hydroxydopamine-induced oxidative stress by increasing expression of hemeoxygenase-1 in a phosphatidylinositol 3-kinase-dependent manner. J BiolChem 2003;278:13898-904.
- Tong H, Imahashi K, Steenbergen C, Murphy E. Phosphorylation of glycogen synthase kinase-3 beta during preconditioning through a phosphatidylinositol-3-kinase–dependent pathway is cardioprotective. Circ Res 2002;90:377-9.
- Hausenloy DJ, Tsang A, Mocanu MM, Yellon DM. Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. Am J Physiol Heart Circ Physiol 2005;288:H971-6.
- Corresponding Author:
- H. M. Soni Department of Physiology, University of Tennessee Health Science Centre, Memphis, TN−38163, USA E-mail: dranitalmcp@gmail.com
| Date of Received | 7 September 2011 |
| Date of Revised | 18 July 2012 |
| Date of Accepted | 20 July 2012 |
| Indian J. Pharm. Sci., 2012, 74 (4): 281-291 | |
Keywords
Carbon monoxide releasing molecule−2, cardioprotection, ischaemia−reperfusion, Langendorff's heart
Carbon monoxide (CO), a diatomic gas has been recognised as a chemical asphyxiate in the blood and considered to be a toxic gas [1]. CO has recently emerged as an endogenous mediator of many physiological and pathological conditions [2]. CO is generated as an endogenous by−product during the catalytic breakdown of heme. Heme oxygenase (HO) is the rate−limiting enzyme which is responsible for heme breakdown, which in turn produces bilirubin, iron (Fe++) and CO. There are two main isoforms of HO, the inducible HO−1 and the constitutive HO−2. Recently, one more isoform has been identified in rat brains termed as HO−3 which is similar to HO−2, but less efficient heme catalyst [3]. It has been reported that selective expression of HO−1 in heart prevents ischaemia−reperfusion−induced (I/R−induced) cardiac dysfunction and apoptosis [4,5]. Exogenously applied CO showed beneficial effects in many physiological conditions which simulate the role of HO−1 [2]. Nitric oxide (NO), a molecule produced by many cells in the body, has several important cardiovascular actions which are well known since decades. NO is primarily produced by vascular endothelial cells. This endothelial−derived NO has several important functions which include relaxation of the vascular smooth muscle (vasodilation), inhibition of platelet aggregation (antithrombotic), and inhibition of leukocyte−endothelial interactions (antiinflammatory). These actions involve NO−stimulated formation of cyclic guanosine monophosphate (cGMP). Nitrodilators are drugs that mimic the actions of endogenous NO by releasing NO or forming NO within tissues. Recently, CO has been recognised as a potential therapeutic agent for many cardiovascular disorders [6]. Administration of CO in gaseous form is not considered to be a good approach as there is no control on the release pattern and concentration which may produce toxicity. Recently, CO−releasing molecules (CORMs) have been developed which provide feasibility for the delivery of CO in a controlled and predictable manner [7]. Tricarbonyldichlororuthenium (II) dimer known as carbon monoxide−releasing molecule−2 (CORM−2) is considered as CO donor and is able to release CO in a controlled manner due to its predictable release kinetics [8]. Motterlini et al. have developed a water−soluble molecule namely, tricarbonyl dichloro (glycinato) ruthenium (II) also referred to as CO−releasing molecule−3 (CORM−3) [9]. CORM−3 has been studied for its cardioprotective potential but mechanism of cardioprotection by CO donor has not been completely understood. Accumulating evidence suggests that p38 mitogen−activated protein kinase (p38 MAPK) activation is essentially involved in the cytoprotective, antiinflammatory, antiapoptotic, and antiproliferative effects of CO [10,11]. There is little information regarding the ability of HO−1 and CO to modulate the protein kinase C (PKC) and phosphatidylinositol 3−kinase (PI3K) pathways.
CO, when used in low concentrations, can exert anti−inflammatory, anti−proliferative, anti−apoptotic and anti−thrombotic activity in various models of cellular injury [12−15]. In the previous work, we demonstrated that CORM−2 (50 μM) protects the rat heart against I/R injury. We have also demonstrated that cardioprotective effect by CORM−2 may be attributed to the activation of KATP channel [16]. In the present study, we investigated the mechanism(s) of CORM−2−mediated cardioprotection using Langendorff’s perfused rat heart model and our aim was to study the role of p38 MAPK, PKC and PI3K in CORM−2−mediated cardioprotection.
Materials and Methods
Tricarbonyldichlororuthenium (II) dimmer (CORM−2), ruthenium (III) chloride hydrate (RuCl3), chelerythrine (PKC inhibitor), wortmannin (PI3K inhibitor), triphenyltetrazolium chloride (TTC) were purchased from Sigma Chemical, St. Louis, Mo., USA. SB−203580 (p38 MAPK inhibitor) was purchased from Calbiochem, La Jolla, USA. SCIO−469 (Specific p38 MAPK alpha inhibitor) was a generous gift from the Chemistry Department, Zydus Research Centre, Ahmedabad, India. Other reagents were obtained from Sigma chemicals, St Louis, MO, USA. In all experiments, 0.01−0.02% dimethyl sulfoxide (DMSO) in Krebs–Henseleit (K–H) buffer was used to ensure that effects were due to CO and not due to DMSO solvent. Also, to dissociate the effects of CO from the donor molecule, RuCl3 which has the same basic structure as CORM−2 but do not liberate CO was used as negative control and termed as inactive carbon monoxide−releasing molecule (iCORM−2).
Male wistar rats weighing 250−300 g were maintained in AAALAC−accredited, climatecontrolled facilities and allowed free access to food and water. All studies were carried out in accordance with the ‘Guide for the Care and Use of Laboratory Animals.’ The animals were housed in quiet rooms with 12:12 h light–dark cycle (07:00 am−07:00 pm). The protocol for use of animals for conducting this study has been reviewed and approved by the Institutional Animal Ethics Committee (IAEC).
