- *Corresponding Author:
- Junling Liu
Department of Geriatrics, Linyi Geriatric Hospital, Linyi, Shandong 221332, China
E-mail: lw603@njmu.edu.cn
| This article was originally published in a special issue, “Drug Development in Biomedical and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2023:85(5) Spl Issue “67-73” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Osteocytes sense mechanical forces in the extracellular environment, and regulate bone repair and regeneration by converting mechanical stimuli into biochemical signals. However, the underlying mechanism by which osteocytes orchestrate bone formation under conditions of mechanical force remains unclear. This research tested the hypothesis that transcriptional co-activator with PDZ-binding motif plays a key role in regulating osteocyte secretion factors receptor activator of nuclear factor-kappa b ligand and osteoprotegerin under conditions of mechanical stretching. To evaluate how transcriptional co-activator with PDZ-binding motif affected osteocyte secretion, we applied mechanical stretching force to MLO-Y4 cells. Pathway-related specific genes and proteins were evaluated using reverse transcription-polymerase chain reaction, Western blot and immunofluorescence. An enzyme-linked immunoassay was used to detect the secretion of soluble receptor activator of nuclear factor-kappa b ligand and osteoprotegerin. Mechanically induced transcriptional co-activator with PDZ-binding motif nuclear translocations promoted the release of osteoprotegerin and inhibited the expression and secretion of receptor activator of nuclear factor-kappa b ligand in osteocytes. Mechanical stretch forces signal resulting transcriptional co-activator with PDZ-binding motif mediated receptor activator of nuclear factor-kappa b ligand and osteoprotegerin expression and secretion. These results imply that transcriptional co-activator with PDZbinding motif may represent a therapeutic target for bone defect restoration.
Keywords
Osteocytes, osteogenesis, transcriptional co-activator with PDZ-binding motif, receptor activator of nuclear factor-kappa b ligand, dendritic cells
As osteocytes are terminally differentiated osteoblasts embedded in bone matrix[1], and function as major mechanical stimuli sensors and transducers[2,3]. However, the majority of prior research has focused on biological changes in osteocytes under conditions of fluid Flow Shear Stress (FSS)[4,5]. Only a few studies have examined whether osteocytes respond to other different forms of mechanical stimulation, such as mechanical stretching force. As has been shown[6], osteocytes are responsible for sending signals to effector cells, including osteoblasts and osteoclasts, in order to regulate bone resorption and formation. Several molecules, including Fibroblast Growth Factor 23 (FGF23), Receptor Activator of Nuclear Factor-Kappa B Ligand (RANKL) and sclerostin are secreted by osteocytes to orchestrate osteoclastic bone resorption, osteoblastic bone remodeling and mineral homeostasis[7]. Osteocytes appear to be the major source of RANKL during bone formation in vivo[8].
The Hippo pathway reportedly appears to react to extracellular stimuli, and alters the nuclear localization of transcription co-activators. As key downstream effectors of the Hippo pathway, Yes-Associated Protein (YAP) and transcriptional coactivator with Transcriptional Co-Activator with PDZ-Binding Motif (TAZ) are involved in the biological functions and gene regulation of the mammalian mechanical signal transduction pathways[9]. Recent research has linked the nuclear transport of TAZ to mechanical tension stimulation[10], suggesting a regulatory role for Hippo-TAZ pathway during responses to stretch force[11]. The Hippo signaling molecule TAZ and calcium-related ion channel have also been reported to be critical effectors affecting the metabolism and functions of Dendritic Cells (DCs), which experience mechanical tension[12]. Thus, we hypothesized that osteocytes perceive stretching forces and secreted molecules related to Hippo-TAZ signaling.
Here, we demonstrated that stretching induced osteocytes promote the RANKL secretion via Hippo- TAZ signaling.
