- *Corresponding Author:
- Qi Xue Department of Thoracic Surgery, National Clinical Research Center for Cancer, Chinese Academy of Medical Sciences and Peking Union Medical College, Chaoyang, Beijing 100021, China E-mail: xueqi_pumc@163.com
| Date of Received | 28 July 2023 |
| Date of Revision | 17 January 2023 |
| Date of Acceptance | 06 May 2024 |
| Indian J Pharm Sci 2024;86(3):1017-1024 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To explore the role of steroid saponins from the flowers of Cestrum noctumum Linn on lung cancer cell epithelial-mesenchymal transition and the molecular mechanisms. Lung cancer cells (A549) were divided into normal control group, steroid saponins from the flowers of Cestrum noctumum Linn group, microRNA-181a-5p group, microRNA-control group, 40 μg/ml steroid saponins from the flowers of Cestrum noctumum Linn+anti-microRNA-181a-5p group and 40 μg/ml steroid saponins from the flowers of Cestrum noctumum Linn+anti-microRNA-control group. Reverse transcription-quantitative polymerase chain reaction was utilized for detecting ribonucleic acid expression. 3-(4, 5-dimethylthiazol-2-yl)-2, 5 diphenyl tetrazolium bromide, clone formation test and transwell assays were performed to analyze A549 lung cancer cell process. Western blot was conducted for detecting protein expression. Steroid saponins from the flowers of Cestrum noctumum Linn treatment inhibited A549 lung cancer cell proliferation and motility, accompanied by decrease of matrix metalloproteinase 2, matrix metalloproteinase 9, N-cadherin, vimentin as well as beta-catenin expression and increases of E-cadherin and microRNA-181a-5p expression. At the same time, elevating microRNA-181a-5p reduced the number of cell clones, repressed cell ability to motility and decreased matrix metalloproteinase 2, matrix metalloproteinase 9, N-Cadherin and vimentin expressions, whereas elevating microRNA-181a-5p increased E-cadherin expression. Inhibiting microRNA-181a-5p expression can rescue steroid saponins from the flowers of Cestrum noctumum Linn induced effects on epithelial-mesenchymal transition and beta-catenin expression in A549 cells. Steroid saponins from the flowers of Cestrum noctumum Linn inhibited epithelial-mesenchymal transition of lung cancer cells through regulation of microRNA-181a-5p/Wnt/β-catenin pathway.
Keywords
Steroid saponins, Cestrum noctumum Linn, lung cancer, microRNA-181a-5p, epithelial-mesenchymal transition
Lung cancer originates in the lungs and can cause harm to the human body, affecting both men and women[1,2]. Studies have shown that traditional Chinese medicine can reduce adverse reactions in lung cancer patients and improve their quality of life[3]. Developing new anti-lung cancer drugs and investigate the specific mechanisms of traditional Chinese medicine against lung cancer is urgent[4]. Cestrum noctumum (C. noctumum) Linn belongs to Cestrum species that grows in tropical and subtropical regions. It is a perennial shrub with bright yellow flowers that bloom at night and emit a pleasant fragrance. It is commonly used in traditional Chinese medicine to treat cancers[5]. Steroid Saponins from C. noctumum Linn (SSCN) inhibits K562 cell proliferation and promotes apoptosis by suppressing Phosphoinositide 3-Kinase (PI3K)/Protein Kinase B (AKT) signaling pathway[6]. It can also inhibit liver cancer cell line BEL-7404 proliferation and suppress liver cancer xenograft growth[7,8]. But the effects and mechanisms of steroid SSCN on lung cancer cells are still unclear.
