- *Corresponding Author:
- Xiaowen Lu
Department of Endodontics, Ningbo Stomatology Hospital, Ningbo, Zhejiang Province 315000, China
E-mail: zhouxiaoye555@163.com
| Date of Received | 14 November 2022 |
| Date of Revision | 25 August 2023 |
| Date of Acceptance | 07 March 2024 |
| Indian J Pharm Sci 2024;86(2):634-641 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To investigate the mechanism of linagliptin on stromal cell-derived factor 1 and vascular endothelial growth factor protein after molar pulp revascularization in rats. 45 specific-pathogen free male Sprague-Dawley rats were divided into 5 groups based on the average body weight, with 9 rats in each group. The control group did not establish the rat model of molar pulp revascularization, and model group, low-linagliptin group, mediumlinagliptin group, and high-linagliptin group all established the rat model of molar pulp revascularization. On d 1 after modeling, rats in low-linagliptin group, medium-linagliptin group, and high-linagliptin group were gavaged with 1, 2, and 3 mg/ml linagliptin, respectively. Rats in control group and model group were gavaged with 0.9 % normal saline at equal volume, once a day for 28 d. After the last gavage, the rats were detected for the body weight and fasting blood glucose, and superoxide dismutase and serum oxidative stress indicators malondialdehyde were detected by kit; hematoxylin and eosin staining was employed to detect the pathological morphology of dental pulp tissue, immunohistochemistry and immunoblot were applied to detect the positive expression of vascular endothelial growth factor and stromal cell-derived factor 1 and the protein content. No significant differences were found in fasting blood glucose and body weight between the groups (p>0.05). In control group, loose connective tissue was found in the root canal of rats, and a large number of long spindle forming fibrocytes were distributed; in model group, a large number of inflammatory cells are found under the dental pulp, with the majority of neutrophils; in comparison with model group, inflammatory cells in pulp tissue of low-linagliptin group, medium-linagliptin group and high-linagliptin group gradually decreased, and scattered restorative dentin could be found in the pulp, and there was no inflammatory expression in the middle and lower tissues of the pulp. Dipeptidyl peptidase 4 inhibitor linagliptin has no effect on rats’ body weight and blood glucose after revascularization, but it can improve the level of oxidative stress and accelerate revascularization and perhaps its mechanism links to the up-regulation of vascular endothelial growth factor and stromal cell-derived factor 1 expression.
Keywords
Linagliptin, revascularization, vascular endothelial growth factor, stromal cell, hypoglycemia
Pulp revascularization is a treatment scheme to improve pulp necrosis of young permanent teeth, which is widely used in clinic. During the operation, root canal disinfection and pulp protection are beneficial to improve the activity of mesenchymal stem cells and repair infected or necrotic pulp[1]. When the root canal is filled with blood, the inflammatory reaction of the tooth apex can subside, which is conducive to the repair of the injury and increases the length of the root and the thickness of the canal wall. There are many factors that affect the revascularization after pulp infection, while the specific mechanism needs a further verification[2]. Vascular Endothelial Growth Factor (VEGF) is elevated in human dental pulp infected by bacteria, while its expression content decreases when the inflammatory response is irreversible[3]. As per previous studies, VEGF up-regulation can accelerate the activity of endothelial cells, increase the growth of vascular endothelium, and promote the differentiation of Stem Cells from Human Exfoliated Deciduous (SHED) cells after the up- regulation of VEGF-2 expression in SHED[4]. As a chemokine, Stromal Cell Derived Factor-1 Alpha (SDF-1α) is crucial in bone tissue regeneration and regulating cell activity by binding to its receptor C-X-C Chemokine Receptor type 4 (CXCR- 4). Based on former studies, SDF-1 combined with hydrogel can promote the regeneration of fibroblasts and thus the regeneration of blood vessels after implantation into tooth roots[5].