Materials and Methods
Cell culture:
The osteocyte-like cell line, Murine Long bone Osteocyte-Y4 (MLO-Y4) cells were cultured in Alpha (α)-minimum essential medium (Gibco) which contained 5 % calf serum (Gibco), 5 % fetal bovine serum (ScienCell, United States of America (USA)), and 1 % streptomycin-penicillin (Hyclone, USA). All experiments were performed using cells between passage 3 and 5, and repeated at least triple. All cells were incubated at 37°, 5 % Carbon dioxide (CO2) in standard humidifed CO2 incubators.
Mechanical stretching load:
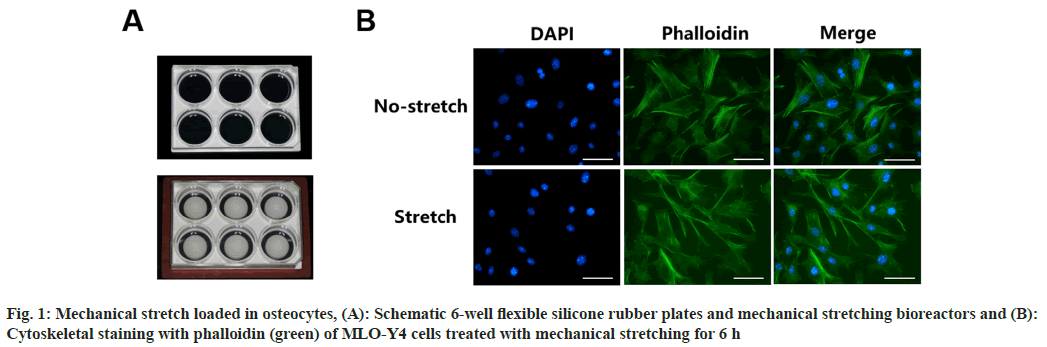
MLO-Y4 cells were seeded in 6-well flexible silicone rubber BioFlex™ plates. After the cells reached about 70 % confluence, the Flexcell® FX-5000™ Tension Plus™ unit (Flexcell International Corp.) was used to apply cyclic sinusoidal continuous mechanical tension was applied (2 % elongation, 0.5 Hz). The control group was incubated under unanimous conditions without mechanical load.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR):
The messenger Ribonucleic Acid (mRNA) expression level was detected by applied Biosystems 7900 RTPCR system using SYBR Green Master Mix. The primer sequences used were summarized in Table 1. The stability of Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) was analyzed, and all data were normalized to GAPDH expression. Quantification of qPCR results was carried out by the 2-ΔΔCT method.
| Gene | Forward primer sequence (5′-3′) | Reverse primer sequence (5′-3′) |
|---|---|---|
| RANKL | CAGCATCGCTCTGTTCCTGTA | CTGCGTTTTCATGGAGTCTCA |
| OPG | AGGCTCGGGTCTTTAACGAAA | CTTGATGGTCAAATAGCACATGC |
| GAPDH | AGGTCGGTGTGAACGGATTTG | TGTAGACCATGTAGTTGAGGTCA |
Table 1: Primer Sequences by RT-PCR
Western blot:
Total protein was isolated using the KeyGEN BioTECH Whole Cell Lysis Assay. The nuclear and cytoplasmic protein of cells was fractionated with NE-PER® nuclear and cytoplasmic extraction reagents (Thermo Fisher Scientific). The total, cytoplasmic and nuclear cell lysate were determined using the Bradford protein assay kit (Beyotime, China). The blocked Polyvinylidene Difluoride (PVDF) membranes were then incubated with the following primary antibodies; Anti-GAPDH (Beyotime, AG019), anti-TAZ (CST, 4883) and p-TAZ (CST, 59971), anti-lamin B1 (Abcam, 16048), anti-Osteoprotegerin (OPG) (Abcam, 9986) and anti-RANKL (Abcam, 9957). Tanon 5200 Chemiluminescence imaging system with Horseradish Peroxidase (HRP) substrate (Millipore) were used to detect the protein bands. The amount of protein expression was compared after normalization with GAPDH and lamin B1.