MicroRNA (miRNA) are a class of non-coding molecules that are involved in various biological processes[9,10]. In relation to lung cancer, miRNA dysregulation has been observed in both the tumor tissues and circulating body fluids of lung cancer patients[11]. Studies have reported that SNHG7 can accelerate lung cancer cell growth through the AKT/mammalian Target of Rapamycin (mTOR) signaling pathway via inhibiting miR-181a-5p[12]. miR-181a-5p is also capacity of inhibiting lung cancer cell proliferation and migration[13]. Wnt1 regulates developmental processes such as tissue patterning and organogenesis. Beta (β)-catenin is an intermediate protein in the Wnt signaling pathway that regulates cell-cell interactions and tissue architecture. They are highly expressed in lung cancer tissue samples and their expression levels are associated with clinical staging and prognosis of patients[14]. In addition, sex determining region Y box 9 (SOX9) Wnt/β-catenin pathway interacted with SOX9 to promote Epithelial-Mesenchymal Transition (EMT) process in lung cancer[15]. Based on the above evidence, we explored the role of SSCN on lung cancer cell EMT and the molecular mechanisms by studying Wnt/β-catenin signaling pathway as well as miR-181a-5p.
Materials and Methods
Materials and reagents:
Lung cancer cells (A549, Shanghai Yaji Biotech), Roswell Park Memorial Institute (RPMI)-1640 medium (Wuhan Yipu Biotech), 3-(4, 5-Dimethylthiazol-2-yl)-2, 5 Diphenyl Tetrazolium Bromide (MTT) assay kit (Shanghai Hefei Biotech), crystal violet (Shanghai Mientei Biotech), Radio-Immunoprecipitation Assay (RIPA) buffer (Shanghai Benshu Biotech), Transwell chambers and Matrigel (Corning Inc., United States of America (USA)), Trizol reagent and quantitative Polymerase Chain Reaction (PCR) reagents (Shanghai Yanhui Biotech).
SSCN isolation:
An appropriate amount of dried night jasmine flowers was soaked in ethanol for 24 h, then heated, refluxed, filtered and concentrated under reduced pressure. This extract was further extracted in sequence with petroleum ether, ethyl acetate and n-butanol. 100 g of the extract obtained using n-butanol was separated using silica gel column chromatography subjected to repeated chromatography and elution and finally recrystallized with methanol. The resulting compound was identified as a steroid saponin.
Cell treatment:
A549 cells were cultured in RPMI-1640 culture medium and treated with various concentrations of SSCN for 2 d. In addition, A549 cells without treatment were used as the NC group. MiR-181a- 5p mimics and negative control were transfected into A549 cells to form the miR-181a-5p group and miR-control group, respectively. MiR-181a- 5p inhibitors and negative control were transfected into A549 cells and then treated with 40 μg/ml SSCN, generating 40 μg/ml SSCN+anti-miR- 181a-5p group and 40 μg/ml SSCN+anti-miRcontrol group.
MTT assay:
Transfected cells were removed from the medium, cleaned with Phosphate Buffer Solution (PBS), centrifuged and then inoculated on 96-well plates. After culture for a specific time, the medium in each well was removed and MTT solution as well as Dimethyl Sulfoxide (DMSO) were added to each well in sequence. The samples were detected by spectrophotometer.
Colony formation assay:
Log phase cells were counted using cell counting plates and the volume of cell suspension corresponding to 800-1000 cells was calculated. The calculated volume of cells was added to each well based on cell density of 800-1000 cells per well. The cell suspension was thoroughly mixed and incubated in a cell culture incubator until cells adhered to the plate. After cell adhesion, the media were replaced according to the experimental design. Cells were cultured for 7-15 d and colony formation was observed. When colonies formed, the cells were then fixed in 4 % paraformaldehyde for at least 30 min. After removal of paraformaldehyde, crystal violet staining was performed for 30 min, followed by rinsing with deionized water. The experimental results were documented by capturing images using a camera.
