- *Corresponding Author:
- Ramadevi Korni
Department of Pharmaceutical Technology, Yalamarty Pharmacy College, Tarluwada, Visakhapatnam-530 052, India
E-mail: ramakalyank@gmail.com
| Date of Submission | 24 March 2017 |
| Date of Revision | 08 March 2018 |
| Date of Acceptance | 13 September 2018 |
| Indian J Pharm Sci 2018;80(6):1003-1010 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The present work was aimed at enhancing the dissolution rate of olanzapine, an antipsychotic agent using the liquisolid technique. Liquisolid system is an innovative technique used for enhancing dissolution rate and bioavailability of poorly soluble drugs. A mathematical model was utilized to formulate liquisolid tablets. Two non-volatile liquids were used in the design of liquisolid tablets, Tween 80 and propylene glycol. The effect of formulation parameters, such as drug:non-volatile liquid ratio and carrier:coating ratio were observed. Microcrystalline cellulose and silica were used as carrier and coating materials, respectively. All liquisolid formulations showed higher drug dissolution rate than directly compressible tablets and marketed tablets. Differential scanning calorimetry revealed that the drug has got solubilised in the liquid vehicle which was further supported by x-ray diffraction studies and scanning electron microscopy. Infrared spectroscopy studies indicated the absence of incompatibility between drug and non-volatile solvents. This study shows that liquisolid technique is a promising alternative for improvement of dissolution rate of water insoluble drug.
Keywords
Olanzapine, liquisolid technique, liquid load factor, non-volatile solvent, carrier material, coating material
Bioavailability of a drug depends on its solubility in gastrointestinal fluids and its permeation across biological membranes. For a drug to be absorbed it must be in solution. So, solubility is the most important parameter for orally administered drugs. The improvement of drug solubility remains one of the most challenging aspects of drug development process. About 40 % of the newly developed drugs are insoluble in water [1]. Numerous conventional and modern approaches like solid dispersions, use of surfactants, inclusion complexes and nanonization are used to improve the solubility of these drugs. Liquisolid technique is one of the most promising approaches for solubility enhancement of poorly soluble drugs.
A liquisolid system refers to formulations formed by conversion of liquid drugs, drug suspensions or drug solutions in non-volatile solvents into dry, nonadherent, free flowing and compressible powder mixtures by blending the suspension or solution with selected carrier and coating materials [2]. Advantage of liquisolid systems is a great number of slightly and very slightly water-soluble and practically waterinsoluble liquid and solid drugs can be formulated into liquisolid systems. Another advantage is that their production is lower than that of soft gelatine capsules. The main limitations of liquisolid systems includes the requirement of solubility of the drug in non-volatile liquid and the problematic formulation of a high dose of poorly water-soluble drugs. Excipients used in formulation of liquisolid systems include non-volatile solvents (propylene glycol, PG), carrier materials (microcrystalline cellulose, Neusilin, Fujicalin) and coating materials (Aerosil) [3].
Olanzapine (OLZ), 2-methyl-4-(4-methyl-1- piperazinyl)-10H-thieno(2,3b)(1,5) benzodiazepine is the most important atypical antipsychotic agent. OLZ belongs to class II of Biopharmaceutical Classification System and has low bioavailability due to its poor solubility in water [4]. Several solubilisation techniques like solid dispersions [5] and inclusion complexes [6] have been applied and reported to enhance the aqueous solubility of OLZ. The present work was aimed at enhancing the dissolution rate of OLZ by development of liquisolid tablets. Effect of three factors namely non-volatile solvent, drug:non-volatile solvent ratio, carrier:coating ratio were observed in this study.
Materials and Methods
OLZ was a gift sample from Dr Reddy’s Laboratories, Hyderabad. PG (PEG 200) was purchased from Nice Chemicals Pvt. Ltd. Cochin, Avicel PH 102 and starch was procured from Yarrow Chem Products, Mumbai, Tween 80 and Aerosil 200 was obtained from Oxford Laboratory, Mumbai.