Enzyme-Linked Immunoassay (ELISA):
The ELISA kits (RayBio Technology) was used to quantify the soluble TRANCE (RANKL) and OPG levels. All procedures were performed in full accordance with the manufacturer's instructions.
Immunofluorescence:
The pretreated MLO-Y4 cells were fixed with 4 % paraformaldehyde for 30 min. 0.3 % Triton X-100 (Beyotime, China) was used for 15 min to permeabilize the cells. Goat serum was used for 60 min to block the cells. The cells were incubated with the primary antibody against TAZ (1:100, cytoskeleton, 4883) at 4° overnight and the secondary antibody for 1 h. The nucleus was stained with 4′,6-Diamidino-2-Phenylindole (DAPI) (Invitrogen, R37605), while the cytoskeleton was stained with phalloidin (cytoskeleton, USA). A Leica fluorescence microscope was used to observe TAZ expression.
Data presentation and statistical analysis:
Data was statistically analyzed through Statistical Package for the Social Sciences (SPSS) 22.0. All data in the figure were expressed as mean±standard deviation. One-way Analysis of Variance (ANOVA) or student's t test were used to evaluate the parameters as appropriate, with p<0.05 being statistically significant.
Results and Discussion
To explore the biological response to mechanical force in osteocytes, we subjected MLO-Y4 cells to cyclic mechanical stretching using 6-well flexible silicone rubber plates (fig. 1A) for 6 h. Cytoskeleton staining showed that after the cells were stretched, the cytoskeleton unfolded and arranged in an orderly manner. The cells changed from random orientation to unidirectional regular arrangement as shown in fig. 1B.
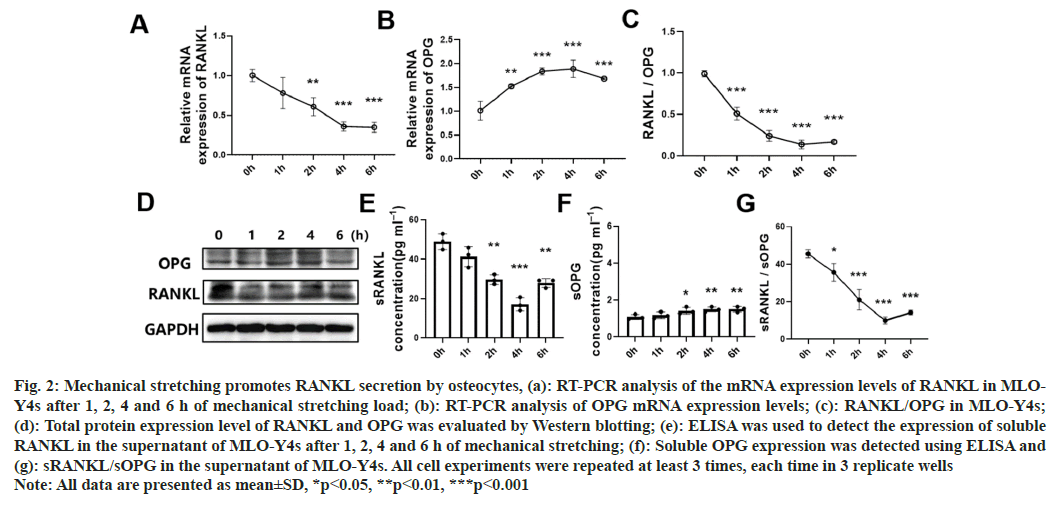
Previous studies have shown that osteocytes are responsible for sending signals to effector cells during bone remodeling in the body and are the major source of RANKL secretion in vivo[3,7]. To evaluate whether mechanical stretching loads affected osteocytemediated Bone Marrow-Mesenchymal Stem/Stromal Cells (BM-MSC) osteogenesis through secretory proteins, we measured the expression and secretion of OPG and RANKL in MLO-Y4 cells. RT-PCR results showed that stretching load could reduce the mRNA expression of RANKL and increase the expression of OPG (fig. 2A-fig. 2C). Similarly, Western blotting results showed that the total protein expression of RANKL was decreased, and the total protein expression of OPG was increased after stretching for 1, 2, 4 and 6 h (fig. 2D). In addition, ELISA results revealed that mechanical stretching could reduce the secretion of soluble RANKL and elevate the release of soluble OPG (fig. 2E-fig. 2G). RANKL exists in several different forms inside and outside the cell[13]. The secreting form of RANKL (sRANKL) can be detected by ELISA. The overall expression of RANKL can be measured by Western blot. Since the main factor in our research was sRANKL, the main conclusion was summarized based on ELISA. Western blot demonstrated the membrane-located and intracellular RANKL, which might result in a peak ahead of time.