Dipeptidyl Peptidase-4 (DPP-4) can regulate the expression of SDF-1 during pulp revascularization. SDF-1 is the substrate of DPP-4. After the two bind to each other, DPP-4 cleaves the terminal residues of SDF-1 and inhibits the expression of SDF-1 and its receptor, thereby losing its activity and chemotaxis[6]. Linagliptin is a DPP-4 inhibitor, which mainly plays a hypoglycemic effect, and has less adverse reactions with higher blood concentration in the body. According to the relevant literature[7], linagliptin can improve molar pulp revascularization and has no significant effect on blood glucose and body weight. However, there are few studies on the expression of SDF- 1 and VEGF in pulp revascularization. This study aimed to explore the effect of linagliptin on the expression levels of VEGF and SDF-1 in rats with pulp revascularization, and to provide reference for the treatment of pulp revascularization by regulating SDF-1 and VEGF levels.
Materials and Methods
Experimental rats:
45 healthy male rats aged 5 w-6 w, weighing (185.51±9.50) g, supplied from the First Affiliated Hospital of Hebei North University, were housed in the animal room. There were 4 rats in each cage. During the experiment, they were free to drink and eat. The experiment was carried out after feeding for a week. Animal License No: SCXK (Ji): 2018- 0013.
Drugs, main reagents and instruments:
DPP-4 inhibitor linagliptin tablets (Boehringer Ingelheim Pharmaceuticals Inc, Germany); VEGF monoclonal antibody (Jiangsu Prosai Biological Co., Ltd.,); SDF-1 monoclonal antibody (PeproTech, United States of America (USA)); stir-in resin (3M, USA); 0.9 % normal saline (Shandong Hualu Pharmaceutical Co., Ltd.,); pentobarbital sodium (Hubei Hongyuan Pharmaceutical Co., Ltd.,); electronic balance (Chengdu Sujing Scientific Equipment Co., Ltd.,); Hematoxylin and Eosin (H&E) staining kit (Beijing Solarbio Science & Technology Co., Ltd.,) and protein electrophoresis apparatus (LKB, Sweden);
Establishment of rat molar pulp revascularization model:
Rats with intact teeth and no disease were selected. Bilateral mandibular first molars were selected as experimental teeth. The mesial and distal root canals of experimental teeth were used as experimental root canals. 1 % sodium pentobarbital (50 mg/ kg) was injected intraperitoneally for anesthesia. The pulp was extracted after drilling with a high-speed turbine. The pulp root canals were soaked in sodium hypochlorite for 10 s and rinsed with Sodium chloride (NaCl) solution. After cleaning the root canals, 1.5 % sodium hypochlorite, 1.7 % Ethylenediaminetetraacetic Acid (EDTA) and NaCl solution were rinsed alternately. The roots were dried with moisture absorbing paper and filled with stir-in resin. After 7 d of revascularization model, the fillings of experimental teeth were removed under general anesthesia, and the root canals were cleaned with 15 file after NaCl irrigation. After drying, the tissues around the periapical tissues were stimulated, and when the blood filled the root canals, the cavities were closed with stir-in resin.
Grouping and intervention methods:
As per the average body weight, a total of five groups containing 45 rats, namely, control group, model group, Low-Linagliptin (L-LIN) group, Medium-Linagliptin (M-LIN) group and High- Linagliptin (H-LIN) group, with 9 rats in each group. The revascularization model was set up in rats of model group, L-LIN group, M-LIN group and H-LIN group. On d 1 after modeling, rats in L-LIN group were gavaged with 1 mg/ml linagliptin, M-LIN group with 2 mg/ml linagliptin, H-LIN group with 3 mg/ml linagliptin, control group and model group were gavaged with 0.9 % normal saline at equal volume, once a day for 28 d.
Blood glucose and body weight detection:
The rat’s body weight and fasting blood glucose in each group were detected after intragastric administration for 28 d. Tail venous blood was drawn, and fasting blood glucose was detected by glucometer. The weight of rats in each group was detected by electronic balance.