Application of mathematical model for design of liquisolid tablets
To calculate the required amounts of carrier and coating materials, a mathematical approach for the formulation of liquisolid systems has been developed by Spireas and Sadu [7]. This approach is based on flowable and compressible liquid retention potential introducing constants for each powder-liquid combination. The Φ-value of a powder represents the maximum amount of a given liquid that can be retained inside its bulk while maintaining its acceptable flowability.
Depending on the excipients ratio (R) of the powder substrate, an acceptable flowing and compressible liquid-solid system can be obtained only if a maximum liquid load on the carrier material is not exceeded. This liquid:carrier ratio is termed ‘liquid-load factor Lf’(w/w) and is defined as the weight ratio of the liquid formulation (W) and the carrier material (Q) in the system, Lf=W/Q. R represents the ratio between the weights of the carrier (Q) and the coating (q) material present in the formulation, R=Q/q. The liquid-load factor that ensures acceptable flowability Φ(Lf) can be determined using the eqn., ΦLf = Φ+φ (1/R), where Φ and φ are the Φ-values of the carrier and coating material, respectively.
Three variables were investigated at different levels to optimize OLZ liquisolid tablets. These variables included, (i) type of non-volatile solvent; Tween 80 and PG, (ii) drug:non-volatile solvent ratios 1:1 and 1:2 and (iii) carrier (Avicel PH 102):coating (Aerosil 200) ratio (R=30, 40 and 50). Starch was used as disintegrant. From literature, the Φ- values for Avicel PH 102 and Aerosil 200 with Tween 80 were 0.16 and 3.33, respectively and with PG were 0.16 and 3.31, respectively [8].
Solubility studies of OLZ in non-volatile solvents
Since selection of non-volatile solvent is an important factor in the formulation of liquisolid tablets, solubility studies were carried out to determine the solubility of OLZ in different non-volatile solvents. The solubility of OLZ was determined in Tween 80, PG and PEG 400 and in distilled water. Ten millilitres of respective solvents were taken in conical flasks and excess amount of OLZ was added and shaken for 48 h at 27° using a rotary shaker (Sisco, India) [9]. The solutions were subjected to centrifugation, supernatants were suitably diluted with 0.1 N HCl and analysed spectrophotometrically using UV/Vis spectrophotometer (Elico, SL 159) at 259 nm for OLZ content [10].
Preparation of liquisolid tablets and directly compressible tablets (DCT)
Twelve formulations of liquisolid tablets were prepared by direct compression method and the composition of the tablets is shown in Table 1. The desired quantity of drug was added to preheated non-volatile solvent (Tween 80/PG) with constant stirring and the solution was mixed thoroughly until a homogenous drug solution was obtained. The resultant liquid medication was converted into a free flowing powder mixture by blending with required quantities of carrier (Avicel PH 102) and coating materials (Aerosil 200) using a standard mixing process that was described by Spireas [11]. Lastly starch (5 % w/w) was added to the powder mixture and thoroughly mixed. The final powder blend was compressed into tablets using rotary tablet machine (Shakti). For comparison, tablet formulation (DCT) was prepared without using any non-volatile solvent. Fifty tablets were prepared for each formulation.