Isolated perfused rat heart preparation
Langendorff’s perfused rat heart model was used for the study as described earlier [16]. Briefly, the rats were heparinised, anaesthetised and their hearts were rapidly excised, placed in ice−cold K-H buffer containing (m mol/l): NaCl 118, KCl 3.2, MgSO4 1.2, NaHCO3 25, NaH2PO4 1.2, CaCl2 1.25 and glucose 11 at pH 7.4. Isolated heart was cannulated via aorta and perfused in the Langendorff’s mode at constant perfusion pressure 70 mmHg using Radnoti Langendorff constant pressure non−recirculating system (Radnoti glass technology Inc., CA, USA). The perfusate was equilibrated with 95% O2 and 5% CO2 and maintained at a temperature of 37°C. Global ischaemia was produced for 30 min followed by 120 min reperfusion.
Experimental protocol
Experiment was designed to determine CORM−2− induced cardioprotection and to observe the role of (a) p38 MAPK, using SCIO−469 as p38 MAPK−alpha inhibitor and SB203580 as p38 MAPK α and β inhibitor, (b) PKC, using chelerythrine as PKC inhibitor and (c) PI3K, using wortmannin as PI3K inhibitor, in CORM−2−mediated cardioprotection. Experimental protocol is illustrated in fig. 1.
Figure 1:Experimental design for CORM-2-mediated cardioprotection and role of various kinases.
The isolated, Langendorff-perfused rat hearts were stabilised for 10 min, perfused for 15 min with K–H buffer. Hearts were treated with
Vehicle or iCORM-2 (inactive form of CORM) or CORM-2 (50 μM) (a, b and c). The treatment with various inhibitors like SCIO-469 (1 μM)
or SB-203580 (10 μM) or chelerythrine (10 μM) or wortmannin (100 nM) was initiated 5 min before CORM-2 administration and continued
during CORM-2 treatment followed by 30 min of global ischaemia and 120 min of reperfusion (d, e and f). Some hearts (g, h and i) pretreated
with CORM-2 and 100 nM wortmannin, the treatment with wortmannin was initiated 5 min before CORM-2 administration and continued
during CORM-2 treatment and throughout reperfusion.
Determination of myocardial injury markers
Levels of lactate dehydrogenase (LDH) and creatine kinase (CK) in coronary effluent were measured before global ischaemia (BGI) and immediately (within 1 min) after reperfusion by commercially available kits (Randox Laboratories Ltd, London, UK) using Rx Daytona analyser (Randox Laboratories, Boston, MA, USA).
Determination of heart rate and coronary flow
For the measurement of ECG two silver electrodes were attached to the aorta and apex of the heart and ECG tracings were recorded using an ECG machine (BPL MK801, Bangalore, India) for monitoring the heart rate (HR). Coronary flow (CF) was measured by collection of coronary effluent BGI and 120 min after reperfusion (120 Rep) in a graduated measuring cylinder.
Assessment of cardiodynamic parameters
For the determination of cardiodynamic parameters, a fluid−filled latex balloon connected to a pressure transducer (Biopac−MP 100; Biopac, Santa Barbara, CA, USA) was inserted in to the left ventricle of the isolated rat heart. Balloon was inflated to achieve left ventricular end−diastolic pressure (LVEDP) of approximately 10 mmHg. AcqKnowledge data acquisition software was used for data collection and to process the left ventricular developed pressure (LVDP), dp/dt max (indices of left ventricular contraction) and dp/dt min (indices of left ventricular relaxation).
Assessment of infarct size
The frozen heart samples were cut into 2−3 mm sections and incubated for 10 min in 1% 2,3,5−triphenyltetrazolium chloride (TTC) in phosphate buffer, pH 7.4 at 37°, followed by a fixation with 10% formal saline for 30 min. Sections were scanned using an HP scanner (HP ScanJet ADF, Colorado, USA). The normal myocardium was stained brick red whereas the infarcted portion remained unstained. Infarct size was measured by Image/J software version 1.37v and expressed as percentage of total area.
Statistical analysis
Results were expressed as mean±SEM. Data were analysed by one−way ANOVA followed by Tukey's multiple comparison tests. All analysis was done using GraphPad Prism software version 4.0. P<0.05 was considered to be statistically significant.
Results
In the present study, we evaluated the role of p38MAPK in CORM−2 mediated cardioprotection. SCIO−469 was used to inhibit p38MAPK−alpha isoform in isolated rat heart. SB203580 was also used to inhibit p38MAPK (inhibits p38 MAPK α and β). Preischaemic exposure of SCIO−469 (1 μM) concomitantly with CORM−2 produced postischaemic recovery in CK, LDH, HR, CF, LVEDP, LVDP, dp/dt max and dp/dt min (Table 1), which was similar to CORM−2 alone.
| Parameter | Time | Groups | ||||||
|---|---|---|---|---|---|---|---|---|
| point | Vehicle | Vehicle | Vehicle | SCIO-469 | SCIO-469 | SCIO-469 | ||
| control | control+iCORM-2 | control+CORM-2 | (1 µM)+vehicle | (1 µM)+iCORM-2 | (1 µM)+CORM-2 | |||
| (50 µM) | (50 µM) | |||||||
| CK (IU/L) | BGI | 17.8±2.4 | 17.5±2.9 | 15.8±2.4 | 18.5±2.0 | 17.5±2.5 | 18.5±2.5 | |
| Imm Rep | 118.3±10.6 | 110.3±6.9 | 21.5±2.5a | 82.3±6.0 | 88.2±6.8 | 22.5±3.5a | ||
| LDH (IU/L) | BGI | 31.7±4.4 | 28.8±3.5 | 34.0±8.1 | 33.3±3.6 | 34.3±3.5 | 65.6±6.1 | |
| Imm Rep | 535.3±59.7 | 557.5±51.6 | 139.5±17.5a | 426.0±41.2 | 422.3±38.9 | 125.6±16.2a | ||
| Heart rate (beats/min) | BGI | 215.8±10.5 | 210.8±11.7 | 214.2±9.8 | 214.2±8.5 | 215.0±8.0 | 210.5±8.5 | |
| 120 Rep | 110.7±5.8 | 110.8±7.7 | 169.5±8.9a | 118.5±7.5 | 120.8±7.6 | 172.3±10.2a | ||
| Coronary flow (ml/min) | BGI | 7.1±0.5 | 7.1±0.6 | 7.1±0.4 | 7.0±0.3 | 7.0±0.2 | 7.2±0.3 | |
| 120 Rep | 2.0±0.2 | 2.0±0.1 | 4.1±0.3a | 2.3±0.1 | 2.4±0.2 | 4.2±0.4a | ||
| LVEDP (% recovery) | 120 Rep | 30.3±3.1 | 30.6±3.2 | 78.5±7.0a | 40.4±2.9 | 40.8±3.0 | 80.1±7.3a | |
| LVDP (% recovery) | 120 Rep | 33.6±3.5 | 32.9±4.1 | 78.0±7.2a | 42.5±3.7 | 43.1±3.5 | 81.2±6.5a | |
| dp/dt max (% recovery) | 120 Rep | 26.8±2.7 | 26.4±3.2 | 54.3±4.3a | 33.5±3.4 | 32.6±3.1 | 55.3±4.2a | |
| dp/dt min (% recovery) | 120 Rep | 23.6±2.4 | 24.0±2.7 | 53.1±4.9a | 30.8±2.6 | 31.1±2.8 | 56.1±4.5a | |
determined before global ischaemia and 120 Rep-values determined 120 min after reperfusion. BGI-Before global ischaemia, Imm Rep-Immediately after
reperfusion, CK=Creatine kinase, LDH=Levels of lactate dehydrogenase, LVEDP=Left vetricular end-diastolic pressure, LVDP=Left ventricular developed pressure.