Oxidative stress:
After intragastric administration, 1 ml of fasting tail venous blood was drawn from rats in each group. The low-speed centrifuge was used to run at 3000 r/min for 15 min, and the supernatant was retained. We detected Malondialdehyde (MDA) with thiobarbituric acid method and Superoxide Dismutase (SOD) with xanthine oxidase method. The experimental procedures were strictly according to the instructions.
Tissue extraction and H&E staining:
We sacrificed the rats by cervical dislocation under anesthesia, the mandible was preserved and put into 10 % formaldehyde solution, fixed overnight, rinsed with tap water, soaked in 14 % EDTA solution for decalcification, dehydrated layer by layer, and after paraffin embedded, cut into 4 μm section for backup. The pulp tissue sections of rats in each group were put into 37° for 15 min, dewaxed with benzophenone, dehydrated layer by layer according to 100 %-95 %-85 % and 75 % ethanol, stained with hematoxylin for 8-10 min, rinsed with running water, treated with 1 % hydrochloric acid alcohol for 10 s, counterstained with eosin staining solution for 10 s, dehydrated layer by layer, and sealed with neutral resin. We observed the rat’s pathological morphology in each group under microscope.
Immunohistochemically detection of SDF-1 and VEGF protein levels in root canal tissues of rats in each group:
Dental crown tissues 4 μm sections, dehydrated and embedded, fixed with formaldehyde, hydrated, and placed at 37.5° for 0.5 h, then added 1 % EDTA solution and blocked with goat serum. After adding VEGF (1:500) and SDF-1 (1:500) antibodies, and adding horseradish peroxidase labeled goat anti-rabbit Immunoglobulin G (IgG) secondary antibody for 0.5 h, counterstained and covered. With faint yellow to sepia color as positive, 5 fields of each immunohistochemically section were randomly selected and analyzed with Mammographic Image Analysis Society (MIAS) medical image analysis system.
Immunoblot detection of SDF-1 and VEGF protein expression in dental pulp tissue:
The pulp tissues of rats in each group were taken, and the total protein of tissues was extracted by precooling radioimmunoprecipitation method, and with Bicinchoninic Acid (BCA) protein, we quantitatively determined the protein concentration. In each group, 80 ng protein was taken for protein electrophoresis by 1.5 % Sodium Dodecyl Sulphate- Polyacrylamide Gel Electrophoresis (SDS-PAGE), semi-dry transferred to Polyvinylidene Chloride (PVDC) membrane, blocked by 5 % bovine serum, added with VEGF (1:1000) and SDF-1 (1:3000) primary antibodies at 4° overnight, added with goat anti-rabbit IgG (1:5000) secondary antibody, exposed by Enhanced Chemiluminescence (ECL) kit, and the electrophoretic bands were analyzed by ImageJ software.
Statistical analysis:
In virtue of GraphPad Prism 8 software, this paper’s data were analyzed, and the experimental data were in line with normal distribution, expressed by (x̄ ±s), compared among multiple groups with one- way Analysis of Variance (ANOVA), compared between groups by Least Significant Difference (LSD) t-test, p<0.05 shows statistically significant difference.
Results and Discussion
After intragastric administration of linagliptin for 28 d, no significant differences were found in body weight and blood glucose among the groups (all p>0.05, see Table 1).
| Group | n | Blood glucose (mmol/l) | Weight (g) |
|---|---|---|---|
| Control | 9 | 6.65±1.20 | 234.05±8.50 |
| Model | 9 | 6.82±1.35 | 232.10±8.65 |
| L-LIN | 9 | 6.89±1.41 | 233.30±9.20 |
| M-LIN | 9 | 6.91±1.33 | 230.50±8.55 |
| H-LIN | 9 | 6.92±1.50 | 231.56±10.05 |
| f | 0.061 | 0.219 | |
| p | 0.993 | 0.926 |
Table 1: Effects of Lidocaine on Body Weight and Fasting Blood Glucose of Rats in Each Group
In comparison with control group, the expression of SOD, an indicator of oxidative stress, in the serum of rats in model group decreased and the expression of MDA increased, with statistical significance (p<0.05). In comparison with model group, the expression of SOD, an indicator of oxidative stress, in the serum of rats increased and the expression of MDA decreased in L-LIN group, statistically significant (p<0.05). In comparison with L-LIN group and M-LIN group, the level of SOD in the serum of rats increased and MDA decreased in H-LIN group, significant statistically (p<0.05). See Table 2 for the expression levels of SOD and MDA.