Transwell assay:
Cells were seeded evenly in 24-well plates according to the experimental requirements, with a Matrigel gel required for invasion assays. When the cells reached a coverage of 80 %-90 %, the culture medium was discarded and the bottom of the wells was washed with sterile PBS 2-3 times. The cells were counted and then resuspended in sterile culture medium. The cell suspension was added to the inner chamber of the Transwell and serum-containing culture medium was added to the outer chamber to ensure consistent drug concentrations between the chambers. The 24-well plate was then placed back into the incubator for continued culture. The inner chamber was fixed with paraformaldehyde. The chamber was then allowed to air dry at room temperature. Under a microscope, the stained Transwell chambers were observed and random fields of view were selected for photography.
Western blot:
Proteins were extracted from each group, separated by polyacrylamide gel electrophoresis (110 V constant current). Protein bands were transferred onto a membrane (200 mA constant current, 1.5 h), incubated with primary antibodies against Matrix Metalloproteinase (MMP)-2, MMP-9, E-cadherin, N-cadherin, vimentin and β-catenin overnight at 4° and then incubated with secondary antibodies (1:2000). The blots were developed and imaged and the grayscale values of protein bands were analyzed to calculate the relative expression levels of proteins compared with β-actin as an internal control.
RT-qPCR:
Extraction of cellular Ribonucleic Acid (RNA) was conducted with a specific kit and then reverse transcription of RNA into complementary Deoxyribonucleic Acid (cDNA) was conducted using commercial kits. PCR amplification was performed using U6 as an internal control. Relative expression level was calculated using the 2-△△Ct method. miR-181a-5p 5'-CAAATTATTGTGGGTTGTC-3' and 5'-TTATGGGTAGATGGGTGA-3'; U6: 5'-CTCGCTTCGGCAGCACA-3' and 5'-AACGCTTCACGAATTTGCGT-3'.
Statistical analysis:
Statistics were analyzed using Statistical Package for the Social Sciences (SPSS) 20.0 software and expressed as mean±standard deviation (x±s). Comparisons were performed using t-tests, oneway analysis of variance or Least Significant Difference (LSD)-t test. p<0.05 indicated statistically significant difference.
Results and Discussion
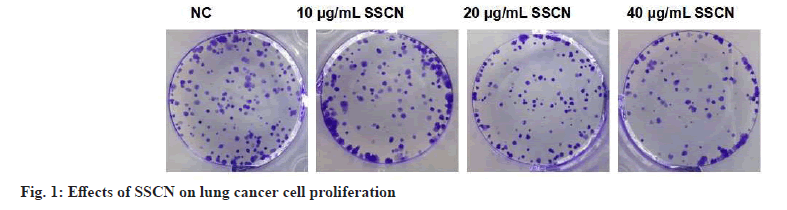
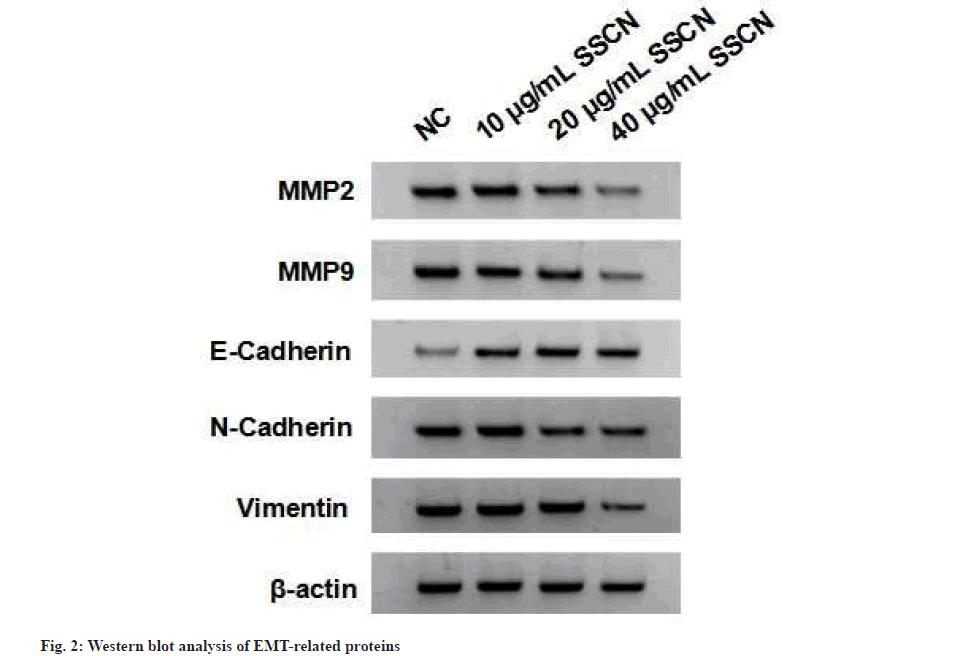
Following treatment with different concentrations of SSCN, there was a decrease in cell viability as well as a reduction in the number of cell clones (fig. 1 and Table 1). After treating A549 cells with various concentrations of SSCN, there was a decrease in A549 cell migration and invasion as well as MMP-2, MMP-9, N-Cadherin and vimentin expression levels and SSCN treatment increased E-cadherin expression (fig. 2 and Table 2).