Fig. 2: Mechanical stretching promotes RANKL secretion by osteocytes, (a): RT-PCR analysis of the mRNA expression levels of RANKL in MLOY4s after 1, 2, 4 and 6 h of mechanical stretching load; (b): RT-PCR analysis of OPG mRNA expression levels; (c): RANKL/OPG in MLO-Y4s; (d): Total protein expression level of RANKL and OPG was evaluated by Western blotting; (e): ELISA was used to detect the expression of soluble RANKL in the supernatant of MLO-Y4s after 1, 2, 4 and 6 h of mechanical stretching; (f): Soluble OPG expression was detected using ELISA and (g): sRANKL/sOPG in the supernatant of MLO-Y4s. All cell experiments were repeated at least 3 times, each time in 3 replicate wells Note: All data are presented as mean±SD, *p<0.05, **p<0.01, ***p<0.001
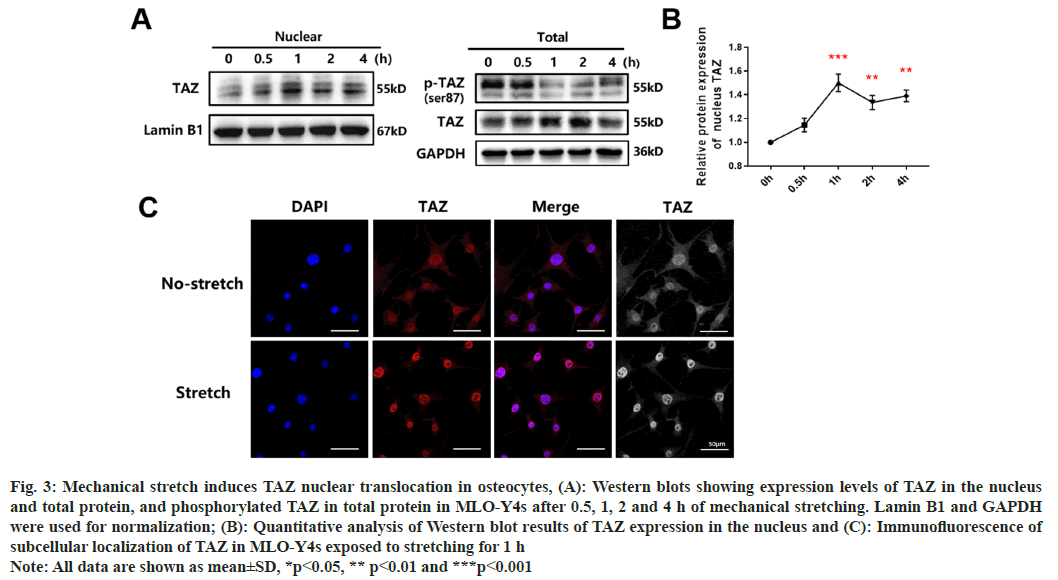
To investigate the molecular signaling pathways that underlie cell-to-cell interaction, we examined potential transcription factors that could explain the regulation of protein secretion. TAZ has been reported to be associated with stretching-related bone mass increase[14]. Moreover, previous research has revealed that TAZ modifies the behavior of cells exposed to mechanical stress, including stretching, through changes in localization and phosphorylation[15,16]. Since the previous studies indicating that the nuclear localization of TAZ is mediated by phosphorylation reactions within the cytoplasm[17], the expression levels of TAZ and phosphorylated TAZ in the nucleus and total protein levels of MLO-Y4 cells exposed to mechanical stretching were measured. Western blots showed that the expression of nuclear and total TAZ was significantly increased and that the expression of total phosphorylated TAZ decreased under conditions of mechanical stretching (fig. 3A). Quantification of nuclear TAZ protein revealed that enhanced nuclear translocation of TAZ reached its highest levels at 1 h (fig. 3B). The subcellular localization of TAZ was further measured using immunofluorescence. Results showed that, after applying mechanical stretching for 1 h, a considerable amount of cytoplasmic TAZ was translocated to the nucleus (fig. 3C). The above results imply that mechanical stretching loads could up-regulate the total expression of TAZ and substantially promote its nuclear translocation.