Table 1: Effects of pharmacological inhibitor of p38 mapk α on cardiac injury parameters in isolated rat heart
| Parameter | Time point | Groups | ||
|---|---|---|---|---|
| SB-203580 | SB-203580 | SB-203580 | ||
| (10 µM)+vehicle | (10 µM)+iCORM-2 | (10 µM)+CORM-2 (50 µM) | ||
| CK (IU/L) | BGI | 20.7±2.4 | 18.3±1.8 | 18.0±1.8 |
| Imm Rep | 103.8±6.5 | 99.5±14.2 | 81.5±7.3b | |
| LDH (IU/L) | BGI | 33.7±3.5 | 30.8±3.1 | 35.8±2.8 |
| Imm Rep | 429.5±41.6 | 428.2±45.7 | 312.0±25.8b | |
| Heart Rate (beats/min) | BGI | 209.2±8.7 | 207.5±8.0 | 210.8±7.2 |
| 120 Rep | 118.7±6.5 | 114.2±5.2 | 125.0±5.7b | |
| Coronary flow (ml/min) | BGI | 7.1±0.2 | 7.1±0.3 | 7.1±0.2 |
| 120 Rep | 2.2±0.2 | 2.3±0.2 | 2.4±0.3b | |
| LVEDP (% recovery) | 120 Rep | 32.9±3.8 | 32.8±3.6 | 35.0±3.2b |
| LVDP (% recovery) | 120 Rep | 38.1±4.2 | 36.4±3.6 | 39.5±4.1b |
| dp/dt max (% recovery) | 120 Rep | 29.2±3.7 | 29.7±3.1 | 32.0±2.8b |
| dp/dt min (% recovery) | 120 Rep | 26.5±3.0 | 27.9±3.2 | 31.8±2.9b |
Table 2:Effects of pharmacological inhibitor of p38 mapk α and β on cardiac injury parameters in isolated rat heart
Figure 2:Representative images of infarct size in different groups after 120 min of ischaemia-reperfusion (I/R) injury in isolated rat heart.
a. vehical control; b. CORM-2 (50 μM); c. SB203580 (10 μM)+CORM-2 (50 μM); d. SCIO-469 (1 μM)+CORM-2 (50 μM); e. chelerythrine (10 μM)+CORM-2 (50 μM); f. wortmannin (100 nM)+CORM-2 (50 μM) (wortmannin preischemic); g. CORM-2 (50 μM)+wortmannin (100 nM) (wortmannin till reperfusion).
There was also significant reduction in % infarct size with SCIO−469 + CORM−2 group (18.1±3.0, P<0.05) versus vehicle treated group (52.5±5.9), which was comparable to CORM−2 alone (18.5±3.1, P<0.05) (figs. 2 and 3). Although SCIO−469 alone showed trend of postischaemic recovery in all above parameters, it did not reach the statistical significance. We have also performed an experiment to find out the role of p38MAPK by using SB203580, a p38MAPK inhibitor which inhibits both α and β isoform. Surprisingly, we found that preischaemic treatment with SB203580 + CORM−2 abolished the improvement in CK, LDH, HR, CF, cardiodynamic parameters (Table 2) and infarct size (figs. 2 and 3).
Figure 3: Effects of SCIO-469 and SB203580 in CORM-2-mediated changes in myocardial infarct size using isolated rat heart model. Values are expressed as mean±SEM (n=6). Values determined 120 min after reperfusion using TTC staining method. ‘a’ indicates P<0.05 versus vehicle control group and ‘b’ indicates P<0.05 versus CORM-2 alone group. ![]() Vehicle Control,
Vehicle Control, ![]() Vehicle Control+i CORM-2,
Vehicle Control+i CORM-2, ![]() Vehicle Control+i CORM-2 (50 μM), (
Vehicle Control+i CORM-2 (50 μM), (![]() ) SCIO-469 (1 μM)+Vehicle,
) SCIO-469 (1 μM)+Vehicle, ![]() SCIO-469 (1 μM)+i CORM-2,
SCIO-469 (1 μM)+i CORM-2, ![]() SCIO-469 (1 μM)+CORM-2 (50 μM) (
SCIO-469 (1 μM)+CORM-2 (50 μM) (![]() ) SB-203580 (10 μM)+Vehicle,
) SB-203580 (10 μM)+Vehicle, ![]() SB- 203580 (10 μM)+i CORM-2, (
SB- 203580 (10 μM)+i CORM-2, (![]() ) SB-203580 (10 μM)+CORM-2 (50 μM)
) SB-203580 (10 μM)+CORM-2 (50 μM)
It has been found that the activation of PKC is responsible for cardioprotective activity against I/R injury. We investigated the role of PKC signalling pathway in CORM−2−induced cardioprotective effect using chelerythrine as PKC inhibitor. Our study results indicated that chelerythrine (10 μM) before and concomitantly with CORM−2 treatment significantly prevented decrease in CK (68.8±6.3 IU/l, P<0.001) and LDH levels (325.5±32.1 IU/l, P<0.05) as compared to CORM−2 alone (CK, 21.5 ± 2.2 IU/l; LDH, 139.5±17.5 IU/l) (Table 3). Similarly, there was significant prevention of postischaemic recovery in HR, CF, LVEDP, LVDP, dp/dt max and dp/dt min (Table 3). CORM−2 (50 μM) alone produced significant reduction in % infarct size (18.5±3.1, P<0.001) as compared to vehicle control (52.5±5.9). When chelerythrine was used to inhibit PKC, % infarct size was found to be 42.5±4.2 (figs. 2 and 4).