| Groups | A value | Cell colony number |
|---|---|---|
| NC | 1.052±0.08 | 131±10.02 |
| 10 μg/ml SSCN | 0.863±0.07* | 102±8.16* |
| 20 μg/ml SSCN | 0.631±0.06* | 70±5.34* |
| 40 μg/ml SSCN | 0.481±0.04* | 61±3.07* |
| F | 53.893 | 179.303 |
| p | 0.000 | 0.000 |
Note: Compared with NC, *p<0.05
Table 1: Effect of SSCN on A549 Cell Proliferation
| Groups | MMP-2 | MMP-9 | Cell migration number | Cell invasion number | E-Cadherin | N-Cadherin | Vimentin |
|---|---|---|---|---|---|---|---|
| NC | 0.86±0.08 | 0.73±0.07 | 315±25 | 186±15 | 0.31±0.03 | 0.76±0.07 | 0.91±0.07 |
| 10 μg/ml SSCN | 0.72±0.07* | 0.63±0.06* | 263±21* | 141±12* | 0.45±0.03* | 0.61±0.05* | 0.78±0.07* |
| 20 μg/ml SSCN | 0.59±0.04* | 0.49±0.03* | 204±18* | 102±8* | 0.66±0.06* | 0.43±0.04* | 0.61±0.05* |
| 40 μg/ml SSCN | 0.43±0.04* | 0.40±0.03* | 164±12* | 76±5* | 0.81±0.07* | 0.35±0.02* | 0.48±0.03* |
| F | 83.586 | 74.883 | 103.08 | 180.806 | 171.35 | 129.543 | 97.182 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: Compared with NC, *p<0.05
Table 2: Effects of SSCN on A549 Cell Migration and Invasion
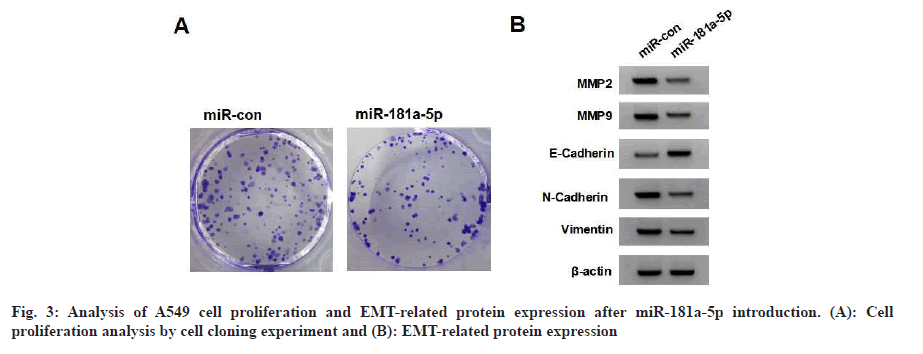
Following treatment with different concentrations of SSCN, there was an increase in miR-181a-5p content (Table 3). MiR-181a-5p mimics increased miR-181a-5p and E-cadherin expression and decreased cell viability, the number of cell clones, migrated and invaded cells and MMP-2, MMP-9, N-cadherin and vimentin expression (fig. 3 and Table 4).