| Group | n | SOD (U/ml) | MDA (mg/ml) |
|---|---|---|---|
| Control | 9 | 50.45±4.30 | 5.25±1.50 |
| Model | 9 | 33.12±2.66a | 13.30±2.20a |
| L-LIN | 9 | 38.09±3.50ab | 11.17±1.90ab |
| M-LIN | 9 | 42.11±3.29abc | 9.50±1.44abc |
| H-LIN | 9 | 46.22±4.05abcd | 8.22±1.36abcd |
| f | 31.720 | 28.410 | |
| p | <0.001 | <0.001 |
Note: Comparing to control group, ap<0.05; comparing to model group, bp<0.05; comparing to L-LIN group, cp<0.05 and comparing to M-LIN, dp<0.05
Table 2: Effects of Lidocaine on Serum Oxidative Stress Indexes of Rats in Each Group
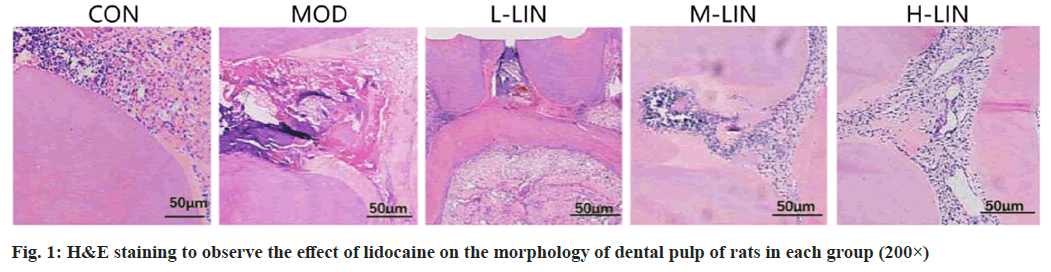
In control group, the root canal of rats was loose connective tissue, and a large number of long spindle forming fibrocytes were distributed. In model group, many inflammatory cells were found under the dental pulp, with the majority of neutrophils. Inflammatory cells in pulp tissue of L-LIN group, M-LIN group and H-LIN group gradually decreased, and scattered restorative dentin could be found in the pulp, and there was no inflammatory expression in the middle and lower tissues of the pulp as shown in fig. 1.
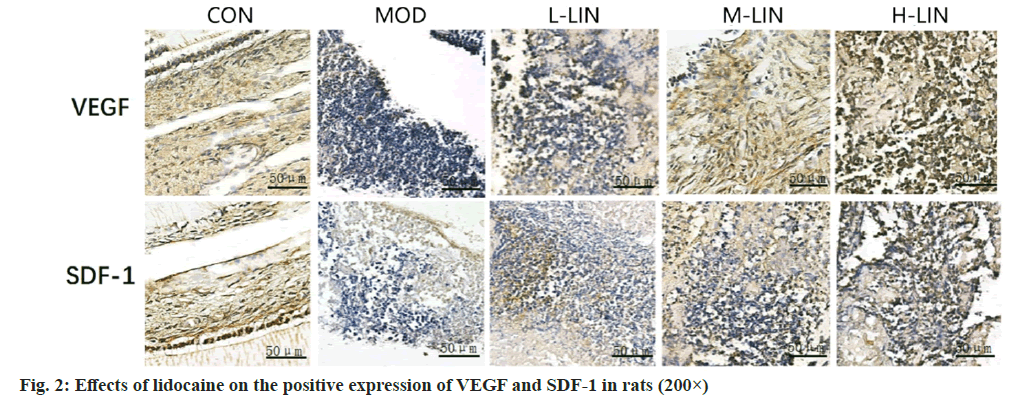
Comparing to control group, the positive expression of VEGF and SDF-1 in rat’s pulp tissue in model group decreased, with statistically significant differences (p<0.05); compared with model group, the positive expression of VEGF and SDF-1 in the pulp tissue of rats in L-LIN group increased, with statistically significant differences (p<0.05); comparing to L-LIN group, the positive expression of VEGF and SDF-1 in the dental pulp tissue of M-LIN group increased, with statistically significant differences (p<0.05); comparing to M-LIN group, the positive expression of VEGF and SDF-1 in the dental pulp tissue of rats in H-LIN group increased, with statistically significant difference (p<0.05). The positive expression of VEGF and SDF-1 as shown in fig. 2 and Table 3.
