- *Corresponding Author:
- J. Dong
Department of Integrative Medicine, Huashan Hospital, Fudan University, 12 Middle Wulumiqi Road, Shanghai, China
E-mail: dongjingcheng2004@126.com
| This article was originally published in a special issue, "Clinical and Experimental Studies on Drug and Intervention Repurposing in China |
| Indian J Pharm Sci 2019:81(4)spl issue1;86-93 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The mechanism of baicalin in airway reconstruction in rats with chronic obstructive pulmonary disease was investigated. The chronic obstructive pulmonary disease models were established in rats through smoke inhalation and instilling lipopolysaccharide. The rats were then divided into the control group and the experimental group, a blank group was used as a reference point of the experiment. Rats in the experiment group received baicalin either at a high dose (80 mg/kg/d, H1) or at a low dose (25 mg/kg/d, H2) to analyze the airway reconstruction functions and antiinflammatory effects of baicalin. During the experiment, the general conditions of the rats were compared. After the experiment, lung tissues of the rats were obtained for Hematoxylin-Eosin staining, and the pathological changes were observed. In addition, the bronchial walls were measured and analyzed. Using immunohistochemical staining, the expression levels of tumor necrosis factor-α and interferon-γ in rat lung tissues were analyzed and compared. Hematoxylin-Eosin staining of lung tissues showed that the rats with chronic obstructive pulmonary disease had severe pathological damages; compared to the blank group, while in rats treated with baicalin, bronchial obstruction, alveolar structure and inflammatory infiltration were improved, and the differences were statistically significant (p<0.05). After baicalin administration, the bronchial wall thickness in chronic obstructive pulmonary disease rats was reduced, collagen deposition in the bronchial epithelium was improved, and airway smooth muscle proliferation was alleviated compared to the control group. Baicalin intervention at high dose decreased the expressions of TNF-α and IFN-γ in chronic obstructive pulmonary disease rats as well as the ratio of Th17/Treg in the lung tissues and improved the balance of the ratio of Th17/Treg. In addition, baicalin treatment reduced the positive cell count of RORγt in the lung tissues of the chronic obstructive pulmonary disease rats, and all these differences were statistically significant (p<0.05). In summary, baicalin administration inhibited airway reconstruction of the chronic obstructive pulmonary disease rats, and the mechanism of the antiinflammatory effect of baicalin appeared to be through the regulation of TNF-α and IFN-γ in lung tissues.
Keywords
Baicalin, COPD, airway reconstruction, antiinflammatory, TNF-α, IFN-γ
Chronic obstructive pulmonary disease (COPD) is a chronic disease, which is characterized by a high incidence, high disability and high mortality rates[1]. Since COPD can cause pulmonary functions to decline progressively and irreversibly, it has a great impact on the daily life of patients. The progression of COPD to the late stage would cause pulmonary heart disease leading to multiple organ failure, which is the main cause of death in COPD[2]. According to statistics from the World Health Organization, COPD has ranked the 4th in all causes of death worldwide and has a trend of increasing annually[3]. Therefore, research on COPD preventive measures and treatment methods has gained the main focus in recent years. The etiology of COPD is not yet clear, which may relate to the risk factors that cause chronic bronchitis, such as tobacco, smoke, occupational dust, air pollution, protease-antiprotease imbalance, and infections. Therefore, the treatment of COPD mainly includes smoking cessation, oxygen therapy, treatment with antiinfectives, glucocorticoids, theophylline and β2 receptor agonists[4]. However, current treatment measures improve only the clinical performance of patients and delay the progression of the disease. Previous studies on COPD have shown that inflammatory cell infiltration occurs in the trachea and bronchial walls of not only COPD patients but also animal models of COPD; the mononuclear cells are more common in the absence of infection and in acute exacerbations, neutrophilic granulocytes are more common[5].