In the heart, activation of the PI3K/Akt pathway has been considered to be cardioprotective during I/R injury. Thus, we hypothesised that the cardioprotective effect of CORM−2 may be attributed to CORM−2−mediated activation of the PI3K pathway in the heart. We initially examined whether addition of PI3K inhibitor, wortmannin (100 nM), before and concomitantly with CORM−2 would block the protection afforded by CORM−2. Langendorff ’s perfused rat hearts were treated with CORM−2 either in the presence or the absence of the PI3K inhibitor, wortmannin. Treatment of the hearts with wortmannin alone showed no significant effect on the recovery of cardiac function, and wortmannin pretreatment (100 nM) also did not inhibit the cardioprotective effect of CORM−2, which was indicated by similar degree of recovery in CK, LDH, HR, CF, LVEDP, LVDP, dp/dt max and dp/dt min (Table 4). There was also a similar pattern of reduction in % infarct size in wortmannin (100 nM) + CORM−2 (50 μM) and CORM−2 treated hearts (figs. 2 and 5). However, addition of 100 nM wortmannin concurrently with CORM−2 as well as during reperfusion would inhibit CORM−2−mediated cardioprotection. Wortmannin alone showed no significant effect on the recovery of CK, LDH, HR, CF and cardiodynamic parameters. Addition of wortmannin concurrently with CORM−2 as well as during reperfusion significantly inhibited CORM−2–induced improvement in postischaemic recovery of CK, LDH, HR, CF, LVEDP, LVDP, dp/dt max and dp/dt min (Table 4). We also found that wortmannin treatment before ischaemia and during reperfusion significantly (18.5±3.1 CORM−2 alone versus 47.2±4.9 CORM−2 + wortmannin, P<0.01) inhibited CORM−2−induced reduction of % infarct size (figs. 2 and 5).
| Parameter | Time point | Groups | ||
|---|---|---|---|---|
| Chelerythrine | Chelerythrine | Chelerythrine | ||
| (10 µM)+vehicle | (10 µM)+iCORM-2 | (10 µM)+CORM-2 (50 µM) | ||
| CK (IU/L) | BGI | 20.7±2.6 | 16.7±1.9 | 20.7±3.0 |
| Imm Rep | 87.3±6.0 | 97.7±7.1 | 68.8±6.3b | |
| LDH (IU/L) | BGI | 39.0±3.4 | 37.0±6.3 | 36.0±3.5 |
| Imm Rep | 419.7±34.1 | 420.0±46.2 | 325.5±32.1b | |
| Heart Rate (beats/min) | BGI | 214.2±7.0 | 213.3±8.6 | 213.3±9.8 |
| 120 Rep | 112.0±6.1 | 112.0±7.4 | 127.5±7.8b | |
| Coronary flow (ml/min) | BGI | 7.1±0.2 | 7.3±0.1 | 7.1±0.1 |
| 120 Rep | 2.3±0.1 | 2.1±0.2 | 2.4±0.3b | |
| LVEDP (% recovery) | 120 Rep | 32.1±3.3 | 34.1±3.5 | 35.1±3.2b |
| LVDP (% recovery) | 120 Rep | 35.6±4.1 | 35.5±3.9 | 39.3±4.5b |
| dp/dt max (% recovery) | 120 Rep | 31.4±4.2 | 29.6±3.1 | 31.3±3.1b |
| dp/dt min (% recovery) | 120 Rep | 28.2±3.4 | 28.6±3.1 | 30.7±2.8b |
Table 3: Effects of pharmacological inhibitor of pkc on cardiac injury parameters in isolated rat heart
| Parameter | Time | Groups | |||||
|---|---|---|---|---|---|---|---|
| point | Wortmannin | Wortmannin | Wortmannin | Vehicle+ | iCORM-2+ | CORM-2(50 | |
| (100 nM)+ | (100 nM)+ | (100 nM)+CORM-2 | wortmannin | wortmannin | µM)+wortmannin | ||
| vehicle | iCORM-2 | (50 µM) | (100 nM) | (100 nM) | (100 nM) | ||
| (preischaemic) | (preischaemic) | (preischaemic) | (postischaemic) | (postischaemic) | (postischaemic) | ||
| CK (IU/L) | BGI | 16.3±2.2 | 20.0±2.4 | 17.7±2.8 | 16.0±1.9 | 20.3±2.7 | 16.0±2.1 |
| Imm Rep | 104.7±6.0 | 118.8±5.8 | 30.5±2.9a | 95.2±7.2 | 103.3±7.6 | 108.8±7.7b | |
| LDH (IU/L) | BGI | 37.0±5.0 | 36.2±6.5 | 33.3±5.0 | 36.2±4.2 | 37.2±5.5 | 35.8±4.9 |
| Imm Rep | 495.3±42.3 | 470.5±44.7 | 126.3±11.7a | 422.8±45.6 | 407.2±47.2 | 343.8±40.9b | |
| Coronary flow | BGI | 7.2±0.2 | 7.2±0.2 | 7.1±0.3 | 7.3±0.3 | 7.2±0.2 | 7.1±0.2 |
| (ml/min) | 120 Rep | 2.1±0.2 | 2.2±0.2 | 4.6±0.3a | 2.2±0.1 | 2.2±0.2 | 2.3±0.2b |
| LVEDP | 120 Rep | 30.1±3.3 | 30.9±3.7 | 70.9±7.5a | 32.3±3.2 | 35.4±3.6 | 36.3±3.1b |
| (% recovery) | |||||||
| LVDP | 120 Rep | 37.0±3.5 | 34.0±3.4 | 72.1±6.7a | 34.9±2.9 | 33.8±2.9 | 42.0±3.8b |
| (% recovery) | |||||||
| dp/dtmax | 120 Rep | 27.1±3.0 | 25.1±2.7 | 55.5±5.2a | 29.9±3.2 | 29.0±3.1 | 32.6±3.0b |
| (% recovery) | |||||||
| dp/dtmin | 120 Rep | 24.1±2.7 | 24.9±2.9 | 53.6±5.6a | 26.3±2.8 | 28.0±2.7 | 31.2±2.8b |
| (% recovery) | |||||||
Table 4: Effects of pharmacological inhibitor of pi3k on cardiac injury parameters in isolated rat heart.