| Group | n | Number of VEGF positive expression | Number of SDF-1 positive expression |
|---|---|---|---|
| Control | 9 | 25.10±8.00 | 18.90±5.12 |
| Model | 9 | 11.40±4.10a | 9.50±1.85 |
| L-LIN | 9 | 18.00±6.20ab | 12.25±3.66ab |
| M-LIN | 9 | 22.30±7.14abc | 14.78±5.14abc |
| H-LIN | 9 | 25.30±5.60abcd | 17.55±4.95abcd |
| f | 6.009 | 6.428 | |
| p | <0.001 | <0.001 |
Note: Comparing to control group, ap<0.05; comparing to model group, bp<0.05; comparing to L-LIN group, cp<0.05 and comparing to M-LIN, dp<0.05
Table 3: Effect of Lidocaine on the Positive Expressions of VEGF and SDF-1 in Each Group of Rats Detected by Immunohistochemistry
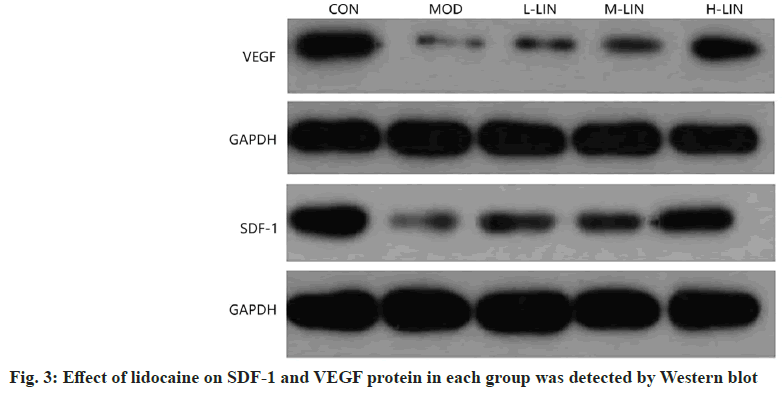
Comparing to control group, SDF-1 and VEGF protein in pulp tissue of model group were decreased, with statistically significant differences (p<0.05); compared with model group, SDF-1 and VEGF protein in pulp tissue of L-LIN group were increased, with statistically significant differences (p<0.05); compared with L-LIN group, SDF-1 and VEGF protein in pulp tissue in M-LIN group increased, with statistically significant differences (p<0.05); compared with M-LIN group, SDF-1 and VEGF protein levels in pulp tissue of rats in H-LIN group increased, with statistically significant differences (p<0.05). See fig. 3 and Table 4 for SDF-1 and VEGF protein levels.