| Formulations | Wt. of OLZ (mg) | Wt. of non-volatile solvent (mg) |
Wt. of MCC (mg) F5 |
Wt. of silica (mg) |
Wt. of starch (mg) |
Total wt. (mg) F9 |
R | Lf | |
|---|---|---|---|---|---|---|---|---|---|
| Tween 80 | PG | ||||||||
| T130 | 10 | 10 | 73.80 | 2.46 | 4.81 | 101.07 | 30 | 0.271 | |
| T140 | 10 | 10 | 82.30 | 2.06 | 5.22 | 109.58 | 40 | 0.243 | |
| T150 | 10 | 10 | 88.11 | 1.76 | 5.49 | 115.58 | 50 | 0.227 | |
| T230 | 10 | 20 | 110.70 | 3.69 | 7.22 | 151.61 | 30 | 0.271 | |
| T240 | 10 | 20 | 123.46 | 3.09 | 7.83 | 164.38 | 40 | 0.243 | |
| T250 | 10 | 20 | 132.16 | 2.64 | 8.24 | 173.04 | 50 | 0.227 | |
| P130 | 10 | 10 | 74.07 | 2.47 | 4.83 | 101.37 | 30 | 0.270 | |
| P140 | 10 | 10 | 82.30 | 2.06 | 5.22 | 109.58 | 40 | 0.243 | |
| P150 | 10 | 10 | 88.50 | 1.77 | 5.51 | 115.78 | 50 | 0.227 | |
| P230 | 10 | 20 | 110.11 | 3.70 | 7.24 | 152.05 | 30 | 0.270 | |
| P240 | 10 | 20 | 123.46 | 3.09 | 7.83 | 164.38 | 40 | 0.243 | |
| P250 | 10 | 20 | 132.74 | 2.65 | 8.27 | 173.66 | 50 | 0.226 | |
| DCT | 10 | - | - | 84.07 | 2.80 | 4.84 | 101.71 | - | |
R is carrier:coating ratio, Lf is liquid load factor, OLZ is olanzapine, PG is propylene glycol, DCT is directly compressible tablets and MT is marketed tablets (Oleanz 10)
Table 1: Composition of liquisolid tablets of olanzapine
The flowability of the powder blends was assessed by determining angle of repose, bulk density, tapped density, Carr’s Index, and Hausner ratio parameters [12]. The hardness [13], uniformity of weight and friability [14] were determined for prepared liquisolid tablets. Five tablets were weighed individually and powdered. The powder equivalent to 5 mg of OLZ was dissolved in 10 ml of 0.1 N HCl and filtered. The drug content was determined by measuring the absorbance at 259 nm after suitable dilution [15].
In vitro dissolution studies
The in vitro release of OLZ from the formulated tablets was carried out using 900 ml of water maintained at 37.0±0.5° with a stirring rate of 50 rpm using rotating paddle tablet dissolution apparatus (USP- LabIndia DS 8000). At regular time intervals, samples of 5 ml were withdrawn and replaced with fresh medium to maintain the volume constant. After filtration and appropriate dilution, the amount of OLZ present in each sample was determined spectrophotometrically at 259 nm and cumulative percent drug release was calculated. To compare the dissolution profiles of formulated products with a marketed tablet, dissolution of Oleanz 10 (Sun Pharma Laboratories Ltd.) was carried out. To compare the dissolution data, dissolution efficiency at 30 min (DE30) was calculated for all the formulations.
Fourier-transform infrared spectroscopy (FTIR) studies
Compatibility between drug and excipients was carried out using FTIR studies. IR spectra were recorded using KBr pellet method using FTIR (Shimadzu, Japan) for pure drug OLZ and formulations T250 and P250.
Differential scanning calorimetry (DSC)
DSC analysis was performed using a Hitachi, STA-7300 DSC Germany, in order to assess the thermal behaviour of pure OLZ and liquisolid compacts. About 5-10 mg of the sample was sealed in the aluminium pans and heated at the rate of 10°/min, covering a temperature range of 50° to 250° under inert atmosphere flushed with nitrogen.
X-ray diffraction (XRD)
The XRD patterns of OLZ and liquisolid formulations T250 and P250 were recorded on PanAlytical, X-Pert pro X-ray diffractometer, Netherlands, using Cu as anode material and Kalpha1 radiation of 1.54060 A°. The data were recorded over a scanning range of °2 θ of 10 to 90.
Scanning electron microscopy (SEM)
The surface characteristics of pure drug and optimized formulations of T250 and P250 were investigated using the scanning electron microscope (JSM-6610LV, Jeol Asia Pte. Ltd., Japan). The sample was placed on a clear glass stub and sputter coated with a thin layer of gold using a vacuum evaporator. Images were examined at 20 kV accelerating voltage.