Baicalin is a flavonoid isolated from the baical skullcap root as the main constituent. Baicalin serves as a key indicator for the quality control of baical skullcap root and its preparations[6]. Baicalin has a wide range of pharmacological effects such as antiallergy, antitumor, antiinflammatory, immune regulatory, antiviral and blood pressure lowering among which the antiinflammatory effect is the most reproducible. The antiinflammatory effect of baicalin is related to the inhibition of prostaglandin E synthesis and regulation of interleukin-2, tumor necrosis factor-α (TNF-α), and nuclear factor-KB levels[7]. In this study, the effect of baicalin on COPD rat models was evaluated to understand the importance of the antiinflammatory effect of baicalin in the treatment of COPD, which could form the basis for clinical drug selection.
Materials and Methods
Animals and grouping:
A total of 32 6-8-week old in-bred healthy male SPF rats weighing 190.10±22 g purchased from the Nanjing Junke Bioengineering Co., Ltd., China, were selected. These rats were adaptively fed with granulated ordinary rat feed and clean tap water for 1 w with constant temperature and humidity (22~24°, 50~60 %). All rats were randomly divided into a blank group (8 rats), a control group (8 rats), and 2 experimental groups (8 rats each) the baicalin high-dosage group (H1) and the baicalin low-dosage group (H2).
COPD rat model development:
Blank group rats were given free access to water and food in a normal environment without any interventions. The control group and the experiment group rats were used to develop COPD rat model through the smoke inhalation method combined with the lipopolysaccharide (LPS) method. On d 1 and d 14 of the experiment, rats in the control group and the experiment group were anesthetized with intraperitoneal injection of 10 % chloral hydrate (300 mg/kg), and the completely non-resistant rats were considered as fully anesthetized. These rats were fixed on the dissection table, and oral cavities fully exposed. A portion of a scalp needle tube with a syringe interface of about 3 cm was cut and was infused with l mm water column. Then, LPS (200 μg/200 μl) was injected into the trachea of rats by tracheal intubation. The ejection of the water column was considered as a successful infusion. The rats were rotated upright for about 20 s for the even distribution of LPS in the rat lung tissues and trachea at all levels. On day 2 to day 13 and from day 15 to day 28, the rats were placed in an inhouse-made organic glass cigarette smoke exposure device (100×80×60 cm) for passive smoking (Jianjun cigarette, tar 12 mg, nicotine 1.0 mg and CO 14 mg, China Tobacco Shandong Industrial Co., Ltd.). The rats were put in the device once a day for 4 consecutive weeks, with 30 min exposure each time. The smoke intensity of each group was kept constant. After passive smoking treatment, the rats in the blank group were allowed to have free access to food and water under the same environment. The diet, activity, respiration, oral and nasal secretions, and reactions of the rats in each group were observed every day. On the 28th d of the experiment, the two groups of rats were executed.
Intervention methods and dosages of baicalin:
On the d 13 of the experiment, rats in the experiment group were given baicalin (80 % pure, Batch No. 110715-201016, Xi’an Zaolutang Pharmaceutical Group Rehabilitation Medicine Co., Ltd.) at different dosages by gavage once a day. Based on the calculation of pure baicalin, the dosage standards of high-dose and low-dose groups were respectively 80 mg/kg/d and 25 mg/kg/d; the baicalin concentrations used for the H1 group and the H2 group were respectively 16 mg/ml and 5 mg/ml, which were formulated as saline suspension. According to the body weights of the rats, the dosage was 1 ml per 200 g of rat weight. The gavage lasted until the 28th d of the experiment. Rats in each group were weighed once a week.
Assessment indicators:
General conditions assessed were, the fur colors, mental states, and behavioral performances of rats in each group every day. Whether the rats had agitation, rapid breathing, sneezing, incontinence, and lip cyanosis were mainly observed. The body weights of the rats in each group were recorded at the beginning of the experiment, and then after 2 w and 4 w, respectively.
Pathological changes of lung tissues were investigated after the blood samples were collected, the rat thoracic cavity was quickly opened, and the lung tissues of the upper, middle and lower lobe of the right lung were respectively placed in a sample vial containing 4 % paraformaldehyde, labeled, and stored at room temperature. At the same time, the left lung was obtained and placed in a sterilized 5 ml EP tube and stored at -80°. The right lung upper lobe tissues of all rats were fixed for 24 h and embedded in paraffin.