| Group | n | VEGF | SDF-1 |
|---|---|---|---|
| Control | 9 | 1.10±0.14 | 1.33±0.06 |
| Model | 9 | 0.25±0.08a | 0.40±0.04a |
| L-LIN | 9 | 0.45±0.10ab | 0.60±0.07ab |
| M-LIN | 9 | 0.60±0.11abc | 0.74±0.09abc |
| H-LIN | 9 | 0.79±0.05abcd | 0.93±0.07abcd |
| f | 94.24 | 244.2 | |
| p | <0.001 | <0.001 |
Note: Comparing to control group, ap<0.05; comparing to model group, bp<0.05; comparing to L-LIN group, cp<0.05 and comparing to M-LIN, dp<0.05
Table 4: Effects of Lidocaine on the Protein Levels OF VEGF and SDF-1 in Each Group of Rats
The purpose of pulp revascularization is to drive the regeneration of endogenous stem cells, and the regeneration of blood vessels in the root canal is conducive to accelerating the generation of new tissues. However, relevant studies have confirmed that the new tissue after pulp revascularization is not completely pulp like tissue, including bone like and periodontal like tissue, which cannot play a therapeutic role[8]. In view of the above problems, how to regulate the tissue composition of new tissues in the process of pulp reconstruction has become the focus and hot spot of medical research.
Linagliptin, a DPP-4 inhibitor, is a common clinical drug to improve blood glucose. The hypoglycemic mechanism of linagliptin is that it can up-regulate the expression of insulin hormone Glucagon Like Polypeptide-1 (GLP-1), increase the expression and release of insulin hormone, and reduce the body circulation’s blood glucose level[9]. The findings from this study showed that the fasting blood glucose and body weight of rats were not significantly changed after 28 d of intragastric administration of linagliptin after revascularization. The mechanism that linagliptin did not significantly change the blood glucose of rats may be that when the blood glucose of the body is at normal concentration, the change of GLP-1 level will not have an effect on blood glucose, which indicates that there is a correlation between GLP-1 expression and blood glucose concentration, and the GLP-1 level changes little and will not cause blood glucose changes after the use of linagliptin. Based on Li et al.[10], no significant change was found in GLP-1 and Glucose-Dependent Insulin Releasing Peptide (GIP) activities under normal blood glucose, and linagliptin could not promote insulin hormone expression without increasing appetite and had little effect on body weight under this environment.
During oxidative stress, the imbalance between the body’s oxidation and antioxidant capacity will secrete a large amount of reactive oxygen species, which will damage tissues and cells. In the case of periodontitis or pulp injury, the body's inflammatory cascade can lead to the deterioration of the condition of inflammatory damaged pulp. SOD and MDA are the main indicators to evaluate oxidative damage, which maintain a dynamic balance in the normal body, but the expression of MDA increases and SOD decreases after pathological damage[11]. Relevant studies have confirmed that pulp stem cell’s elevated MDA expression can produce a large number of oxidation products and inhibit the activity of dental pulp stem cells[12]. This study confirmed that linagliptin given by gavage to rats after the establishment of pulpless root canal model and revascularization can significantly inhibit the expression of MDA, increase the activity of SOD, and thus inhibit oxidative damage. As a GLP-1 receptor agonist, linagliptin can inhibit inflammatory response and oxidative stress injury by reducing the growth of ROS in oxidative active clusters and up-regulating the expression of GLP-1R, thus improving lipid oxidative damage, reducing MDA level and increasing SOD expression[13]. At present, GLP-1 has been confirmed in myocardial ischemia-reperfusion injury to reduce cardiomyocytes oxidative damage by regulating the expression of nuclear transcription factor Nuclear factor erythroid 2-Related Factor 2 (NRF2)[14], but the mechanism of linagliptin inhibiting oxidative stress in pulp injury revascularization needs further study.