| 7 | miR-181a-5p |
|---|---|
| NC | 1.00±0.08 |
| 10 μg/ml SSCN | 1.52±0.12* |
| 20 μg/ml SSCN | 2.13±0.16* |
| 40 μg/ml SSCN | 2.61±0.18* |
| F | 225.761 |
| p | 0.000 |
Note: Compared with NC, *p<0.05
Table 3: Effects Of SSCN on miR-181A-5P Expression
| Groups | miR-181a-5p | A value | Cell colony number | MMP-2 | MMP-9 | Cell migration number | Cell invasion number | E-Cadherin | N-Cadherin | Vimentin |
|---|---|---|---|---|---|---|---|---|---|---|
| miR-con | 1.00±0.10 | 1.048±0.10 | 135±9.76 | 0.87±0.07 | 0.74±0.07 | 319±21 | 181±13 | 0.32±0.03 | 0.74±0.07 | 0.93±0.09 |
| miR-181a-5p | 2.31±0.19* | 0.576±0.05* | 67±5.02* | 0.39±0.03* | 0.34±0.03* | 161±11* | 85±7* | 0.71±0.06* | 0.38±0.03* | 0.45±0.04* |
| t | 18.304 | 12.665 | 18.587 | 18.908 | 15.757 | 19.994 | 19.506 | 17.441 | 14.181 | 14.621 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: Compared with miR-control, *p<0.05
Table 4: Effects of miR-181a-5p on the Proliferation and EMT of A549 Cells
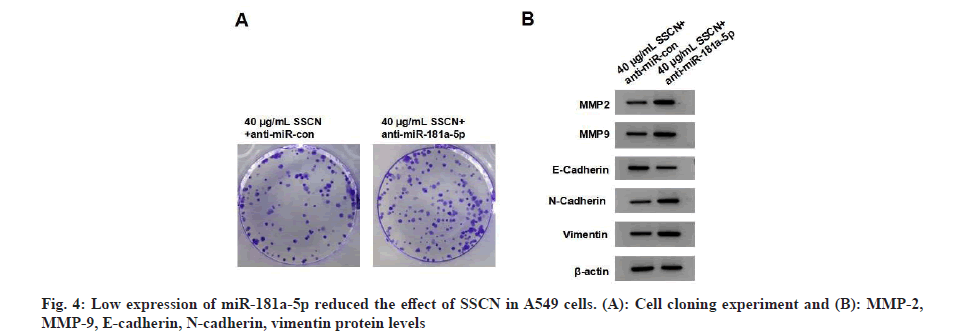
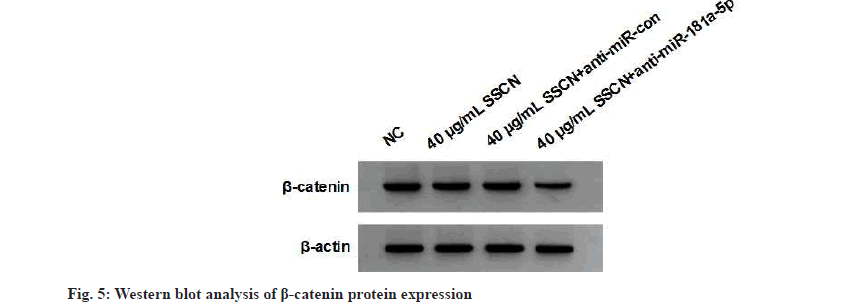
When compared to the 40 μg/ml SSCN+anti-miRcontrol group, the 40 μg/ml SSCN+anti-miRFig 181a-5p group demonstrated a decrease in miR- 181a-5p expression, an increase in cell viability, the number of cell clones, migrated and invaded cells, and MMP-2, MMP-9, N-cadherin and vimentin expression and a decrease in E-cadherin expression (fig. 4 and Table 5). SSCN treatment exhibited a decrease in β-catenin expression, whereas the effect was relieved after anti-miR- 181a-5p treatment (fig. 5 and Table 6).