Figure 4:Effects of chelerythrine in CORM-2-mediated changes in myocardial infarct size using isolated rat heart model.
Values are expressed as mean±SEM (n=6). Values determined 120
min after reperfusion using TTC staining method. ‘a’ indicates P<0.05
versus vehicle control group and ‘b’ indicates P<0.05 versus CORM-2
alone group.![]() Vehicle Control, (
Vehicle Control, (![]() ) Vehicle Control+i CORM-
) Vehicle Control+i CORM-
2, ( ![]() ) Vehicle Control+i CORM-2 (50 μM), (
) Vehicle Control+i CORM-2 (50 μM), (![]() ) Chelerythrine (10
) Chelerythrine (10
M)+Vehicle, ( ![]() ) Chelerythrine (10 M)+i CORM-2, (
) Chelerythrine (10 M)+i CORM-2, (![]() ) Chelerythrine
) Chelerythrine
(10 M)+i CORM-2 (50 μM)
Discussion
CO is produced endogenously by HO enzyme as a product in the catabolism of heme to CO, biliverdin and iron. A connection between NO and cardiovascular diseases has been demonstrated since decades whereas CO has been emerged as cardioprotective agent in recent years. Considerable evidence supports the protective role for the HO−1/CO system against coronary artery I/R injury. Pharmacological induction of HO−1 significantly reduces infarct size and the incidence of reperfusion arrhythmias following myocardial I/R, whereas cardiac tissue damage is exacerbated by HO inhibitors [17−19]. Similarly, cardiac specific overexpression of HO−1 protects against I/R−induced cardiac dysfunction and apoptosis in isolated perfused heart preparations [4,5]. Yet et al. proposed that in vivo myocardial ischaemia and reperfusion showed infarct size of 14.7% in transgenic mice as compared to 56.5% in wild−type mice [5]. In addition, isolated hearts from heterozygote HO−1 knockout mice demonstrated an increased susceptibility to I/R injury as compared to wild type controls [20].
Figure 5:Effects of wortmannin in CORM-2-mediated changes in myocardial infarct size using isolated rat heart model.
Values are expressed as mean±SEM (n=6). Wortmannin treatment was initiated 5 min before CORM-2 administration and continued during CORM-2 treatment and in some hearts throughout reperfusion. Values determined 120 min after reperfusion using TTC staining method. ‘a’ indicates P<0.05 versus vehicle control group and ‘b’ indicates P<0.05 versus CORM-2 alone group. ![]() Vehicle Control,
Vehicle Control, ![]() Vehicle Control+i CORM-2,
Vehicle Control+i CORM-2, ![]() Vehicle Control+i CORM-2 (50 μM),
Vehicle Control+i CORM-2 (50 μM), ![]() Wortmannin (100 nM)+Vehicle, (
Wortmannin (100 nM)+Vehicle, (![]() ) Wortmannin (100 nM)+i CORM-2,
) Wortmannin (100 nM)+i CORM-2, ![]() Wortmannin (100 nM)+i CORM-2 (50 μM),
Wortmannin (100 nM)+i CORM-2 (50 μM), ![]() ) Vehicle+ Wortmannin (100 nM),
) Vehicle+ Wortmannin (100 nM),![]() ) i CORM-2+ Wortmannin (100 nM),
) i CORM-2+ Wortmannin (100 nM), ![]() CORM-2 (50 μM)+ Wortmannin (100 nM)
CORM-2 (50 μM)+ Wortmannin (100 nM)
Recent studies indicated that CO can also confer cytoprotective actions in the heart. Treatment of isolated cardiac cells or hearts with a CO donor preserves cell viability and myocardial performance against hypoxia−reoxygenation damage [9]. Similarly, the administration of a CO donor at the time of reperfusion reduced infarct size in an in vivo murine model of coronary occlusion [21]. Mice receiving a short infusion of CO are protected against MI for up to 72 h, which is equivalent to the protection afforded by ischaemic preconditioning [22]. Inhalation of CO also protects against myocardial I/R injury in rats, safeguards the heart during reperfusion after cardiopulmonary bypass in pigs and attenuates I/R injury following cardiac transplantation [6,23,24]. Tricarbonyldichlororuthenium (II) dimmer known as CORM−2, a lipid−soluble molecule, which delivers CO in a controlled manner and simulates the cytoprotective action of HO−1 derived CO in biological systems. Subsequently, tricarbonyldichloro (glycinato) ruthenium (II) (CORM−3), a water soluble form has been developed and it has demonstrated protection against cardiac I/R injury [25].