Results and Discussion
The liquid vehicles used in liquisolid systems should be orally safe, inert, and not highly viscous and preferably water miscible non-volatile organic solvents. In the current work, four liquid vehicles were chosen for solubility studies. All the liquid vehicles chosen for solubility studies are not highly viscous which is evident from the reported viscosities at 25° (90 mPa s for PEG 400, 58.1 mPa s for PG, 425 mPa s for Tween 80). The liquid vehicles are soluble in water and have high boiling points (>100°). The solubility of OLZ in different solvents was presented in Table 2. OLZ, which belongs to class II of BCS, is practically insoluble in water and its reported water solubility values are 0.03988 mg/ml [16], 0.043 mg/ml and 0.0942 mg/ml [17]. The solubility of OLZ in water was found to be 0.044 mg/ml which is in accordance to the literature cited and the drug showed enhanced solubility in non-volatile solvents. The solubility of OLZ was highest in PG followed by Tween 80 and PEG 400. The solubility of a drug in the solvents is affected by different physicochemical properties of the solvents, such as hydrophilicity, polarity, viscosity, chemical structure and molecular weight [18]. Even though Tween 80 has high hydrophilicity due to its high hydrophile-lipophile balance value of 15 compared with other liquid vehicles used, it had the lower solubilisation effect than PG. This may be due to other physicochemical properties such as high viscosity (425 mPas), high molecular weight (1310 g/mol) and extremely branched chemical structure [19]. Thus, Tween 80 and PG were chosen to develop liquisolid tablets. Higher drug solubility in the liquid vehicle results in reduced requirement of carrier and coating materials.
| Solvents | Solubility (mg/ml) |
|---|---|
| Water | 0.015±0.001 |
| PG | 3.370±0.421 |
| Tween-80 | 2.773±0.364 |
| PEG-400 | 1.130±0.102 |
Table 2: Solubility studies of olanzapine at 27°
The poor flow properties of powders pose a major challenge in tablet manufacturing industry. Uniform tablet weight and assay are not assured due to variability in filling from hopper into die cavity. Angle of repose is a characteristic of the internal friction or cohesion of the particles. The value of the angle of repose will be high if the powder is cohesive and low if the powder is non-cohesive. The results of micromeritic properties of prepared powder blends were given in Table 3. Very poor (T210-T250) to poor (T110-T150) flow properties were observed with Tween 80-based liquisolid powders and poor (P210-P250) to passable (P110-P150) flow properties were observed with liquisolid powders based on PG. The increasing order of flow properties of liquisolid powders with respect to non-volatile solvent is Tween 80<PG. The results were analogous to the earlier results [20]. With the decrease in drug:non-volatile liquid ratio, which implies an increase in concentration of drug in non-volatile solvents, the flow characteristics of powders improved. The flow properties increased with increase in drug concentration: 33.33<50 %. All the liquisolid tablets with higher excipient ratio (R=50) showed enhanced flow properties compared to lower excipient ratios due to increase in the amount of Avicel PH 102 from R=30 to R=50. Avicel PH 102 has good flow properties and the increase in its amount in the formulation increased flow properties. Carrier:coating ratio has enhanced the flow properties in the following order: 30<40<50.
| Formulations | Carr’s index (%) ± SD |
Hausner ratio ± SD |
Angle of repose (degrees) ± SD |
|---|---|---|---|
| T130 | 33.85 ± 0.33 | 1.51 ± 0.02 | 44.56 ± 0.57 |
| T140 | 31.51 ± 0.41 | 1.46 ± 0.01 | 41.44 ± 0.43 |
| T150 | 21.20 ± 0.37 | 1.41 ± 0.01 | 35.91 ± 0.42 |
| T230 | 37.55 ± 0.23 | 1.58 ± 0.01 | 56.16 ± 0.34 |
| T240 | 36.38 ± 0.49 | 1.57 ± 0.02 | 51.90 ± 0.41 |
| T250 | 30.48 ± 0.38 | 1.54 ± 0.01 | 44.61 ± 0.26 |
| P130 | 22.54 ± 0.49 | 1.32 ± 0.03 | 37.88 ± 0.58 |
| P140 | 20.11 ± 0.14 | 1.28 ± 0.01 | 34.25 ± 0.43 |
| P150 | 16.64 ± 0.35 | 1.21 ± 0.01 | 28.93 ± 0.37 |
| P230 | 30.05 ± 0.35 | 1.53 ± 0.02 | 46.76 ± 0.53 |
| P240 | 25.08 ± 0.47 | 1.52 ± 0.01 | 38.69 ± 0.26 |