Two blocks from the 2 groups were randomly selected, sectioned, and stained with Hematoxylin-Eosin (HE) staining solution. Under the 100X optical microscope, the pathological changes in lung tissues of rats in each group were observed, in which the alveolar structures, the bronchial and arteriole morphologies, and the inflammatory cell infiltration were mainly compared.
Measurement and analysis of bronchial walls was made by selecting 5 intact circular bronchial smooth muscle cross-sectional sections from the HE-stained and processed sections. The ratio of the minimum diameter to the maximum diameter of the trachea was ≥0.5. Under a 400-fold optical microscope, the Image Pro Plus 7.0 analysis software was utilized to determine the bronchial basement membrane circumference (PBM) and the wall area (WAT), and calculate the WAT/PBM value of each bronchus, the average value was taken as the bronchial wall thickness of the rat. In addition, the areas of stain positive signal (area +) and PBM were determined, and the area +/PBM value of each bronchus was calculated and averaged as the smooth muscle layer thickness. In addition, the area of Masson stain positive signal (Wcol) was measured, and the Wcol/PBM value of each bronchus was calculated and the average value was taken to represent the degree of sub-epithelial collagen deposition in rats.
Expressions of TNF-α and IFN-γ in lung tissues were evaluated by immunohistochemical staining of TNF-α and IFN-γ after the pathological HE staining of rat lung tissues was treated with an antioffset agent to prevent dissection (reagent kits of Wuhan Boster Biological Technology). The pathological images of rat lung tissues after immunohistochemical staining were processed and quantified. The images were collected by Olympus microscope and a computer image acquisition camera. The specimens of immunohistochemical staining were analyzed by using the Bs Image Station microscopic image analysis software. One section of each rat in each group was selected for observation. Four fields of view (×200) were randomly selected from the stained area of each section. The positive staining average gray value and the average gray value of TNF-α and IFN-γ immunohistochemical expression of TNF-α and IFN-γ of pathological sections of rat lung tissues were measured and calculated.
The Th17/Treg ratio in the lung tissues were measured. The IL-17+ cell was marked as Th17, and the transcription factor wing-headed transcription factor P3 (Foxp3) positive cell Foxp3+ was marked as Treg; under a high power microscope, the cells were counted; 5 regions were selected for each specimen, and the positive cell count in 1 mm2 view was recorded.
Foxp3 and RORγt were detected through expression of Foxp3 and RORγt in the nuclei and the cytoplasm of inflammatory cells such as lymphocytes around the airway, lung tissues, and lung stroma; their positive stains were brown, and the positive cell counts of Foxp3 and RORγt under a high power view were recorded.
Relationship mechanism of TNF-α, IFN-γ and COPD airway inflammation:
TNF-α was mainly derived from inflammatory cells such as activated mononuclear macrophages, mast cells, and activated T lymphocytes, which was a multifunctional cytokine. During the inflammatory response process of COPD, TNF-α could induce the endothelial cells to express adhesion molecules, therefore, the white blood cells adhere to the vascular endothelium, causing a large number of white blood cells to accumulate in the inflammation site. The appropriate amount of TNF-α could participate in the activation of inflammatory cells in immune cells thereby exert antiinfective and antitumor effects. The high expression of TNF-α enhanced the permeability of micro-vascular walls and chemotherapeutic neutrophils thereby causing the blood coagulation at the inflammation sites; therefore, the local lymphocytes continued to proliferate and infiltrate.
IFN-γ was a type-II interferon and was a small molecule polypeptide that regulated cell function. Low doses of IFN-γ enhance immunity, while high dosages of IFN-γ inhibit immune function. According to previous studies, IFN-γ enhanced the action of TNF on vascular endothelial cells by stimulating macrophages, neutrophils, and vascular endothelial cells, and could unbalance the protease-antiprotease system, thereby reacting on the COPD airway inflammation response. Since IFN-γ had the important cellular immune regulation function, the severity of the disease was also related to IFN-γ. Therefore, IFN-γ was also an important indicator for the evaluation of immune function in patients with COPD.