It has been emphasised that CO can influence many signalling pathways, especially p38 MAPK. To date, four isoforms of the p38 MAPK family have been identified: p38α, p38β, p38γ and p38δ [26−29]. It was found that p38α and p38β are ubiquitously expressed whereas p38γ and p38δ are differentially expressed depending on tissue type. Activation of p38α is not only dependent on stimulus but also on cell type [30]. Despite all four p38 MAPK group members displaying similar activation profiles, differences have been observed in the kinetics and level of activation of these isoforms [30,31]. Many laboratories showed that expression of HO−1 or exposure of endothelial cells (ECs) to exogenous CO enhances p38 MAPK activation by TNF−α [32]. Specific inhibition of p38 MAPK activation by SB203580 or through overexpression of a p38 MAPK−dominant negative mutant abrogates the antiapoptotic effect of HO−1. On the other hand, evidence exists for the concomitant activation of p38 MAPK and apoptosis induced by a variety of agents, such as nerve growth factor withdrawal and Fas ligation [33]. In Jurkat T cells, Fas/CD95−induced cell death is augmented by exposure to CO, and this occurs in part via inhibition in the activity of MAPK [34,35]. Thus, the role of p38 MAPK in apoptosis is cell type− and stimulus−dependent. Although p38 MAPK signalling can promote cell death in some cell lines, it may enhance survival, cell growth, and differentiation in others [31]. Kim et al. suggested a critical role for the β−isoform of p38 MAPK in mediating the effects of CO on cytoprotection and Hsp70 expression because these effects were abrogated in ECs by SB 203580, a selective inhibitor of α and β isoforms, and in p38β−null fibroblasts [10]. Further, Zhang et al. reported that CO inhibits apoptosis via p38 MAPK−STAT or Akt/PKB−STAT pathways during I/R injury [36]. Owing to the complexity of upstream signalling pathways of p38 MAPK in response to stimuli, it is possible that the intensity or duration of the kinase activation could be dependent on the specific signalling pathways that they utilize. Recently, Kohmoto et al. demonstrated that perioperative exposure of donors and recipients to CO at a low concentration can impart potent antiinflammatory and cytoprotective effects in rat lung I/R injury following extended cold preservation and transplantation [37]. The protective effects of CO seen in this study appear to be, at least in part, mediated through the activation p38 MAPK. We performed the study to assess the role of p38 MAPK in cardioprotective effect of CORM−2 using pharmacological inhibitors of p38 MAPK such as SCIO−469 (p38 MAPK alpha inhibitor) and SB203580 (α and β p38 MAPK inhibitor) in rat model of I/R injury. Pretreatment with SCIO−469 (1 μM) followed by CORM−2 (50 μM) showed similar patterns of recovery in cardiac parameters such as CK, LDH, HR, CF rate, LVEDP, LVDP, dp/dt max, dp/dt min and infarct size as observed with CORM−2 alone. Paradoxically, pretreatment with SB203580 (10 μM) followed by CORM−2 significantly prevented postischaemic recovery in myocardial injury markers CK and LDH. Addition of SB203580 (10 μM) significantly abolished the improvement in HR, CF, LVEDP, LVDP, dp/dt max and dp/dt min as compared to CORM−2 (50 μM) group. Postischaemic rat hearts treated with CORM−2 showed significantly reduced infarct size as compared to vehicle control. On the other hand, when SB203580 (10 μM) was used to inhibit p38 MAPK, infarct size was found to be significantly increased which indicates the significant prevention of cardioprotective effect of CORM−2 once p38 MAPK has been inhibited by SB203580. These results indicated that cardioprotective effect by CORM−2 may not dependent on p38 MAPK alpha activation. But when we used specific inhibitor of p38 MAPK α and β, SB203580, cardioprotection was abolished, indicating that there might be an activation of p38 MAPK β by CORM−2 treatment during I/R injury in isolated rat heart.
Activation of PKC has previously been also shown to be important in cardioprotection during ischaemia [38,39]. Relatively few studies have implied roles for protein kinases A/G/C in HO−1 transcriptional regulation. In primary rat hepatocytes and vascular smooth muscle, treatment with dibutylated cAMP and other agonists of protein kinase A (PKA) activated HO−1 transcription [40]. In contrast, HO−1 expression was induced by the general PKC inhibitor chelerythrine in human aortic ECs. Inhibitor studies have also implied a contributory role for PKCs in the activation of HO−1 by oxidised phospholipids [41]. We performed the study to find out the role of PKC in cardioprotective effect of CORM−2 using the pharmacological inhibitor of PKC chelerythrine in rat model of cardiac I/R injury. Pretreatment with chelerythrine (10 μM) followed by CORM−2 (50 μM) showed significant prevention of postischaemic recovery in myocardial injury markers CK and LDH. Pretreatment with chelerythrine (10 μM) prevented the improvement in HR, CF, LVEDP, LVDP, dp/dt max and dp/dt min as compared to CORM−2 (50 μM) group. Postischaemic rat hearts treated with chelerythrine (10 μM) followed by CORM−2 (50 μM) showed significant increase in infarct size as compared to CORM−2 alone. These results indicated that PKC catalytic inhibitor chelerythrine blocks CORM−2−mediated cardioprotection.
A limited number of studies have examined the role of the PI3K cell survival pathway in the context of HO−1 gene regulation. PI3K, a ubiquitous lipid−modifying enzyme consisting of a p85 regulatory subunit and a p110 catalytic subunit, responds to activation by diverse stimuli including growth factors, cytokines and cytotoxic agents. Inhibitors of p38 MAPK and PI3K blocked the activation of HO−1 by 15d−PGJ2 in human lymphocytes [42]. Stimulation of PI3K/Akt by nerve growth factor and haemin in dopaminergic neuronal cells enhanced HO−1 gene expression in an Nrf2−dependent fashion and provided protection against oxidative stress [43]. PI3K/Akt activation has been associated with the cardioprotective effect of ischaemic preconditioning, which protects the myocardium from injury produced by subsequent sustained ischaemia [44]. The PI3K/Akt and STAT pathways are known to play critical roles in mediating cell proliferation, differentiation and apoptosis. There is little information on the role of PI3K/Akt and STAT pathways in mediating apoptotic signals during I/R injury, and also little is known about the ability of CO to modulate these pathways. Previous data by Zhang et al. showed that CO requires p38 MAPK activation to exert an antiapoptotic effect during I/R injury in Rat pulmonary artery endothelial cell (PAEC) and reported associations amongst MAPKs, PI3K/Akt, and STATs [36]. To best of our knowledge, there are no reports available regarding the role of PI3K in CORM−2−mediated cardioprotection in heart. Thus, we hypothesised that the cardioprotective effect of CORM−2 may be associated with activation of the PI3K/Akt pathway in the heart. We, therefore, initially examined whether addition of PI3K inhibitor before and concomitantly with CORM−2 would block the protection afforded by CORM−2. Inhibition of PI3K activity by wortmannin (100 nM) both concomitantly with CORM−2 treatment (before ischaemia) and continued during reperfusion blocked CORM−2−mediated recovery of cardiac function, whereas inhibition only before ischaemia did not significantly block CORM−2−mediated recovery of cardiac functions such as CK, LDH, HR, CF, cardiodynamics (LVEDP, LVDP, dp/dt max, dp/dt min) and infarct size indicating that PI3K activity is required for CORM−2−mediated cardioprotection during reperfusion. Therefore, we tested the importance of the PI3K pathway in CORM−2−mediated recovery of cardiac function when the hearts were treated with CORM−2 at reperfusion. Based on these data, we conclude that PI3K activity during reperfusion is critical for CORM−2−mediated cardioprotection. Our finding is similar to the findings of Hausenloy et al. who showed that the cardioprotective effects of ischaemic preconditioning can be blocked by addition of the PI3K inhibitor LY294002 at the start of reperfusion [45].