| P250 | 18.08 ± 0.23 | 1.44 ± 0.02 | 34.51 ± 0.47 |
| DCT | 16.27 ± 0.54 | 1.23 ± 0.01 | 28.26 ± 0.46 |
SD is standard deviation for n=3 observations, DCT is directly compressible tablets
Table 3: Micromeritic properties of precompression powders
In the formulation of liquisolid tablets, the liquid vehicle itself acts as a binder. The compactness of liquisolid tablets is due to the presence of hydroxyl groups in the molecular structure of liquid vehicle, which leads to hydrogen bonding between solvent and other excipients in the formulations [21]. High specific surface area (SSA) and high liquid absorption capacity are the important criteria for selection of a carrier in a liquisolid system. MCC PH 102 was used as a carrier because it has a large SSA of 1.18 m2/g when compared to other general carriers, like lactose (0.35 m2/g), sorbitol (0.37 m2/g), and starch (0.6 m2/g) [22]. Fluid penetration into a porous material is a capillary driven flow. The penetration rate of a liquid into a porous material is based on the balance between capillary and opposing viscous forces as given by the Eqn., L2 = 2mγcosθt/kη, where, L is the penetrating length at time t, m is the pore size, γ is the surface tension of the liquid, θ is the contact angle between the liquid and solid, η is the viscosity of the liquid and k is a constant that depends on the pore shape [23]. The small pore size restricts the entry of liquid vehicle with large molecular weight and extremely branching structure. A high surface tension between porous particles and liquid vehicle leads to a high contact angle, which maximises the diffused amount of liquid vehicle. Avicel PH 102 has a pore size of 100 μm. All the liquisolid powders were compressed into tablets.
The percent friability (Table 4) of all the formulations was within IP limits. Tablets should be sufficiently hard to resist breaking while handling and yet should be soft to disintegrate when taken orally. The hardness of the formulations was in the range of 2.1 to 2.9 kg/cm2. Tablets prepared with Tween 80 were slightly harder than tablets prepared with PG. All formulations passed uniformity of weight according to IP 2007. The drug content of the formulations was in the range of 95.51 to 99.28 % and was found to be within IP limits (Table 4) [24].
The dissolution profiles of liquisolid tablets, DCT and MT were present in Figure 1A and B. It was apparent that liquisolid tablets showed better dissolution than DCT and MT. The enhanced drug release in liquisolid tablets might be due to the reported three mechanisms. The mechanisms include increased surface area of drug, increased drug solubility and improved wettability of drug particles. The liquisolid tablets contain the drug in molecularly dispersed state. Therefore, the surface area of the drug available for dissolution is also increased. In the microenvironment, that is, at the interface between an individual liquisolid particle and the dissolution medium, the amount of liquid vehicle diffusing out of a single liquisolid particle along with the drug particle acts as a cosolvent. Liquid vehicle can act as a surface active agent and reduces interfacial tension between tablet surface and dissolution medium. Therefore the wetting of drug particles is improved [25]. The dissolution of liquisolid tablets increased with increase in R value. Liquisolid tablets with higher R values contain high amount of MCC PH102 and low quantity of Aerosil 200, which could be associated with enhanced wicking, disintegration and disaggregation properties [26]. The formulations containing 33.33 % drug concentration showed higher drug release compared to 50 % drug concentration as former formulations contained high concentration of non-volatile liquid when compared to latter formulations. The DE30 values for marketed tablets and tablets without non-volatile solvent were 19.07 and 23.10, respectively and the values obtained for liquisolid formulations were higher than these values. From all the results obtained, formulation P250 formulated with PG as non-volatile solvent, drug concentration 33.33 % and R value 50 was considered as optimum formula.