| Groups | miR-181a-5p | A value | Cell colony number | MMP-2 | MMP-9 | Cell migration number | Cell invasion number | E-Cadherin | N-Cadherin | Vimentin |
|---|---|---|---|---|---|---|---|---|---|---|
| 40 μg/ml SSCN+anti-miR-contrrol | 1.00±0.08 | 0.488±0.04 | 64±5.10 | 0.42±0.03 | 0.38±0.03 | 161±11 | 73±6 | 0.83±0.07 | 0.36±0.03 | 0.47±0.04 |
| 40 μg/ml SSCN+anti-miR-181a-5p | 0.43±0.04* | 0.986±0.08* | 127±9.16* | 0.86±0.07* | 0.75±0.07* | 286±21* | 167±12* | 0.38±0.03* | 0.76±0.07* | 0.95±0.09* |
| t | 19.118 | 16.703 | 18.027 | 17.332 | 14.575 | 15.818 | 21.019 | 17.726 | 15.757 | 14.621 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: Compared with 40 μg/ml SSCN+anti-miR-control, *p<0.05
Table 5: miR-181a-5p Knockdown reduced the effect of SSCN in A549 Cells
| Groups | β-catenin |
|---|---|
| NC | 0.85±0.08 |
| 40 μg/ml SSCN | 0.54±0.05* |
| 40 μg/ml SSCN+anti-miR-con | 0.52±0.05 |
| 40 μg/ml SSCN+anti-miR-181a-5p | 0.93±0.09# |
| F | 81.846 |
| p | 0.000 |
Note: Compared with NC group, *p<0.05 and compared with 40 μg/ml SSCN, #p<0.05
Table 6: Western BLOT analysis of Β-Catenin Protein Expression
Steroid saponins are important active components found in natural products and have a wide range of anti-tumor effects[16,17]. It has been reported that extracts from night jasmine flowers can effectively hinder cervical cancer cell proliferation and induce apoptosis[18]. The n-butanol extract from night jasmine leaves can significantly inhibit gastric cancer cell growth through the components C6 as well as C7[19]. We investigated the effects of SSCN on lung cancer cells by treating A549 cells with different concentrations of SSCN. There is a reduction in the number of cell clones and a decrease in migration and invasion. MMP-2 and MMP-9 expression were also reduced, indicating that SSCN inhibited lung cancer cell growth and motility. EMT is a fundamental biological process by which epithelial cells acquires a more motile, fibroblast-like phenotype that allows them to migrate and invade surrounding tissues. E-cadherin is considered an epithelial cell marker, while other two proteins including N-cadherin and vimentin are considered mesenchymal marker proteins[20].
We discovered that SSCN inhibited EMT process in lung cancer cells by analyzing EMT-related proteins.
As reported, miR-181a-5p, a microRNA showed a low expression in lung cancer sufferers[21]. In addition, NEAT1 can promote lung cancer cell proliferation through miR-181a-5p[22]. We discovered its overexpression reduced cell viability, the number of cell clones and migration and invasion of A549 cells. Moreover, SSCN increased its expression in A549 cells, suggesting that it interacts with miR-181a-5p to modulate lung cancer progression. Wnt/β-catenin signaling pathway was involved in cancer progression. Sanguinarine inactivated the pathway to inhibit lung adenocarcinoma cell motility[23]. Total flavonoids inhibit the growth of lung cancer by regulating the pathway[24], indicating that traditional Chinese medicine can influence lung cancer by modulating Wnt/β-catenin signaling pathway. Herein, SSCN displayed its ability to inhibit the Wnt/β-catenin signaling pathway. Further results confirmed inhibiting miR-181a-5p expression were able to reduce the effects of SSCN on lung cancer cell malignancy and β-catenin expression. Here are some possible mechanisms responsible for Wnt/β- catenin signaling pathway in lung cancer:
The pathway can induce EMT in lung cancer cells. Activation of the pathway can down regulate E-cadherin, a key epithelial marker and upregulate N-cadherin[25], promoting an invasive and migratory phenotype in lung cancer cells.