Pulp injury is resulted from many factors. It has been confirmed that pulp injury is related to oxidative inflammatory injury and growth factor imbalance. VEGF can promote angiogenesis and improve the activity of dental pulp stem cells, which is used as the main indicator to evaluate the health of pulp tissue[15]. Animal experiments showed that the establishment of revascularization in rats with pulp injury can up-regulate the expression of VEGF in rat pulp tissue, promote pulp stem cell’s differentiation in the matrix, and improve the regeneration and repair ability of dental tissue[16]. SDF-1 is a member of chemokine CXC family, and CXCR4 is the only receptor of SDF-1. After interaction, it forms the SDF-1/CXCR4 axis, which acts in regulating downstream signaling pathways[17]. It is confirmed from relevant studies that SDF-1/CXCR4 plays a chemotactic role in promoting tissue repair and regeneration after pulp inflammatory injury. Han et al.[18] showed that migration and proliferation abilities of human pulp cells cultured in vitro after intervention with SDF-1 significantly increased by the way of concentration depending. This study confirmed that intragastric administration of linagliptin to rats after revascularization can increase VEGF and SDF-1 expression in pulp tissue, thus playing a role in promoting pulp revascularization and tissue regeneration. Studies have confirmed that linagliptin is a DPP-4 inhibitor and GLP-1 is a natural substrate of DPP-4. Linagliptin can reduce DPP-4 expression and play a role in regulating GLP-1 signaling. Linagliptin increases the expression of GLP-1, thereby increasing the expression of VEGF in ischemic tissues, which is involved in vascular regeneration. SDF-1 is one of the targets of DPP-4. DPP-4 can recognize and degrade its amino acid and alanine residues, thereby inhibiting SDF-1 activity[19]. Lin et al.[20] showed that the use of linagliptin in rats with pulp injury and revascularization can increase SDF- 1 expression level in the serum of rats, improve pulp injury, accelerate the movement of pulp stem cells to the site of pulp injury, and promote pulp regeneration.
This study still has some limitations. Owing to the limitation of time and funds, control group and cell experiment were not established, and the test method was single. In the future, we should strengthen cooperation, increase the experimental grouping and methods, enrich the experimental content, and provide a reference scheme for improving the of molar pulp revascularization. In conclusion, DPP-4 inhibitor linagliptin has no effect on rat’s body weight and blood glucose after revascularization, but it can improve the level of oxidative stress and accelerate revascularization, and perhaps its mechanism links to the up- regulation of VEGF and SDF-1 expression.
Author’s contributions:
Ye Zhou and Yongjing Liu have contributed equally to this work.
Conflict of interests:
The authors declared no conflict of interests.
References
- Siddiqui Z, Sarkar B, Kim KK, Kadincesme N, Paul R, Kumar A, et al. Angiogenic hydrogels for dental pulp revascularization. Acta Biomater 2021;126:109-18.
[Crossref] [Google Scholar] [PubMed]
- Nakakura-Ohshima K, Quispe-Salcedo A, Sano H, Hayasaki H, Ohshima H. The effects of reducing the root length by apicoectomy on dental pulp revascularization following tooth replantation in mice. Dental Traumatol 2021;37(5):677-90.
[Crossref] [Google Scholar] [PubMed]
- Dronova TA, Babyshkina NN, Zavyalova MV, Slonimskaya EM, Cherdyntseva NV. Vascular Endothelial Growth Factor Receptor 2 (VEGFR2) contributes to tamoxifen resistance in estrogen-positive breast cancer patients. Mol Biol 2021;55(1):102-8.
[Crossref] [Google Scholar] [PubMed]
- Liang C, Liao L, Tian W. Stem cell-based dental pulp regeneration: Insights from signaling pathways. Stem Cell Rev Rep 2021;17(4):1251-63.
[Crossref] [Google Scholar] [PubMed]
- Kwon MY, Ghanta S, Ng J, Tsoyi K, Lederer JA, Bronson RT, et al. Expression of stromal cell-derived factor-1 by mesenchymal stromal cells impacts neutrophil function during sepsis. Crit Care Med 2020;48(5):e409.
[Crossref] [Google Scholar] [PubMed]
- Li M, Wang Z, Xia H, Yu L, Hu Z. Vildagliptin and G-CSF improved angiogenesis and survival after acute myocardial infarction. Arch Med Res 2019;50(3):133-41.