Fig. 3: Mechanical stretch induces TAZ nuclear translocation in osteocytes, (A): Western blots showing expression levels of TAZ in the nucleus
and total protein, and phosphorylated TAZ in total protein in MLO-Y4s after 0.5, 1, 2 and 4 h of mechanical stretching. Lamin B1 and GAPDH
were used for normalization; (B): Quantitative analysis of Western blot results of TAZ expression in the nucleus and (C): Immunofluorescence of
subcellular localization of TAZ in MLO-Y4s exposed to stretching for 1 h
Note: All data are shown as mean±SD, *p<0.05, ** p<0.01 and ***p<0.001
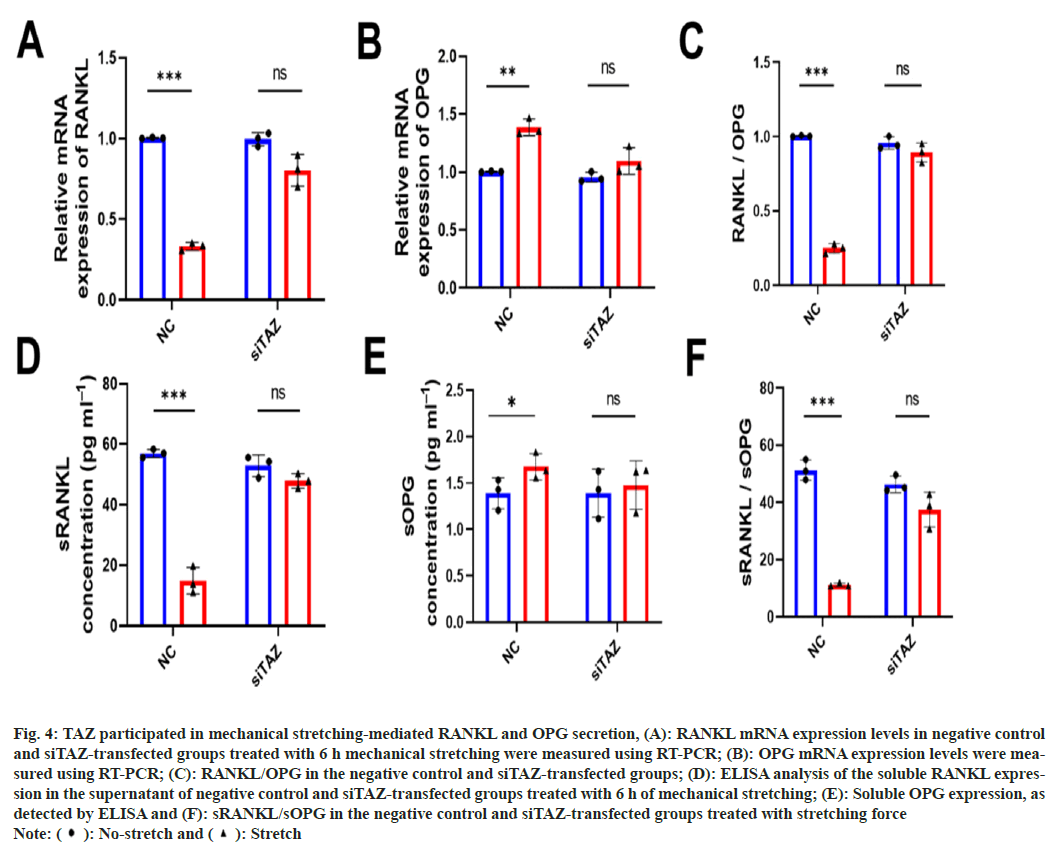
Next, to evaluate whether TAZ affected mechanical stretching-mediated RANKL and OPG secretion, we treated negative controls and siTAZ-transfected MLO-Y4s with stretching force. mRNA expression levels of RANKL and OPG were detected using RT-PCR, and results showed that the expression of RANKL did not decrease after stretching load in siTAZ-transfected MLO-Y4 cells (fig 4A-fig. 4C). The release of soluble RANKL and OPG was also simultaneously measured using ELISA. As expected, secretion expression was consistent with RT-PCR results as shown in fig. 4D-fig. 4F. These results illustrate that mechanically-induced TAZ nuclear translocation could promote the release of OPG and inhibit the secretion of RANKL in osteocytes.