Statistics analysis:
The SPSS 20.0 statistics software was applied to perform the statistical analysis. The measurement data were expressed as the mean±the standard deviation and were submitted to the homogeneity test of variance. P>0.05 indicated the homogeneity of variance, and the pair comparisons between each mean number were analyzed using one-way ANOVA. P<0.05 indicated inhomogeneity of variance, and the one-way ANOVA should be corrected by Welch. The pair comparisons of multivariate analysis were performed by the Least Significant Difference (LSD) method. P<0.05 indicated the statistical significance of the difference.
Results and Discussion
In the blank group, the color of rat fur was normal and glossy; the respiration was stable; no symptoms such as salivation and wheezing were observed; no oral and nasal secretions were observed; the claws and nails were shiny; rats were in good mental states, with good responses and normal activities; the body weights of rats were normal and showed steady growth trends, and the urine and feces were normal during the experiment. In the COPD model control group, the color of rat fur was dull; the respiration was fast and rapid; symptoms of salivation were observed; at about 3 w of COPD induction, the respiratory rate increased, symptoms such as cough, shortness of breath, and difficulty in breathing were aggravated, and the color of claws and nails was dark and not glossy; the rats were in poor mental states and were less likely to move; the body weights gain was reduced and the rat feces were loose.
Within the first 2 w of COPD induction, the general conditions and body weight of rats in the COPD model group had no obvious differences compared to the blank group. After 2 w of COPD induction, the rats gradually developed symptoms of fatigue and weakness, fur became dull; however, no significant differences in the intakes of food and water were seen compared with the blank group; at the 2nd w of COPD model construction, the body weights of rats in the blank group were compared with that in the control group, and the differences were not statistically significant (p>0.05). By the 4th w, the body weights of rats in the control group and the blank group were 236.2±13.0 and 281.3±32.7 g, respectively, and the differences was statistically significant (p<0.05); the body weights of rats in the HI group and the H2 group were 278.1±28.5 and 264.5±19.6 g, respectively, compared to the control group, the differences were statistically significant (p<0.05). There were no significant differences between the other groups (p>0.05). The weight changes in rats in each group were shown in Table 1.
| Group | Week 1 | Week 2 | Week 4 |
|---|---|---|---|
| The blank group | 214.6±7.8 | 239.5±10.9 | 281.3±32.7 |
| The control group | 204.3±11.0 | 220.3±11.2 | 236.2±13.0* |
| H1 | 212.5±9.2 | 237.4±12.3 | 278.1±28.5 |
| H2 | 210.6±10.2 | 236.1±10.7 | 264.5±19.6 |
| F | - | - | 1.996 |
| P Value | - | - | P<0.05 |
Data expressed mean weight in g±standard deviation n=8. In the 4th week, respectively compared with the blank group and the H1 group, *p<0.05; other group comparisons, p>0.05
Table 1: Body weight changes of rats in each group
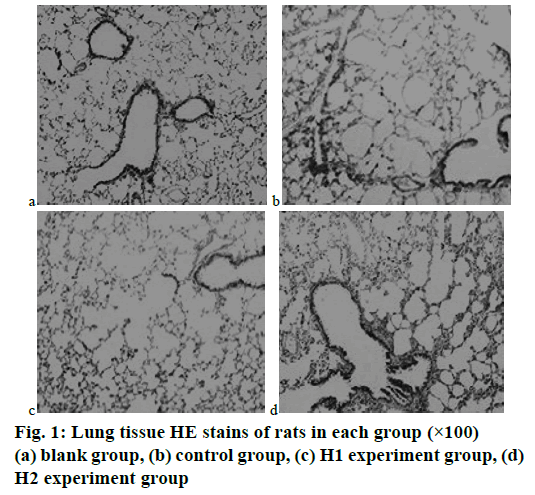
In the blank group of rats, the bronchial structures of the rats were intact, and the mucosa epithelial cells (ciliated cells, cup-shaped fine cells, muscle cells) were arranged neatly, and no obstructions, no stenosis, no embolism, and scar formation were found in the tracheas and bronchial lumen; the pulmonary alveoli and the interval structures of pulmonary alveoli of rats were normal, without congestion, edema, or glandular hyperplasia. The results are shown in fig. 1a.