The pathway maintains the stem cell-like properties of Cancer Stem Cells (CSCs) in lung cancer[26,27]. Activation of the pathway in CSCs may enhance self-renewal capacity and promote tumor initiation and progression. These data demonstrate the Wnt/β-catenin signaling pathway was involved in the regulation of the SSCN/miR-181a-5p in lung cancer cell malignancy. In summary, SSCN inhibits lung cancer cell EMT through the miR- 181a-5p/Wnt/β-catenin signaling pathway.
Conflict of interests:
The authors declared no conflict of interests.
References
- Abu Rous F, Singhi EK, Sridhar A, Faisal MS, Desai A. Lung cancer treatment advances in 2022. Cancer Invest 2023;41(1):12-24.
[Crossref] [Google Scholar] [PubMed]
- Lee JH, Saxena A, Giaccone G. Advancements in small cell lung cancer. Semin Cancer Biol 2023;93:123-8.
[Crossref] [Google Scholar] [PubMed]
- Wei Z, Chen J, Zuo F, Guo J, Sun X, Liu D, et al. Traditional Chinese Medicine has great potential as candidate drugs for lung cancer: A review. J Ethnopharmacol 2023;300:115748.
[Crossref] [Google Scholar] [PubMed]
- Cheng W, Chen X. Research progress on mechanism of traditional Chinese medicine against non-small cell lung cancer. Chin J Exp Tradit Med Formula 2020;26(24):234-41.
- Lu H, Zhong Z, Zhao S. Study on the inhibition of Steroid saponins from Cestrum noctumum Linn on human hepatocellular carcinoma using nude mice. Lishizhen Med Mater Med Res 2010;21(4):1704-5.
- Luo F, Zhao S, Zhong Z. Inhibitory effect of steroid saponins from the flowers of Cestrum noctumum Linn on K562 cell proliferation and its regulatory role in thePI3K/AKt pathway. Pract J Cancer 2013;28(6):583-6.
- Zhao S, Nong Z, Ye H. Mechanism investigation of three Steroid saponins from Cestrum noctumum Linn-mediated inhibitory effect on human hepatocellular carcinoma cell proliferation. Chin J Exp Tradit Med Formula 2013;19(7):212-6.
- Nong Z, Zhao S, Zhang M. Investigation of Steroid saponins from Cestrum noctumum Linn on human hepatocellular carcinoma using nude mice. Chin J Exp Tradit Med Formula 2013;19(17):239-43.
- Iacomino G. miRNAs: The road from bench to bedside. Genes 2023;14(2):314.
- Chakrabortty A, Patton DJ, Smith BF, Agarwal P. miRNAs: Potential as biomarkers and therapeutic targets for cancer. Genes 2023;14(7):1375.
[Crossref] [Google Scholar] [PubMed]
- Frydrychowicz M, Kuszel Ł, Dworacki G, Budna-Tukan J. MicroRNA in lung cancer-a novel potential way for early diagnosis and therapy. J Appl Genet 2023;64(3):459-77.
[Crossref] [Google Scholar] [PubMed]
- Li L, Ye D, Liu L, Li X, Liu J, Su S, et al. Long noncoding RNA SNHG7 accelerates proliferation, migration and invasion of non-small cell lung cancer cells by suppressing miR-181a-5p through AKT/mTOR signaling pathway. Cancer Manag Res 2020;12:8303-12.
[Crossref] [Google Scholar] [PubMed]
- Ma Z, Qiu X, Wang D, Li Y, Zhang B, Yuan T, et al. MiR-181a-5p inhibits cell proliferation and migration by targeting Kras in non-small cell lung cancer A549 cells. Acta Biochim Biophys Sin 2015;47(8):630-8.