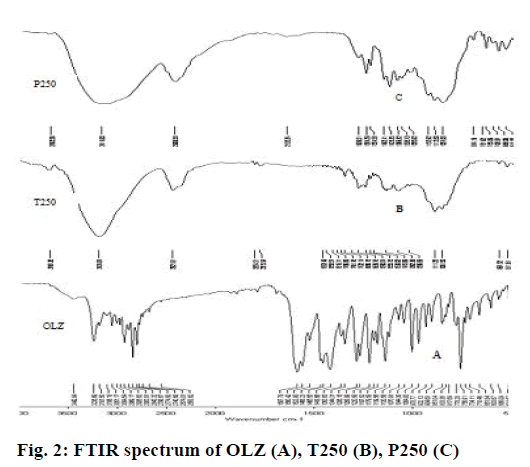
FTIR spectra of OLZ showed characteristic absorption peaks at 3239 cm-1 (NH and OH stretching), 2929 cm-1 (CH stretching), 1587 (C=C stretching), 1421 cm-1 (C=N stretching) and 1287 cm-1 (CN stretching) [27]. The presence of these peaks in liquisolid tablets confirm the absence of drug-excipients interactions (Figure 2A, B and C) as observed in earlier findings [28].
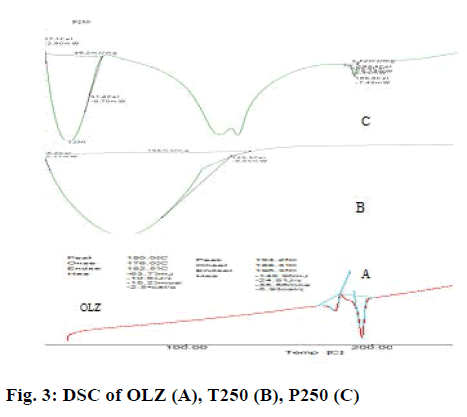
In the DSC curve (Figure 3A), pure OLZ has a sharp endothermic peak at 194.25°, which correspond to the melting point of OLZ [29]. The thermal behaviour of liquisolid systems (Figure 3B and C) show the shifting of endothermic peak to lower temperatures, which clearly indicates that crystalline nature of drug is completely lost [30].
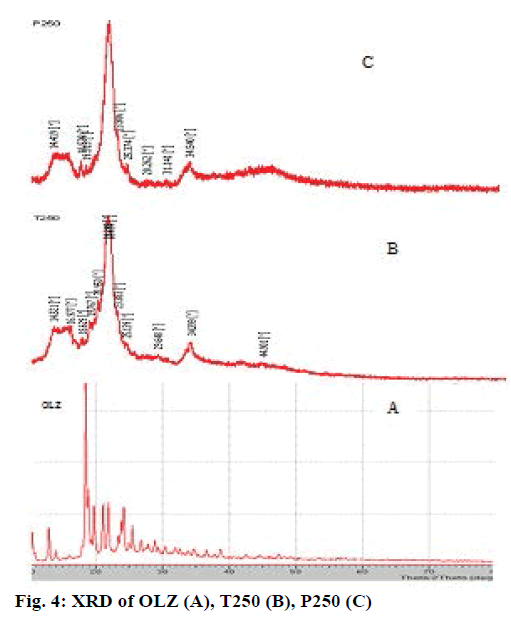
Changes in the physical form of the drug particles are important since they affect the dissolution rate and bioavailability. Hence, this aspect of OLZ in liquisolid compacts was studied. The XRD of OLZ in Figure 4A shows the presence of sharp intense peaks indicative of its high crystalline character [31]. XRD patterns of liquisolid powders (Figure 4B and C) showed the reduction in intensity of these distinct peaks, which reveals that OLZ has been completely converted to solubilised form as observed in the earlier results [32].