In the control group of rats the bronchial mucosa congestion, edema, cilia lodging, reduction, adhesion, and loss were observed; a large number of inflammatory cell infiltration was seen in the lumen, mainly neutrophils and lymphocytes; smooth muscle hyperplasia and elastic fiber rupture were observed, and interstitial lymphocyte infiltration was seen; the stenosis or obstruction of tracheal lumen was more common in small airways, the endocrine secretion increased, the alveolar walls became thinner, the alveolar cavities were enlarged, and some were merged into pulmonary bullae. The results are shown in fig. 1b.
In the experiment group H1 the degeneration and necrosis of tracheal epithelial cells, and the changes in cilia were less than that of the control group. Improvements of the lesions were obvious. Only a small amount of lymphocyte infiltration around the lumen was seen, which was a chronic inflammatory infiltration, and lymphoid follicles were seen. The results are shown in fig. 1c. In the experiment group H2, there were different degrees of inflammatory cell infiltration in the lumen of the trachea and around the lumen. Some of the pulmonary alveoli were dilated, the lung interval structures were narrowed, the endobronchial bronchus cavities were narrowed, the alveolar walls became thinner, the alveolar cavities were enlarged, and a small number of pulmonary bullae were formed, but the condition was improved compared with the control group. The results were shown in fig. 1d. Therefore, the intervention of baicalin appeared to improve the histopathological changes in lung tissues of COPD rats.
The airway reconstruction was an important factor that led to the further development of COPD. The basic manifestations included the thickening of the bronchial walls, the increased deposition of sub-epithelial collagen, and the thickening of the smooth muscle layer. In addition, α-smooth muscle actin (α-SMA) was the structural basis for the contractile movement of smooth muscle cells and was therefore considered to be an important target of smooth muscle cells. In the experiment, the bronchial wall thickness (WAT/PBM), the sub-epithelial collagen deposition (Wcol/PBM), and the smooth muscle layer thickness (α-SMA(+)area/ PBM) were measured to further analyze the inhibition mechanism of baicalin on airway reconstruction in COPD rats. Table 2 showed the comparisons of trachea morphologies of rats in each group.
| Group | WAT/PBM (μm2/mm) | Wcol/PBM (μm2/mm) | α-SMA(+)area/PBM (μm2/mm) |
|---|---|---|---|
| The blank group | 51.23±1.94 | 19.88±1.90 | 12.21±1.58 |
| The control group | 84.07±2.89# | 79.80±3.77# | 50.15±2.04# |
| H1 | 63.85±1.62* | 55.32±3.02* | 16.03±1.12* |
| H2 | 70.01±1.40* | 59.22±2.88* | 19.14±1.49* |
Data expressed as mean±standard deviation of n=8. Compared with the blank group, #p<0.05; compared with the model control group, *p<0.05
Table 2: Comparison of rat trachea morphology in each group
It could be seen from the data in Table 2 that the thickness of the bronchial walls of rats in the model control group was (84.07±2.89) μm2/μm, the Wat value was significantly decreased after baicalin administration, and the H1 group was better than the H2 group (p<0.05). The level of sub-epithelial collagen deposition of rats in the model control group was (79.80±3.77) μm2/μm, the Wcol value was significantly decreased after baicalin administration, and the H1 group was better than the H2 group (p<0.05). The thickness of the smooth muscle layer of rats in the model control group was (50.15±2.04) μm2/μm, the α-SMA(+)area value was significantly decreased after baicalin administration, which was close to that of rats in the blank group, and the H1 group was better than the H2 group (p<0.05). Thus, it can be concluded that baicalin could improve the deposition of collagen in the airway epithelium of COPD rats and could alleviate the proliferation of airway smooth muscle. In addition, baicalin also inhibited the expression of α-SMA protein in the bronchial smooth muscle of COPD rats. Combined with HE staining results in the bronchus of rats in this experiment, the airway wall edema, epithelial cell shedding, bronchial lumen stenosis, and other phenomena in the COPD model group were very obvious, indicating that airway reconstruction was a necessary factor for COPD airflow limitation. However, after the administration of baicalin, the above conditions of the experimental group were improved to different extents, and the improvement of the H1 group was better than that of the H2 group, which indicated that baicalin had certain inhibition effects on airway reconstruction in COPD rats.