[Crossref] [Google Scholar] [PubMed]
- Wei HN, Bai SE, Wu ZL, Lin WH, Zheng CF, Chen WX. Exploration of the safety of linagliptin in the treatment of pulp revascularization. J Guangxi Med Univ 2019;36(4):551-4.
- Sousa MG, Xavier PD, Cantuária AP, Porcino RA, Almeida JA, Franco OL, et al. Host defense peptide IDR-1002 associated with ciprofloxacin as a new antimicrobial and immunomodulatory strategy for dental pulp revascularization therapy. Microb Pathog 2021;152:104634.
[Crossref] [Google Scholar] [PubMed]
- Rigamonti AE, Leoncini R, de Col A, Tamini S, Cicolini S, Abbruzzese L, et al. The appetite- suppressant and GLP-1 stimulating effects of whey proteins in obese subjects are associated with increased circulating levels of specific amino acids. Nutrients 2020;12(3):775.
[Crossref] [Google Scholar] [PubMed]
- Li YL. Effect of linagliptin combined with insulin detemir in the treatment of patients with T2DM poorly controlled by metformin combined with pioglitazone. Med J Chin People's Health 2020;32(8):53-5.
- Jakubiak GK, Osadnik K, Lejawa M, Kasperczyk S, Osadnik T, Pawlas N. Oxidative stress in association with metabolic health and obesity in young adults. Oxid Med Cell Longev 2021;2021:1-9.
[Crossref] [Google Scholar] [PubMed]
- Kaufman G, Skrtic D. N-acetyl cysteine modulates the inflammatory and oxidative stress responses of rescued growth-arrested dental pulp microtissues exposed to TEGDMA in ECM. Int J Mol Sci 2020;21(19):7318.
[Crossref] [Google Scholar] [PubMed]
- Mosquera JE, Torres N, Restrepo J, Ruz-Pau C, Suryanarayanan S. Linagliptin induced pancreatitis. AACE Clin Case Rep 2020;6(1):e37-9.
- Fu J, Xu W, Zhang Y, Sun H, Zhao J. Luteolin modulates the NF-E2-related factor 2/glutamate-cysteine ligase pathway in rats with spinal cord injury. J Med Food 2021;24(3):218-25.
[Crossref] [Google Scholar] [PubMed]
- Wen R, Wang X, Lu Y, Du Y, Yu X. The combined application of rat bone marrow mesenchymal stem cells and bioceramic materials in the regeneration of dental pulp-like tissues. Int J Clin Exp Pathol 2020;13(7):1492.
[Google Scholar] [PubMed]
- Zhang X, Yang JL, Yu Q, Liu YL. Effect of pulp revascularization on TGF-β1 and VEGF expression in root tissue of delayed replantation. J Pract Radiography 2015;31(11):1890-921.
- Weisberg EL, Sattler M, Azab AK, Eulberg D, Kruschinski A, Manley PW, et al. Inhibition of SDF-1-induced migration of oncogene-driven myeloid leukemia by the L-RNA aptamer (Spiegelmer), NOX-A12, and potentiation of tyrosine kinase inhibition. Oncotarget 2017;8(66):109973.
[Crossref] [Google Scholar] [PubMed]
- Han BY, Mu R, Zhu L. Effect of IGF-1 gene transfection on mineralization of human dental pulp stem cells in vitro. J Med Postgraduates 2020;33(9):919-25.
- Kawakita E, Koya D, Kanasaki K. CD26/DPP-4: Type 2 diabetes drug target with potential influence on cancer biology. Cancers 2021;13(9):2191.
[Crossref] [Google Scholar] [PubMed]
- Lin X, Wei HN, Chen WX. Effects of linagliptin on SDF-1 expression and pulp tissue regeneration after pulp revascularization. J Guangxi Med Univ 2020;37(4):593-8.