Fig. 4: TAZ participated in mechanical stretching-mediated RANKL and OPG secretion, (A): RANKL mRNA expression levels in negative control
and siTAZ-transfected groups treated with 6 h mechanical stretching were measured using RT-PCR; (B): OPG mRNA expression levels were measured
using RT-PCR; (C): RANKL/OPG in the negative control and siTAZ-transfected groups; (D): ELISA analysis of the soluble RANKL expression
in the supernatant of negative control and siTAZ-transfected groups treated with 6 h of mechanical stretching; (E): Soluble OPG expression, as
detected by ELISA and (F): sRANKL/sOPG in the negative control and siTAZ-transfected groups treated with stretching force
Note: ( ): No-stretch and (
): No-stretch and ( ): Stretch
): Stretch
Cellular mechanical transduction activities triggered by mechanical stimuli, including fluid shear force, compression and tension, regulate the maintenance of bone homeostasis, bone remodeling and tissue repair[18]. As the most abundant resident cells in bone, the role of osteocytes in bone homeostasis and metabolism has aroused significant interest. It has been suggested that osteocytes sense mechanical cues in the extracellular environment and transfer them into biochemical signals to regulate bone repair and regeneration[19,20]. However, how they might perceive mechanical loads is still a subject of ongoing research. Osteocytes produce extracellular signals to regulate other cells, including osteoblasts and osteoclast, to mediate bone formation, bone resorption and bone renewal[21], but how osteocytes affect other cells under mechanical stretching loads has not yet been elucidated. Our research aimed to uncover the mechanism underlying these processes.
Here, we observed decreased RANKL secretion from stretch-exposed MLO-Y4 cells. In vitro research revealed that fluid shear force induces enhanced bone formation and reduction of bone resorption by promoting Wnt1 expression via the activation YAP1 and TAZ in osteocyte-like MLO-Y4 cells[14]. Therefore, we considered it very important to explore the in-depth mechanism of bone cell sensing of mechanical stretching. A previous study by Sasaki revealed that mechanical stretch activates downstream AKT signaling, which then down-regulates the sclerostin expression and leads to decreased bone formation[14]. Here, we applied mechanical stretching loads to osteocyte-like MLO-Y4 cells and found that the osteocyte-like MLO-Y4 cells responded to cyclic mechanical stretching by decreasing RANKL secretion. TAZ regulates the RANKL secretion by nuclear transport after dephosphorylation[11]. Monitoring the expression of TAZ in nucleus helped us detect mechanical force related signal transport. The overall phosphorylation level of TAZ was detected to determine whether mechanical stretching force regulating the dephosphorylation or the nuclear transport process. Our results revealed that p-TAZ was downregulated and TAZ was slightly upregulated in a view of whole protein level. TAZ translocation occurs within osteocytes from the cytoplasm to the nucleus after mechanical stretching force loading, driving the secretion of downstream signaling molecules.