In summary, we demonstrated that Langendorff’s perfused rat hearts treated with CORM−2 exhibited significant cardioprotection. In this study, we sought to further delineate the signal transduction pathways utilised by CORM−2 during I/R injury. We utilised isolated rat heart model to delineate the ability of CORM−2 to modulate various pathways during I/R injury. We demonstrate that activation of β isoform of p38 MAPK is important for CORM−2−mediated cardioprotection. Furthermore, CORM−2−mediated activation of the PKC signalling pathway is required for cardioprotective effect of CORM−2 during I/R injury. Furthermore, using chemical inhibitor of PI3K, we showed that CORM−2 enhances PI3K activation during reperfusion to prevent I/R injury in heart (fig. 6).
Acknowledgment
The auhors thank Dr. Ajay Sharma (Masson Eye Institute, Columbia, MO 65212, USA) for his valuable guidance and support. The authors also thank the management of Zydus Research Centre for all support.
References
- Chance B, Erecinska M, Wagner M. Mitochondrial responses to carbon monoxide toxicity. Ann N Y AcadSci 1970;174:193-204.
- Otterbein LE, Soares MP, Yamashita K, Bach FH. Hemeoxygenase-1: Unleashing the protective properties of heme. Trends Immunol 2003;24:449-55.
- Perrella MA, Yet SF. Role of hemeoxygenase-1 in cardiovascular function. Curr Pharm Des 2003;9:2479-87.
- Vulapalli SR, Chen Z, Chua BH, Wang T, Liang CS. Cardioselective overexpression of HO-1 prevents I/R-induced cardiac dysfunction and apoptosis. Am J Physiol Heart Circ Physiol 2002;283:H688-94.
- Yet SF, Tian R, Layne MD, Wang ZY, Maemura K, Solovyeva M, et al. Cardiac-specific expression of hemeoxygenase-1 protects against ischemia and reperfusion injury in transgenic mice. Circ Res 2001;89:168-73.
- Fujimoto H, Ohno M, Ayabe S, Kobayashi H, Ishizaka N, Kimura H, et al. Carbon monoxide protects against cardiac ischemia–reperfusioninjury in vivo via MAPK and Akt–eNOS pathways. ArteriosclerThrombVascBiol 2004;24:1848-53.
- Motterlini R, Mann BE, Johnson TR, Clark JE, Foresti R, Green CJ. Bioactivity and pharmacological actions of carbon monoxide-releasing molecules. Curr Pharm Des 2003;9:2525-39.
- Józkowicz A, Huk I, Nigisch A, Weigel G, Dietrich W, Motterlini R, et al. Hemeoxygenase and angiogenic activity of endothelialcells: Stimulation by carbon monoxide and inhibition by tin protoporphyrin-IX. Antioxid Redox Signal 2003;5:155-62.
- Clark JE, Naughton P, Shurey S, Green CJ, Johnson TR, Mann BE, et al. Cardioprotective actions by a water-soluble carbon monoxide-releasing molecule. Circ Res 2003;93:e2-8.
- Kim HP, Wang X, Nakao A, Kim SI, Murase N, Choi ME, et al. Caveolin-1 expression by means of p38beta mitogen-activated protein kinase mediates the antiproliferative effect of carbon monoxide. ProcNatlAcadSci USA 2005;102:11319-24.
- Otterbein LE, Choi AM. Hemeoxygenase: Colors of defense against cellular stress. Am J Physiol Lung Cell Mol Physiol 2000;279:L1029-37.
- Ryter SW, Choi AM. Hemeoxygenase-1/carbon monoxide: From metabolism to molecular therapy. Am J Respir Cell MolBiol 2009;41:251-60.
- Shin DY, Chung J, Joe Y, Pae HO, Chang KC, Cho GJ, et al. Pretreatment with CO-releasing molecules suppresses hepcidin expression during inflammation and endoplasmic reticulum stress through inhibition of the STAT3 and CREBH pathways. Blood 2012;119:2523-32.
- Winburn IC, Gunatunga K, McKernan RD, Walker RJ, Sammut IA, Harrison JC. Cell damage following carbon monoxide releasing molecule exposure: Implications for therapeutic applications. Basic Clin PharmacolToxicol 2012;111:31-41.
- Kramkowski K, Leszczynska A, Mogielnicki A, Chlopicki S, Fedorowicz A, Grochal E, et al. Antithrombotic properties of water-soluble carbon monoxide-releasing molecules. ArteriosclerThrombVascBiol 2012;32:2149-57.
- Soni H, Patel P, Rath AC, Jain M, Mehta AA. Cardioprotective effect with carbon monoxide releasing molecule-2 (CORM-2) in isolated perfused rat heart: Role of coronary endothelium and underlying mechanism. VasculPharmacol 2010;53:68-76.
- Clark JE, Foresti R, Sarathchandra P, Kaur H, Green CJ, Motterlini R. Hemeoxygenase-1-derived bilirubin ameliorates postischemic myocardialdysfunction. Am J Physiol Heart Circ Physiol 2000;278:H643-51.
- Hangaishi M, Ishizaka N, Aizawa T, Kurihara Y, Taguchi J, Nagai R, et al. Induction of hemeoxygenase-1 can act protectively againstcardiac ischemia/reperfusion in vivo. Biochem Biophys Res Commun 2000;279:582-8.
- Masini E, Vannacci A, Marzocca C, Pierpaoli S, Giannini L, Fantappié O, et al. Hemeoxygenase-1 and the ischemia-reperfusion injury in the rat heart. ExpBiol Med (Maywood) 2003;228:546-9.
- Yoshida T, Maulik N, Ho YS, Alam J, Das DK. H (mox-1) constitutes an adaptive response to effect antioxidant cardioprotection: A study with transgenic mice heterozygous for targeted disruption of the Hemeoxygenase-1 gene. Circulation 2001;103:1695-701.