[Crossref] [Google Scholar] [PubMed]
- Zheng Q, Xu H, Zhang X, Wang H, Wang J, Wang C, et al. Expression and significance of Wnt/β-catenin signaling pathway in vitro natural degenerationmodel of endplate chondrocytes in rats. Zhonghua Yi Xue Za Zhi 2014;94(31):2464-7.
[Crossref] [Google Scholar] [PubMed]
- Huang JQ, Wei FK, Xu XL, Ye SX, Song JW, Ding PK, et al. SOX9 drives the epithelial–mesenchymal transition in non-small-cell lung cancer through the Wnt/β-catenin pathway. J Transl Med 2019;17(1):1-3.
[Crossref] [Google Scholar] [PubMed]
- Lin J, Wang W, Zou H. Biosynthesis and physiological activity of phytosteroid saponins. Fujian Agric Sci Technol 2017;1(10):57-9.
- Liu X, Yu J, Liu M. Research progress on the biological activity of steroid saponins in recent 10 years. Chin Med 2015;40(13):2518-23.
- Lu H, Qin Q, Dai C. Effects of the flower extract of Cestrum noctumum Linn on proliferation and apoptosis of cervical cancer cells. World Latest Med Inform 2020;20(68):269-78.
- Wu D, Liao W, Huang J. Effects of C6 and C7 in n-butanol extract from the leaves of Cestrum noctumum Linn on proliferation and apoptosis of human gastric cancer cell SGC7901. China Pharm 2015;26(31):4342-4.
- Yu K, Li H, Zhang L. The role and mechanism of SOX4-mediated EMT in lung cancer. Acta Univ Med Anhui 2020;55(2):182-9.
- Xue WX, Zhang MY, Li R, Liu X, Yin YH, Qu YQ. Serum miR-1228-3p and miR-181a-5p as noninvasive biomarkers for non-small cell lung cancer diagnosis and prognosis. Biomed Res Int 2020;2020:9601876.
[Crossref] [Google Scholar] [PubMed]
- Li S, Yang J, Xia Y, Fan Q, Yang KP. Long noncoding RNA NEAT1 promotes proliferation and invasion via targeting miR-181a-5p in non-small cell lung cancer. Oncol Res 2018;26(2):289.
[Crossref] [Google Scholar] [PubMed]
- Jia YA, Min CH, Minghua LI, Jingru QI. Effects of sanguinarine on invasion, migration and Wnt/β-catenin signaling pathway of lung adenocarcinoma cells. Cancer Res Prev Treat 2019;46(12):1057-61.
- Han L, Fang S, Li G, Wang M, Yu R. Total flavonoids suppress lung cancer growth via the COX-2-mediated Wnt/β-catenin signaling pathway. Oncol Lett 2020;19(3):1824-30.
[Crossre] [Google Scholar] [PubMed]
- Yang S, Liu Y, Li MY, Ng CS, Yang SL, Wang S, et al. FOXP3 promotes tumor growth and metastasis by activating Wnt/β-catenin signaling pathway and EMT in non-small cell lung cancer. Mol Cancer 2017;16:1-2.
[Crossref] [Google Scholar] [PubMed]
- Shu X, Chen M, Liu SY, Yu L, Sun LX, Sun LC, et al. Palladin promotes cancer stem cell‐like properties in lung cancer by activating Wnt/Β‐Catenin signaling. Cancer Med 2023;12(4):4510-20.
[Crossref] [Google Scholar] [PubMed]
- Tung CH, Wu JE, Huang MF, Wang WL, Wu YY, Tsai YT, et al. Ubiquitin-specific peptidase 5 facilitates cancer stem cell-like properties in lung cancer by deubiquitinating β-catenin. Cancer Cell Int 2023;23(1):207.
[Crossref] [Google Scholar] [PubMed]