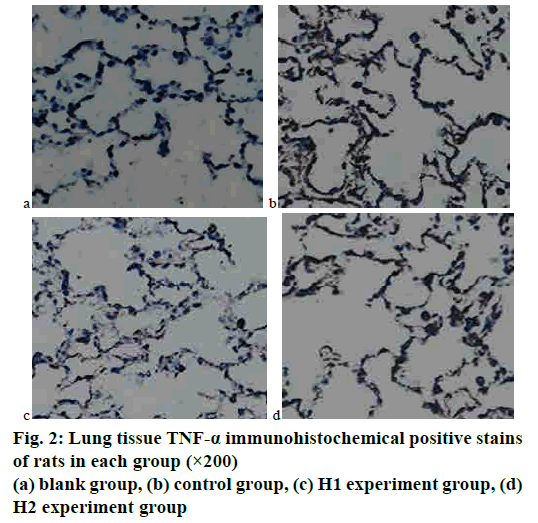
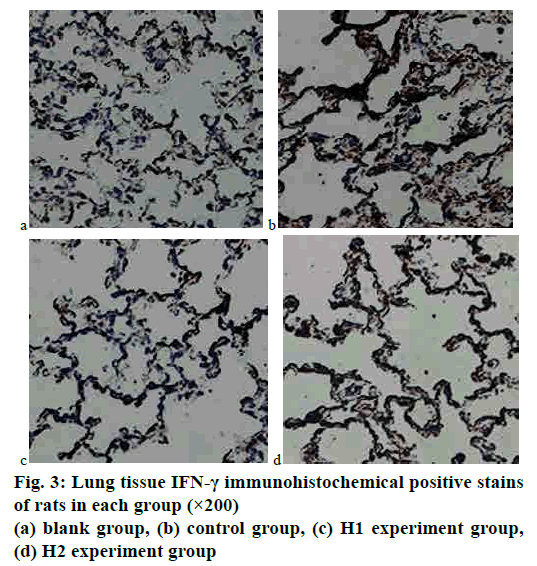
The immunohistochemical staining results of lung tissues of rats in each group were analyzed. It could be seen by the naked eye under an optical microscope that the positive expressions of TNF-α in rat lung tissues were brown, which were mainly distributed in the cell membrane, cytoplasm, and bronchial mucosa. The expressions of IFN-γ in rat lung tissues were brown, which were mainly expressed in the cell membrane and bronchial mucosa. The gray values of images were detected by using an image analysis system immunohistochemical analysis software. The results showed that the gray values of TNF-α and IFN-γ in the COPD model rat control group were the smallest, and the gray values of that in the experiment group increased with the increase of the dosage of baicalin. Tables 3 and 4 showed the comparison of average gray values of lung tissue TNF-α and IFN-γ immunohistochemical stains of rats in each group. The immunohistochemical results of TNF-α and IFN-γ were shown in figs. 2 and 3.