- Guo Y, Stein AB, Wu WJ, Tan W, Zhu X, Li QH, et al. Administration of a CO-releasing molecule at the time of reperfusion reduces infarct size in vivo. Am J Physiol Heart Circ Physiol 2004;286:H1649-53.
- Stein AB, Guo Y, Tan W, Wu WJ, Zhu X, Li Q, et al. Administration of a CO-releasing molecule induces late preconditioning against myocardial infarction. J Mol Cell Cardiol 2005;38:127-34.
- Lavitrano M, Smolenski RT, Musumeci A, Maccherini M, Slominska E, Di Florio E, et al. Carbon monoxide improves cardiac energetics and safeguards the heart during reperfusion after cardiopulmonary bypass in pigs. FASEB J 2004;18:1093-5.
- Akamatsu Y, Haga M, Tyagi S, Yamashita K, Graça-Souza AV, Ollinger R, et al. Hemeoxygenase-1-derived carbon monoxide protects hearts fromtransplant associated ischemia reperfusion injury. FASEB J 2004;18:771-2.
- Motterlini R, Clark JE, Foresti R, Sarathchandra P, Mann BE, Green CJ. Carbon monoxide-releasing molecules: Characterization of biochemical and vascular activities. Circ Res 2002;90:E17-24.
- Lechner C, Zahalka MA, Giot JF, Møller NP, Ullrich A. ERK6, a mitogen-activated protein kinase involved in C2C12 myoblast differentiation. ProcNatlAcadSci USA 1996;93:4355-9.
- Li Z, Jiang Y, Ulevitch RJ, Han J. The primary structure of p38 gamma: A new member of p38 group of MAP kinases. Biochem Biophys Res Commun 1996;228:334-40.
- Jiang Y, Gram H, Zhao M, New L, Gu J, Feng L, et al. Characterization of the structure and function of the fourth member of p38 group mitogen-activated protein kinases, p38delta. J BiolChem 1997;272:30122-8.
- Kumar S, McDonnell PC, Gum RJ, Hand AT, Lee JC, Young PR. Novel homologues of CSBP/p38 MAP kinase: Activation, substrate specificity and sensitivity to inhibition by pyridinylimidazoles. Biochem Biophys Res Commun 1997;235:533-8.
- Nebreda AR, Porras A. p38 MAP kinases: Beyond the stress response. Trends BiochemSci 2000;25:257-60.
- Zarubin T, Han J. Activation and signaling of the p38 MAP kinase pathway. Cell Res 2005;15:11-8.
- Ryter SW, Morse D, Choi AM. Carbon monoxide: To boldly go where NO has gone before. Sci STKE 2004;2004:RE6.
- Kummer JL, Rao PK, Heidenreich KA. Apoptosis induced by withdrawal of trophic factors is mediated by p38 mitogen-activated protein kinase. J BiolChem 1997;272:20490-4.
- Song R, Zhou Z, Kim PK, Shapiro RA, Liu F, Ferran C, et al. Carbon monoxide promotes Fas/CD95-induced apoptosis in Jurkat cells. J BiolChem 2004;279:44327-34.
- Pae HO, Oh GS, Choi BM, Chae SC, Kim YM, Chung KR, et al. Carbon monoxide produced by hemeoxygenase-1 suppresses T cell proliferation via inhibition of IL-2 production. J Immunol 2004;172:4744-51.
- Zhang X, Shan P, Alam J, Fu XY, Lee PJ. Carbon monoxide differentially modulates STAT1 and STAT3 and inhibits apoptosis via a phosphatidylinositol 3-kinase/Akt and p38 kinase-dependent STAT3 pathway during anoxia-reoxygenation injury. J BiolChem 2005;280:8714-21.
- Kohmoto J, Nakao A, Stolz DB, Kaizu T, Tsung A, Ikeda A, et al. Carbon monoxide protects rat lung transplants fromischemia-reperfusion injury via a mechanism involving p38 MAPK pathway. Am J Transplant 2007;7:2279-90.
- Ooie T, Takahashi N, Nawata T, Arikawa M, Yamanaka K, Kajimoto M, et al. Ischemia-induced translocation of protein kinase C-ε mediates cardioprotection in the streptozotocin-induced diabetic rat. Circ J 2003;67:955-61.
- Inagaki K, Hahn HS, Dorn GW II, Mochly-Rosen D. Additive protection of the ischemic heart ex vivo by combined treatment with δ-protein kinase C inhibitor and ε-protein kinase C activator. Circulation 2003;108:869-75.
- Immenschuh S, Kietzmann T, Hinke V, Wiederhold M, Katz N, Muller-Eberhard U. The rat hemeoxygenase-1 gene is transcriptionally induced via the protein kinase Asignaling pathway in rat hepatocyte cultures. MolPharmacol 1998;53:483-91.
- Krönke G, Bochkov VN, Huber J, Gruber F, Blüml S, Fürnkranz A, et al. Oxidized phospholipids induce expression of human hemeoxygenase-1 involving activation of cAMP-responsive element-binding protein. J BiolChem 2003;278:51006-14.
- Alvarez-Maqueda M, El Bekay R, Alba G, Monteseirín J, Chacón P, Vega A, et al. 15-deoxy-delta 12,14-prostaglandin J2 induces hemeoxygenase-1 gene expression in a reactive oxygen species-dependent manner in human lymphocytes. J BiolChem 2004;279:21929-37.
- Salinas M, Diaz R, Abraham NG, Ruiz de Galarreta CM, Cuadrado A. Nerve growth factor protects against 6-hydroxydopamine-induced oxidative stress by increasing expression of hemeoxygenase-1 in a phosphatidylinositol 3-kinase-dependent manner. J BiolChem 2003;278:13898-904.
- Tong H, Imahashi K, Steenbergen C, Murphy E. Phosphorylation of glycogen synthase kinase-3 beta during preconditioning through a phosphatidylinositol-3-kinase–dependent pathway is cardioprotective. Circ Res 2002;90:377-9.
- Hausenloy DJ, Tsang A, Mocanu MM, Yellon DM. Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. Am J Physiol Heart Circ Physiol 2005;288:H971-6.