Since RANKL/OPG acts as a critical signal axis for osteocytes by regulating bone homeostasis[3,8], we measured the secretion levels of soluble RANKL and OPG in the supernatant of the MLO-Y4 cells subjected to mechanical stretching forces. RANKL can be secreted by shedding the extracellular domain from the cytomembrane into the culture medium[6]. Li’s results indicated that oscillating fluid flow stimulation of osteocytes reduces the RANKL/ OPG mRNA ratio[22]. Similarly, we observed a down-regulation of both the RANKL/OPG mRNA and secretion ratios. We subsequently verified that knockdown of TAZ inhibits the expression and secretion of RANKL and reduces the RANKL/ OPG expression ratio. The TAZ signaling pathway regulates RANKL secretion. Notably, it has been reported that compressive forces can increase RANKL expression and secretion, in osteocytes and further induce osteoclastogenesis, which is contrary to our results. These differences may have arisen because of discrepancies in the forms of mechanical force load[3,23].
Taken together, we determined that TAZ regulates osteocyte secretion mechanical stretch force. Mechanical tension can activate TAZ nuclear translocation, thereby reducing RANKL and the RANKL/OPG intracellular expression and extracellular secretion ratios in osteocytes. In light of the important role of TAZ in transmitting mechanical forces and regulating the bone formation, we suspect that TAZ may be a potential target for bone remodeling and regulation. We hope to discover more effective interventions for TAZ to accelerate bone reconstruction. In the future, we will attempt to accelerate the treatment process by targeting drugs that activate these pathways, thereby achieving bone synthesis metabolism.
Mechanically stretching force induced TAZ nuclear translocation can promote the release of OPG in bone cells and inhibit the intracellular expression and extracellular secretion of RANKL. TAZ may represent the therapeutic target for bone defect repair.
Conflict of interests:
The authors declared no conflict of interests.
References
- Bonewald LF. The amazing osteocyte. J Bone Miner Res 2011;26(2):229-38.
[Crossref] [Google Scholar] [PubMed]
- Li MC, Chow SK, Wong RM, Qin L, Cheung WH. The role of osteocytes-specific molecular mechanism in regulation of mechanotransduction–A systematic review. J Orthop Transl 2021;29:1-9.
[Crossref] [Google Scholar] [PubMed]
- Li W, Zhao J, Sun W, Wang H, Pan Y, Wang L, et al. Osteocytes promote osteoclastogenesis via autophagy-mediated RANKL secretion under mechanical compressive force. Arch Biochem Biophys 2020;694:108594.
[Crossref] [Google Scholar] [PubMed]
- Deepak V, Kayastha P, McNamara LM. Estrogen deficiency attenuates fluid flow-induced [Ca2+]i oscillations and mechanoresponsiveness of MLO-Y4 osteocytes. FASEB J 2017;31(7):3027-39.
[Crossref] [Google Scholar] [PubMed]
- Liao C, Cheng T, Wang S, Zhang C, Jin L, Yang Y. Shear stress inhibits IL-17A mediated induction of osteoclastogenesis via osteocyte pathways. Bone 2017;101:10-20.
[Crossref] [Google Scholar] [PubMed]
- Lau E, Al-Dujaili S, Guenther A, Liu D, Wang L, You L. Effect of low-magnitude, high-frequency vibration on osteocytes in the regulation of osteoclasts. Bone 2010;46(6):1508-15.
[Crossref] [Google Scholar] [PubMed]
- Chen H, Senda T, Kubo KY. The osteocyte plays multiple roles in bone remodeling and mineral homeostasis. Med Mol Morphol 2015;48(2):61-8.
[Crossref] [Google Scholar] [PubMed]
- Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med 2011;17(10):1231-4.
[Crossref] [Google Scholar] [PubMed]
- Hong W, Guan KL. The YAP and TAZ transcription co-activators: Key downstream effectors of the mammalian Hippo pathway. Semin Cell Dev Biol 2012;23(7):785-93.
[Crossref] [Google Scholar] [PubMed]
- Li W, Zhao J, Wang J, Sun L, Xu H, Sun W, et al. ROCK-TAZ signaling axis regulates mechanical tension-induced osteogenic differentiation of rat cranial sagittal suture mesenchymal stem cells. J Cell Physiol 2020;235(9):5972-84.
[Crossref] [Google Scholar] [PubMed]
- Cai X, Wang KC, Meng Z. Mechanoregulation of YAP and TAZ in cellular homeostasis and disease progression. Front Cell Dev Biol 2021;9:673599.
[Crossref] [Google Scholar] [PubMed]
- Chakraborty M, Chu K, Shrestha A, Revelo XS, Zhang X, Gold MJ, et al. Mechanical stiffness controls dendritic cell metabolism and function. Cell Rep 2021;34(2):108609.
[Crossref] [Google Scholar] [PubMed]
- Ikeda T, Kasai M, Suzuki J, Kuroyama H, Seki S, Utsuyama M, et al. Multimerization of the receptor activator of nuclear factor-κB ligand (RANKL) isoforms and regulation of osteoclastogenesis. J Biol Chem 2003;278(47):47217-22.
[Crossref] [Google Scholar] [PubMed]
- Li X, Han L, Nookaew I, Mannen E, Silva MJ, Almeida M, et al. Stimulation of Piezo1 by mechanical signals promotes bone anabolism. Elife 2019;8:e49631.
[Crossref] [Google Scholar] [PubMed]
- Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, et al. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 2013;154(5):1047-59.
[Crossref] [Google Scholar] [PubMed]
- Byun MR, Jeong H, Bae SJ, Kim AR, Hwang ES, Hong JH. TAZ is required for the osteogenic and anti-adipogenic activities of kaempferol. Bone 2012;50(1):364-72.
[Crossref] [Google Scholar] [PubMed]
- Kanai F, Marignani PA, Sarbassova D, Yagi R, Hall RA, Donowitz M, et al. TAZ: A novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. EMBO J 2000;19(24):6778-91.
[Crossref] [Google Scholar] [PubMed]
- Ren L, Yang P, Wang Z, Zhang J, Ding C, Shang P. Biomechanical and biophysical environment of bone from the macroscopic to the pericellular and molecular level. J Mech Behav Biomed Mater 2015;50:104-22.
[Crossref] [Google Scholar] [PubMed]
- Moriishi T, Fukuyama R, Ito M, Miyazaki T, Maeno T, Kawai Y, et al. Osteocyte network; a negative regulatory system for bone mass augmented by the induction of RANKl in osteoblasts and Sost in osteocytes at unloading. PloS One 2012;7(6):e40143.
[Crossref] [Google Scholar] [PubMed]
- Li X, Kordsmeier J, Xiong J. New advances in osteocyte mechanotransduction. Curr Osteoporos Rep 2021;19(1):101-6.
[Crossref] [Google Scholar] [PubMed]
- Cabahug-Zuckerman P, Frikha-Benayed D, Majeska RJ, Tuthill A, Yakar S, Judex S, et al. Osteocyte apoptosis caused by hindlimb unloading is required to trigger osteocyte RANKL production and subsequent resorption of cortical and trabecular bone in mice femurs. J Bone Mineral Res 2016;31(7):1356-65.
[Crossref] [Google Scholar] [PubMed]
- Li J, Rose E, Frances D, Sun Y, You L. Effect of oscillating fluid flow stimulation on osteocyte mRNA expression. J Biomech 2012;45(2):247-51.
[Crossref] [Google Scholar] [PubMed]
- Yashima Y, Kaku M, Yamamoto T, Izumino J, Kagawa H, Ikeda K, et al. Effect of continuous compressive force on the expression of RANKL, OPG and VEGF in osteocytes. Biomed Res 2020;41(2):91-9.
[Crossref] [Google Scholar] [PubMed]