| Group | Number | TNF-a | IFN-γ |
|---|---|---|---|
| The blank group | 8 | 135.24±4.01 | 107.88±7.03 |
| The control group | 8 | 60.25±2.13 | 58.37±2.28 |
| The H1 experiment group | 8 | 115.87±5.25 | 97.45±3.63 |
| The H2 experiment group | 8 | 68.94±5.03 | 69.64±2.76 |
| F | - | 358.43 | 138.42 |
| P Value | - | p<0.05 | p<0.05 |
Table 3: Comparison of average gray values of lung tissue TNF-a and IFN-g immunohistochemical stains of rats in each group (X±S)
| The blank group | The control group | H1 | H2 | |
|---|---|---|---|---|
| Th17/Treg | 0.61±0.08 | 4.33±0.78 | 2.35±0.56 | 1.95±0.70 |
Table 4: The comparison of the TH17/Treg ratio in lung tissues of rats in each group
TNF-α could indirectly reflect the degree of inflammation in the lungs. According to the TNF-α immunohistochemical maps of fig. 2, the TNF-α expression was the weakest in the blank group, and that in the experimental group was significantly weaker than that in the COPD model rat control group; in the experiment group, the intensity of TNF-α expression decreased with the increase of the dosage of baicalin, which approached to that in the blank group. The differences among groups were statistically significant (p<0.05). IFN-γ was expressed in the epithelial cell membrane and bronchial mucosa and had antiinflammatory and antiviral functions. According to the IFN-γ immunohistochemical maps of fig. 3, the expression of IFN-γ was the weakest in the blank group, and the expression of IFN-γ in the experiment group was significantly weaker than that in the COPD model rat control group; in the experiment groups H1 and H2, with the increase of baicalin administration dosage, the intensity of IFN-γ expression gradually decreased, and that in H1 became closer to that in the blank group. The differences among groups were statistically significant (p<0.05).
Th17 cells and Treg cells were a group of cells that antagonized each other and were associated with the deterioration of lung function. Therefore, in the experiment, IL-17+ cells were compared with Foxp3+ cells in the lung tissues of each rat in each group. The ratio of IL-17+ cells to Foxp3+ cells in the blank group was (0.61±0.08). The ratio of IL-17+ cells to Foxp3+ cells in the COPD model rats after being smoked was (4.33±0.78), which was significantly higher than that in the blank group, and the differences were statistically significant (p<0.05). After the intervention of baicalin, the ratio of IL-17+ cells to Foxp3+ cells in H1 and H2 groups were (2.35±0.56) and (1.95±0.70), respectively, which were significantly lower than that of the control group, and the differences were statistically significant (p<0.05). Therefore, the imbalance of Th17/Treg ratio was important for the occurrence and development of COPD. After the intervention of baicalin, the imbalance of Th17/Treg ratio could be improved.
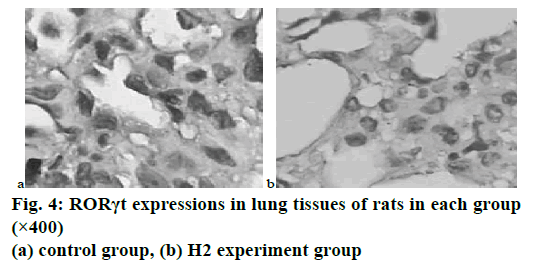
The results of immunohistochemical stains indicated that Foxp3 and RORγt were mainly expressed in the nuclei and cytoplasm of inflammatory cells such as lymphocytes around the airway, lung tissues, and lung stroma. In the control group of COPD rats after smoking treatment, the number of RORγt positive cells in lung tissue was significantly increased compared with the blank group, while the number of RORγt positive cells in the H1 and H2 groups after the intervention of baicalin was significantly decreased, and the differences were statistically significant (p<0.05). In the experiment, the number of Foxp3 positive cells in each group did not change much, and the differences were not statistically significant (p>0.05). Fig. 4 showed the expression of RORγt in the lung tissues of the COPD model rats in the control group and the H2 group after baicalin intervention.
The experiment had proved that the antiinflammatory mechanism of baicalin in COPD rats and the regulation of TNF-α and IFN-γ expressions in rat lung tissues were related, and baicalin had significant effects on balancing the Th17/Treg ratio in COPD rats.