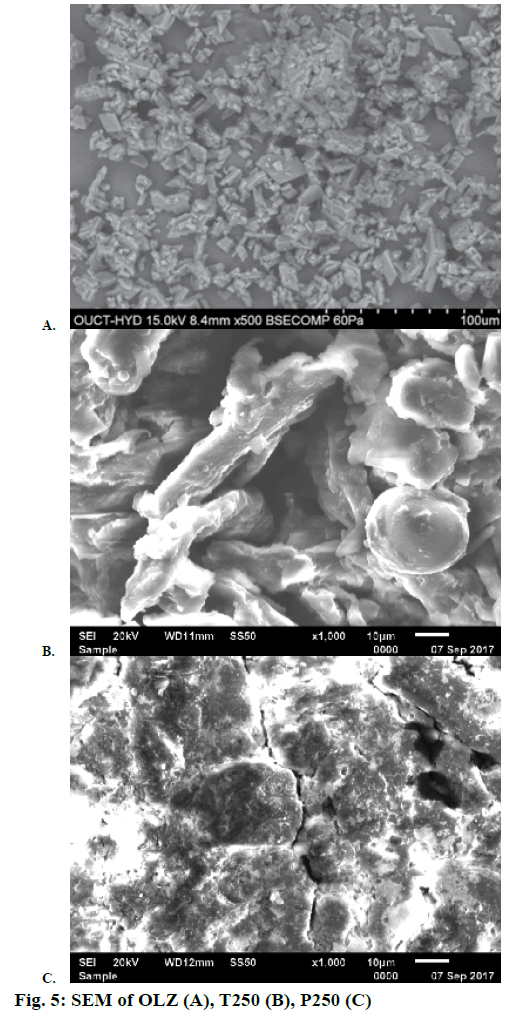
The SEM photomicrograph, in Figure 5A, showed the presence of irregular shaped crystals of OLZ. The photomicrographs of T250 and P250 (Figures 5B and C) signify the complete disappearance of crystals of OLZ, which indicates that the drug was totally solubilised in the liquid vehicles [33].
In the current study, an attempt was made to enhance dissolution rate of OLZ by liquisolid technique. The objective of the study was achieved as observed from various results. Both PG and Tween 80 improved dissolution rate of OLZ and PG proved to be a better non-volatile solvent with higher dissolution profiles and flow properties than Tween 80. The conversion of drug from crystalline state to molecularly dispersed state was clearly indicated in DSC, XRD and SEM studies. It can be concluded that liquisolid technique could be a promising strategy in improving dissolution rate of poorly soluble drug OLZ.
Acknowledgements
The authors thank Dr. Reddy’s Laboratories, Hyderabad for providing OLZ as gift sample for this work. They also thank Y. V. V. S. Viswanath, Chairman, Yalamarty Educational Trust for providing facilities to carry out this research work.
References
- Gowthamarajan K, Sachin KS. Dissolution testing for poorly soluble drugs: a continuing perspective. Dissolut Technol 2010;24-32.
- Tiong N, Elkordy AA. Effects of liquisolid formulations on dissolution of naproxen. Eur J Pharm Biopharm 2009;73:373-84.
- Vraníková B, Gajdziok J. Liquisolid systems and aspects influencing their research and development. Acta Pharm 2013;63:447-65.
- Callaghan JT, Bergstrom RF, Ptak LR, Beasley CM. Olanzapine – pharmacokinetic and pharmacodynamic profile. Clin Pharmacokinet 1999;37:177-93.
- Krishnamoorthy V, Suchandrasen, Prasad VPR. Physicochemical characterization and in vitro dissolution behavior of olanzapine-mannitol solid dispersions. Braz J Pharm Sci 2012;48:243-55.
- de Freitas MR, Rolim LA, Soares MF, Rolim-Neto PJ, de Albuquerque MM, Soares-Sobrinho JL. Inclusion complex of methyl-β-cyclodextrin and olanzapine as potential drug delivery system for schizophrenia. Carbohydr Polym 2012;89:1095-100.
- Spireas S, Sadu S. Enhancement of Prednisolone dissolution properties using liquisolid compacts. Int J Pharm 1998;166:177-88.
- Altememy DRJ, Altememy JJ. Formulation and evaluation of meloxicam liquisolid compact. Int J Pharm Sci 2016;6:453-63.
- Elkordy AA, Essa EA, Dhuppad S, Jammigumpula P. Liquisolid technique to enhance and to sustain griseofulvin dissolution: Effects of choice of non-volatile liquid vehicles. Int J Pharm 2012;434:122-32.
- Joseph E, Balwani G, Nagpal V, Reddi S, Saha RN. Validated UV spectrophotometric methods for the estimation of olanapine in bulk, pharmaceutical formulations and preformulation studies. Braz J Pharm Sci 2015;6(3):181-90.
- Spireas. Liquisolid systems and methods of preparing same. United States patent US6423339B1. 2002 July 23.
- Aulton ME. Pharmaceutics: The Design and Manufacture of Medicines. 3rd ed. London, United Kingdom: Churchill Livingstone; 2007. p. 355-6.