COPD is a relatively common chronic disease that can be prevented and treated, which is usually caused by prolonged exposure to toxic particles or gases thereby inducing the abnormalities in the tracheal and alveolar structures at all levels. The main features of COPD are chronic airway inflammatory reactions, including cough, wheezing, increased airway secretions, and airway reconstruction, which ultimately lead to decreased lung function. At present, COPD has ranked the 4th position in all causes of death worldwide and has a trend of increasing annually. Therefore, COPD is becoming an important health issue of concern to all human beings worldwide.
It is believed that the important reason for the incomplete reversibility of COPD airflow at that time is airway reconstruction, which has received increasing attention in recent years. Airway reconstruction begins with epithelial damages and inflammatory responses, and changes in the epithelial phenotype continue to exacerbate this process, further causing lung damages. Repeated stimulation of chronic airway inflammation can cause airway reconstruction. The stimulation of various harmful particles can cause inflammatory cells to produce inflammatory mediators. The inflammatory mediators act on airway structural cells and cause abnormal repairs, which in turn can cause the thickening of airway wall fibers and the narrowing of the airway lumen. The abnormal repair is repeated, allowing airway reconstruction to continue to progress, which ultimately results in an irreversible restriction of airflow.
In summary, the COPD rat model was developed through a combination of smoke inhalation method and the LPS method, which were then divided into the control group and the experiment group; in addition, a blank group was used as the reference object of the experiment to analyze the antiinflammatory effects of baicalin on COPD. In the experiment, the wall area of the basement membrane was used to quantify the wall area, and the tube diameter was calculated and compared. The results showed that the bronchial wall thickness of rats in the COPD model control group was significantly thicker than that in the blank group, and the bronchial wall thickening of the rats after administration of baicalin was significantly less than that of the untreted model rats, indicating that baicalin had certain effects on inhibiting the airway reconstruction of COPD rats. In addition, the effects of inflammatory mediator expressions on COPD airway reconstruction were analyzed and were confirmed by experiments that the anti-inflammatory mechanism of baicalin in COPD rats and the regulation of TNF-α and IFN-γ expressions in rat lung tissues were related.
Acknowledgements:
This work was supported by National Natural Science Foundation of China (No.81403148).
References
- Zhang X, Bao W, Fei X, Zhang Y, Zhang G, Zhou X, et al. Progesterone attenuates airway remodeling and glucocorticoid resistance in a murine model of exposing to ozone. Mol Immunol 2018;96:69-77.
- Perry MM, Tildy B, Papi A, Casolari P, Caramori G, Rempel KL. The anti-proliferative and anti-inflammatory response of COPD airway smooth muscle cells to hydrogen sulfide. Respir Res 2018;19(1):85.
- Wang Y, Jia M, Yan X, Cao L, Barnes PJ, Adcock IM, et al. Increased neutrophil gelatinase-associated lipocalin (NGAL) promotes airway remodelling in chronic obstructive pulmonary disease. Clin Sci 2017;131(11):1147-59.
- Ye WJ, Xu WG, Guo XJ, Han FF, Peng J, Li XM, et al. Differences in airway remodeling and airway inflammation among moderate-severe asthma clinical phenotypes. J. Thorac Dis 2017;9(9);2904-14.
- Tajti G, Gesztelyi R, Pak K, Papp C, Keki S, Szilasi ME, et al. Positive correlation of airway resistance and serum asymmetric dimethylarginine level in COPD patients with systemic markers of low-grade inflammation. Int J Chron Obstruct Pulmon Dis 2017;2:873-84.
- Wada Y, Kitaguchi Y, Yasuo M, Ueno F, Kawakami S, Fukushima K, et al. Diversity of respiratory impedance based on quantitative computed tomography in patients with COPD. Int J Chron Obstruct Pulmon Dis 2018;13:1841-9.
- De Rose V, Molloy K, Gohy S, Pilette C, Greene CM. Airway Epithelium Dysfunction in Cystic Fibrosis and COPD. Mediators Inflamm 2018;2018: 1309746.