- Khar RK, Vyas SP, Ahmed FJ, Jain GK. The Theory and Practice of Industrial Pharmacy. 4thed. New Delhi: CBS Publishers and Distributors Pvt. Ltd; 2015. p. 481-83.
- Indian Pharmacopoeia. Vol. 1. New Delhi: Controller of Publication, Govt. of India, Ministry of Health and Family Welfare; 2007. p. 182-3.
- Maheswarappa MK, Desai PD. Design and in vitro evaluation of mouth dissolving tablets of olanzapine. Asian J Pharm 2011;5:107-13.
- Olanzapine, Compound Summary for CID 4585. Pubchem, Open chemistry database. Available from: http://pubchem.ncbi.nlm.nih.gov/compound/olanzapine
- Olanzapine, identification. Available from: https://www.drugbank.ca/drugs/DB00334.
- Spireas S, Sadu S. Enhancement of prednisolone dissolution properties using liquisolid tablets. Int J Pharm 1998;166:177-88.
- Khanfar M, Sheikh Salem M, Kaddour F. Preparation of sustained-release dosage form of venlafaxine HCl using liquisolid technique. Pharm Dev Technol 2014;19(1):103-15.
- Yehia SA, El-Ridi MS, Tadros MI, El-Sherif NG. Enhancement of the oral bioavailability of fexofenadine hydrochloride via cremophor EL based liquisolid tablets. Adv Pharm Bull 2015;5(4):569-81.
- Hentzchel CM, Sakmann A, Leopard CS. Suitability of various excipients as carrier and coating materials for liquisolid compacts. Drug Dev Ind Pharm 2011;37:1200-7.
- Javadzadeh Y, Siahi MR, Asnaashari S, Nokhodchi A. An investigation of physicochemical properties of piroxicam liquisolid compacts. Pharm Dev Technol 2007;12:337-43.
- Lerk CF, Bolhius GK, de Boer AH. Effect of microcrystalline cellulose on liquid penetration and disintegration of directly compressed tablets. J Pharm Sci 1979;68:205-11.
- Indian Pharmacopoeia. Vol. 3. New Delhi: Controller of Publication, Govt. of India, Ministry of Health and Family Welfare; 2007. p. 1471-2.
- Nokhodchi A, Hentzschel CM, Leopold CS. Drug release from liquisolid systems: speed it up, slow it down. Expert Opin Drug Deliv 2011;8(2):191-205.
- Shashidher B, Madhusudhanarao Y, Venkateswarlu V. The Liquisolid technique: an overview. Brz J Pharm Sci 2011;47(3):475-82.
- Hiriyanna SG, Basavaiah K, Goud PSK, Dhayanithi V, Raju K, Pati HN. Identification and characterization of olanzapine degradation products under oxidative stress conditions. Acta Chromatogr 2008;20(1):81-93.
- Devender RK, Karthik YJ, Raju J, Suresh B. Competence of raloxifene hydrochloride loaded liquisolid compacts for improved dissolution and intestinal permeation. J Drug Deliv Sci Technol 2015;30:232-41.
- Mudit D, Ashwini GK, Parthasarathi KK. Enhancing the aqueous solubility and dissolution of olanapine using freeze drying. Braz J Pharm Sci 2011;47(4):743-9.
- Kapure VJ, Pande VV, Deshmukh PK. Dissolution enhancement of rosuvastatin calcium by liquisolid compact technique. J Pharm 2013;2013:315902.
- Tiwari M, Chawla G, Bansal AK. Quantification of olanzapine polymorphs using powder X-ray diffraction technique. J Pharm Biomed Anal 2007;43:865-72.
- Amrit BK, Indrajeet DG, Avinash HH, Pandurang ND, Satish BB. Dissolution rate enhancement of fenofibrate using liquisolid tablet technique. Lat Am J Pharm 2009;28(2):219-25.
- Fahim JS, Sachin LT, Umesh BK. Design and development of liquisolid compact of candesartan cilexetil to enhance dissolution. J Pharm Res 2013;381-8.
 T130;
T130;  T140;
T140;  T150;
T150;  T230;
T230;  T240;
T240;  T250;
T250;  DCT;
DCT;  MT. B:
MT. B: