- *Corresponding Author:
- Hemlata Nimje
Department of Pharmaceutical Chemistry, JSPM’s Charak College of Pharmacy and Research, Wagholi, Pune, Maharashtra 412207, India
E-mail: hemanimje@gmail.com
| Date of Received | 09 May 2023 |
| Date of Revision | 28 February 2024 |
| Date of Acceptance | 26 August 2024 |
| Indian J Pharm Sci 2024;86(4):1268-1276 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
N-nitrosodimethylamine is a carcinogenic agent accidentally found in ranitidine formulations. For the products to be safe for human use and quality control test, the estimation of N-nitrosodimethylamine in ranitidine is significantly important. The goal of this study was to use liquid chromatography with tandem mass spectrometric technique for the development and validation of N-nitrosodimethylamine in ranitidine drug and tablets. The proposed approach was utilized to estimate routine analysis of N-nitrosodimethylamine in ranitidine drug and tablets. The injection volume 20 µl and wavelength 254 nm was selected for N-nitrosodimethylamine and ranitidine quantification. Binary mobile phase in a gradient mode was used for the study. The mixture of solvent A (0.1 % formic acid in water) and solvent B (0.1 % formic acid in methanol) was used as the mobile phase. The flow rate of 0.6 ml/min in gradient mode with complete run time of 14 min was used for the analysis. For N-nitrosodimethylamine analysis multiple reaction monitoring transitions were 75.80/44.20 (quantitative analysis) and 75.80/58.20 (qualitative analysis). N-nitrosodimethylamine retention time (tR) was found at 1.25 min. For N-nitrosodimethylamine, the limit of detection was 0.3 ng/ml and limit of quantitation was found to be 1 ng/ml. The linearity range for N-nitrosodimethylamine was decided as 1, 2, 5, 10, 20 and 50 ng/ml from its intensity response. The regression value observed in a linearity graph was 0.9999. The method was specific (no interference of any peak), validated for its accuracy (98.07 to 100.22±1.7565), precision (98.64 to 99.44±0.9083) and robustness (does not effect on developed method by slight change in flow rate and column temperature). In the validation investigation, the quality control levels lower quality control (4 ng/ml), middle quality control (8 ng/ml) and higher quality control (40 ng/ml) were used. Newly developed and validated method for N-nitrosodimethylamine in ranitidine by liquid chromatography with tandem mass spectrometry was accurate, precise and sensitive. The developed method was further used for routine analysis of N-nitrosodimethylamine in ranitidine drug and tablets.
Keywords
N-nitrosodimethylamine, ranitidine, liquid chromatography-tandem mass spectrometry, validation study
Ranitidine HCl (RAN) is a regularly prescribed over-the-counter medication for acid reflux and heartburn. By chance or accidently, the chemical contaminant N-nitrosodimethylamine (NDMA) has been found in pharmaceutical prescription items[1]. NDMA is a confirmed carcinogen contaminant[2-4]. Food and Drug Administration (FDA) tested valsartan, losartan, metformin and RAN for NDMA and other nitroso impurities in September 2019[5,6]. Exposure to NDMA is made more likely by the numerous components found in different formulations[7]. NDMA contaminant in very low concentrations (below 0.3 ppm) was reported to be found during the RAN production process[8]. United States FDA’s (US FDA) ongoing investigation indicates that a number of RAN brands may contain high levels of NDMA contamination as a result of high temperature storage or production methods[1]. Products containing RAN are available in the other markets but are withdrawn from US market because of high levels of NDMA contamination[9]. FDA is working to make sure that the contaminants doesn't exceed above 0.3 ppm per day and will be safe for human use[10,11]. Various foods and water have both been shown to contain trace amounts of NDMA[12,13]. Therapeutic Goods Administration Laboratory has reported testing of 135 batch samples of RAN for NMDA, but no method for studying the creation and validation of NDMA in RAN has been documented[14]. Chemically RAN is N'-[2-[[[5-[(dimethylamino) methyl]-2-furanyl]methyl]thio]ethyl]-N-methyl- 2-nitro-1,1-ethenediamine (C13H22N4O3S) and molecular weight is 314.4 g/mol[15-17]. NDMA is N-nitrosodimethylamine (C2H6N2O) and molecular weight is 74.08 g/mol. Structure of RAN and NDMA are shown in fig. 1. NDMA is a yellow oily liquid, volatile and susceptible to photolytic breakdown due to its absorption of ultraviolet light. NDMA is stored in cool, dark and well closed container at 2°-8°. Using head space analysis and liquid injection mode, Alshehri et al., reported an analytical method for NDMA in RAN product estimation by Headspace Solid-Phase Microextraction coupled with Gas Chromatography-Mass Spectrometry (HS-SPMEGC- MS)[18]. In this study, it was found that as RAN is susceptible to high temperature, using Gas Chromatography (GC) for NDMA was problematic. As a method of extraction and introduction into the GC, Solid-Phase Microextraction (SPME) was evaluated in this work to determine its usefulness. Though HS-SPME-GC-MS is reported specifically for estimation of NDMA in RAN formulations, the availability of this highly sophisticated hyphenated technique limits the utility in routine analysis. The present work proposes Liquid Chromatographytandem Mass Spectrometry (LC-MS/MS) method as an alternative to the reported method for estimation of NDMA in RAN tablets. Yokoo et al., reported forced degradation investigation for RAN and its related impurities using High Performance Liquid Chromatography (HPLC)[19]. This study investigated the root cause of presence of NDMA in RAN by forced degradation study and found that not only RAN produced NDMA, but the impurities present in RAN also lead to presence of NDMA[19]. In the present study the quantification and validation of NDMA as an impurity has been performed using LC-MS/MS method. A study published by US FDA reports quantification of NDMA using multiple reaction monitoring on a triple quadrupole mass spectrometer[20], however, no validation of analytical method for estimation of NDMA in RAN is reported. Angrish et al., reported the testing of NDMA in RAN[21]. In this study, LC-MS/MS parameters for method development and validation were reported as per US FDA procedure. Yamamoto et al., reported separation of NDMA from drug substances using Solid-Phase Extraction (SPE)-LC-MS/MS[22]. It includes sample pretreatment for NDMA removal in drug substances and drug products using SPE prior to Liquid Chromatography-Mass Spectrometry (LC-MS) analysis. Various drugs studies for separation of NDMA as impurity from RAN, metformin, nizatidine, valsartan and telmisartan was reported. The main objective of the present work was to develop simple, precise, accurate, fast and robust LC-MS/MS method for estimation of NDMA in RAN formulations. The proposed method was successfully validated as per International Council for Harmonisation (ICH) guidelines. Hence, the method can be applied for routine analysis in its quality control for RAN. This systematic study on identification of NDMA as an impurity in RAN will help to estimate the intrinsic stability of RAN drug and its formulation.
Materials and Methods
Materials:
The chemicals used for present work included formic acid (Merck life science private limited, India), water (Fisher scientific, India) of HPLC grade. The methanol LC-MS grade (J. T. Baker, Mumbai, India) was used throughout analysis. RAN active pharmaceutical ingredient and NDMA were procured from Clearsynth Lab, Mumbai, India. Tablet formulations A (Rantac 150, JB Chemicals and Pharmaceuticals Ltd., Mumbai, India) and B (Aciloc 150, Cadila Pharmaceuticals Ltd., Ahmadabad, India) were purchased from local market. The labeled claim of the RAN for each tablet was 150 mg.
Instrumentation:
Shimadzu Liquid Chromatography (LC) 20AD LC system and the 4000 QTrap mass spectrometer SCiex (MDS SCIEX) were utilised in the LCMS/ MS procedure. All data collection and result processing were performed by the analyst 1.4.2 software. The LC system is made up of the various components such as auto sampler SIL-HTC, degasser DGU20A3, column oven CTO-10AS VP, binary pump LC20AD and reservoir tray. Thermo Hypersil Gold C18 column with 3 μm particle size and 4.6×100 mm dimensions size was used for separation.
Preparation of standard stock solutions:
A standard stock solution of RAN (1 mg/ml) was prepared by dissolving 10 mg of drug RAN in 5 ml HPLC grade water. It was then transferred into 15 ml glass centrifuge tube, mixed and volume made up to 10 ml with HPLC grade water. A vortex mixer was used to obtain a clear solution. A stock solution of NDMA (1 mg/ml) was prepared by dissolving accurately weighed 1 mg NDMA in 1 ml HPLC grade water (diluent) in a volumetric flask. From these working stock solutions, 10 ng/ ml solution was prepared for analysis.
Preparation of RAN standard solution and NDMA mixture:
The mixture of RAN standard solution and NDMA (100 μg/ml for RAN and 10 ng/ml for NDMA) was prepared using HPLC grade water as a diluent. The mixture was passed through a membrane filter (0.45 μm) after being sonicated for 10 min.
Chromatographic and mass spectrometric conditions:
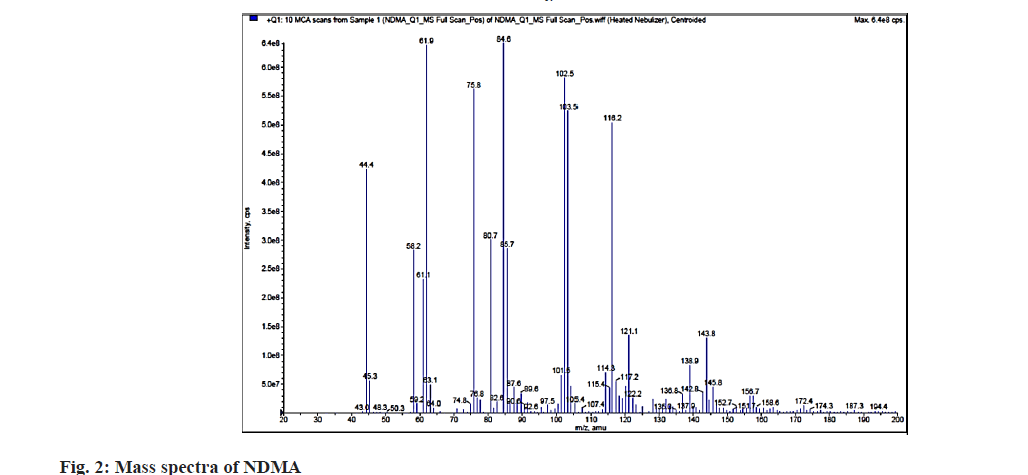
For the present work, the LC was performed on Thermo Hypersil Gold C18 column having column temperature 40°. The combination of 0.1 % formic acid in water (solvent A) and 0.1 % formic acid in methanol (solvent B) was selected as mobile phase in gradient mode of separation pattern (Table 1), with the flow rate 0.6 ml/min and total run time for mobile phase was 14 min throughout the analysis. For filtration of mobile phase, 0.45 μm disposable membrane filter was used. Mobile phase was degassed by ultrasound sonicator for 10 min before use in the analysis. The volume of injection was 20 μl and the detection was carried out by photodiode array detector. The atmospheric pressure chemical ionisation probe was employed in the positive mode of the multiple reaction monitoring scan under the mass spectrometric settings. The collision gas (6 psi), curtain gas (20 psi), ion source gas 1 (40 psi) and nebulizer current 4 mA was used. To conduct this study, a turbo ion spray interface running in positive ionization mode was used. Collision Energy 25 eV, collision cell exit potential 15 V, declustering potential 60 V and entrance potential 10 V were used. The pressure of the drying nitrogen gas was 35 psi and temperature of 400° was used. MS parameters for quantitative analysis used are m/z 75.80/44.20 and qualitative analysis m/z 75.80/58.20 based on the mass spectra of NDMA (fig. 2).
| Time (min) | Pump A with Solvent A (%) | Pump B with Solvent B value (%) |
|---|---|---|
| 0.01 | 95 | 5 |
| 3 | 80 | 20 |
| 9 | 0 | 100 |
| 14 | 95 | 5 |
Table 1: Lc Gradient Program for Mobile Phase
Preparation of RAN tablet solution:
For sample solution of RAN, 20 tablets having 150 mg label claim (Rantac 150) were weighed accurately. Average weight was calculated and the tablets were crushed to finely ground powder using mortar and pestle. The sample solution of RAN (100 μg/ml) was prepared by dissolving equivalent to 1 mg of RAN tablet powder in 5 ml HPLC grade water. It was then transferred into 15 ml glass centrifuge tube, mixed and volume made up to 10 ml with HPLC grade water. The sample was shaken with mechanical wrist action shaker for 40 min. The sample was centrifuged for 15 min at 4500 rpm after extraction. The supernatant was filtered using a 0.22 μm polyvinyl difluoride syringe filter. The first 1 ml was discarded. For LC-MS analysis, the filtered sample solution was transferred to an HPLC vial.
Validation of proposed method:
Lower Quality Control (LQC), Middle Quality Control (MQC) and Higher Quality Control (HQC) of standard NDMA were prepared for the study of linearity, Limit of Detection (LOD), Limit of Quantitation (LOQ), ruggedness, precision in terms of repeatability, reproducibility and accuracy study using different concentrations. The concentrations selected were in the range of 1, 2, 5, 10, 20, and 50 ng/ml. LQC set as 4 ng/ml as an internal quality control, MQC 8 ng/ml and HQC 40 ng/ml for injecting in LC-MS/MS for quality control. These were then spiked to the RAN and then injected in LC-MS/MS. With the use of the Analyst 1.4.2 application software, the calibration curve was plotted between the areas of peak vs. different concentrations. To resolve the target chemicals into a single peak, the signal to noise ratio of injections at progressively decreasing concentrations was taken into account while calculating LOD. The lowest amount of analyte in a sample that can be quantitatively quantified with enough precision and accuracy is LOQ of a specific analytical method. In order to calculate precision in terms of reproducibility, the Relative Standard Deviation (RSD) was measured after injecting NDMA into LC-MS/MS from various vials six times. Analyzing several injections from a single vial helped to study the test for method reproducibility. The experiment had six replicates of LQC and HQC for intraday precision and 5 consecutive days over the period of a week for interday precision. The results were expressed in terms of relative standard deviation. In conditions of change in mobile phase flow rate, ruggedness was found to be within the range of RSD. Solution stability was determined using the comparison between fresh solution and bench top solution (after 48 h) and auto sample solution (after 48 h). As per the ICH guidelines, a recovery study for NDMA was performed by adding different concentrations of 10 ng/ml, 20 ng/ml and 30 ng/ml in the known standard solutions. Different levels 50 %, 100 % and 150 % were used for recovery study.
Results and Discussion
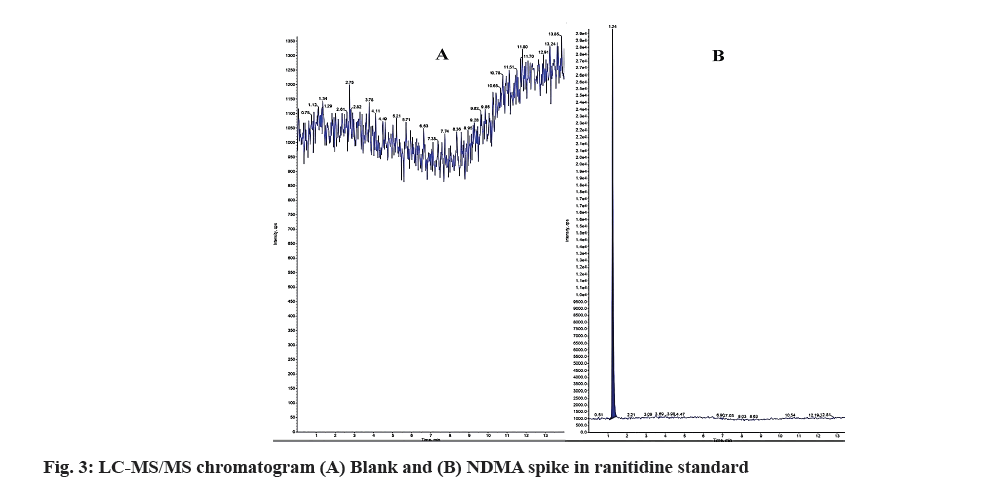
The main aim of the proposed LC-MS/MS method is to separate the related impurity from the RAN in the purchased marketed formulation of parent substance by Reversed-Phase High Performance Liquid Chromatography (RP-HPLC) and quantify NDMA by triple quadruple mass spectrometer. RAN is freely soluble in water and hence chosen for the separation. The mobile phase solvent A (0.1 % formic acid in water) and solvent B (0.1 % for formic acid in methanol) were selected based on trial and error. The stationary phase C18 column (Thermo Hypersil Gold) with dimension 4.6×100 mm, having particle size 3 μm and injection volume 20 μl was selected for NDMA and RAN quantification. Initially, the temperature of column was 25°, but for change of pressure column temperature was increased 40° for reduction of retention time for both NDMA and RAN. Various isocratic and gradient modes of separation were tried. Finally gradient mode of separation was chosen in which different proportions of solvent A and solvent B with respect to time was selected as shown in Table 1. The flow rate for mobile phase was 0.6 ml/min which was maintained throughout the analysis for 14 min. The standard solutions of RAN and NDMA showed the maximum absorbance at 254 nm, when they were scanned over 200 to 400 nm. Combined RP-HPLC chromatogram for RAN and NDMA was estimated for the separation study. For LC-MS/MS, the MS parameter 75.80/44.20 was chosen for quantitative analysis of NDMA and 75.80/58.20 was chosen for qualitative analysis of NDMA. After injection of NDMA solution, LCMS/ MS gives a chromatogram with single peak at retention time at 1.25 min shown in the (fig. 3).
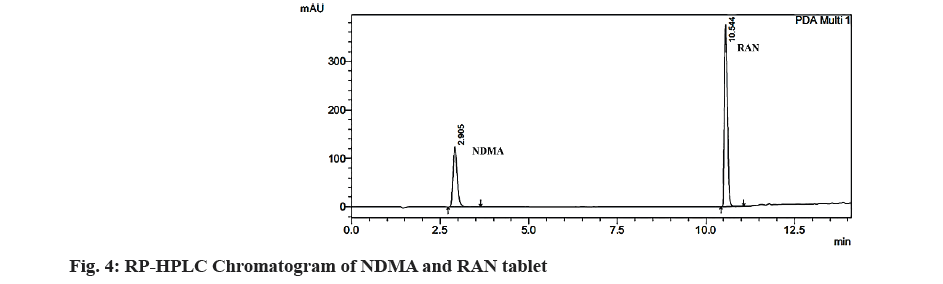
The commercial RAN tablet formulations (Rantac 150 and Aciloc 150) were analyzed and RAN concentrations were estimated from peak area and height with respect to the NDMA. The tailing factors of NDMA and RAN were observed as 1.235 and 1.165 respectively by RP-HPLC. The resolution was found to be 38.29, a theoretical plate of above 10 000 (60450 for Rantac 150 tablet and 60212 for Aciloc 150 tablet) was obtained. The related impurity NDMA (10 μg/ml) was separated from RAN (100 μg/ml) shown in fig. 4 and data of the system suitability test are shown in Table 2.
| Parameters | NDMA | RAN tablet (Rantac 150) | RAN tablet (Aciloc 150) |
|---|---|---|---|
| Retention time (min) | 2.905 | 10.544 | 10.496 |
| Area of peak | 1006523 | 2206898 | 2216532 |
| Height of peak | 124183 | 374653 | 374254 |
| Theoretical plates | 2610.35 | 60450.62 | 60212.12 |
| Tailing factor | 1.235 | 1.165 | 1.16 |
| Resolution | - | 38.29 | 38.28 |
Table 2: Results for System Suitability Parameters for Ndma and Ran Tablets
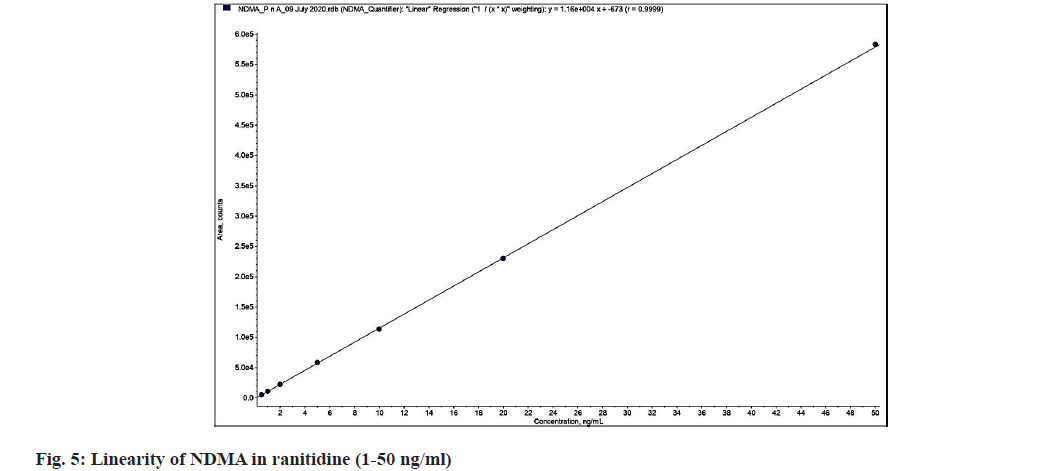
The linearity of NDMA was investigated using a calibration curve and a correlation coefficient analysis of the drug concentration and response area over the calibration range. The linearity ranged from 1-50 ng/ml, with a regression coefficient value of 0.9999 as shown in fig. 5.
The accuracy and precision of the present method selected for the given concentration range were studied for different concentrations ranging from 1-50 ng/ml with quality control levels of LQC, MQC and HQC. The repeatability and reproducibility of NDMA was performed for 4, 8 and 40 ng/ml with six repetitions. It showed acceptable results for precision (RSD) of the given method as shown in Table 3. The results of an accuracy study for NDMA concentrations of 10, 20 and 30 ng/ml at 50 %, 100 % and 150 % levels were added in the known RAN standard solutions (Table 4).
|
QC level |
Amount added (ng/ml)* | Interday concentration found (ng/ml)* | Amount found (%±SD) | % RSD | Intraday concentration found (ng/ml)* | Amount found (%±SD) | % RSD |
|---|---|---|---|---|---|---|---|
|
LQC |
4 | 3.94 | 98.50±1.21 | 1.23 | 3.97 | 99.33±1.78 | 1.79 |
|
MQC |
8 | 7.93 | 99.18±0.92 | 0.92 | 7.89 | 98.64±1.03 | 1.05 |
|
HQC |
40 | 39.61 | 99.03±0.54 | 0.55 | 39.77 | 99.44±0.90 | 0.91 |
Note: * replicates six sample analysis determinations; SD: Standard Deviation and RSD: Relative Standard Deviation
Table 3: Data Results for Assessment of Precision for Ndma
| RAN amount taken (µg/ml) | NDMA amount added* (ng/ml) | NDMA amount recovered* (ng/ml) | % Recovery±S.D. | % RSD |
|---|---|---|---|---|
| 20 | 10 | 9.80 | 98.07±1.75 | 1.79 |
| 20 | 20 | 19.96 | 99.80±1.59 | 1.59 |
| 20 | 30 | 29.81 | 99.38±1.72 | 1.73 |
Note: * replicates six sample analysis determinations; SD: Standard Deviation and RSD: Relative Standard Deviation
Table 4: Recovery Study for Ndma
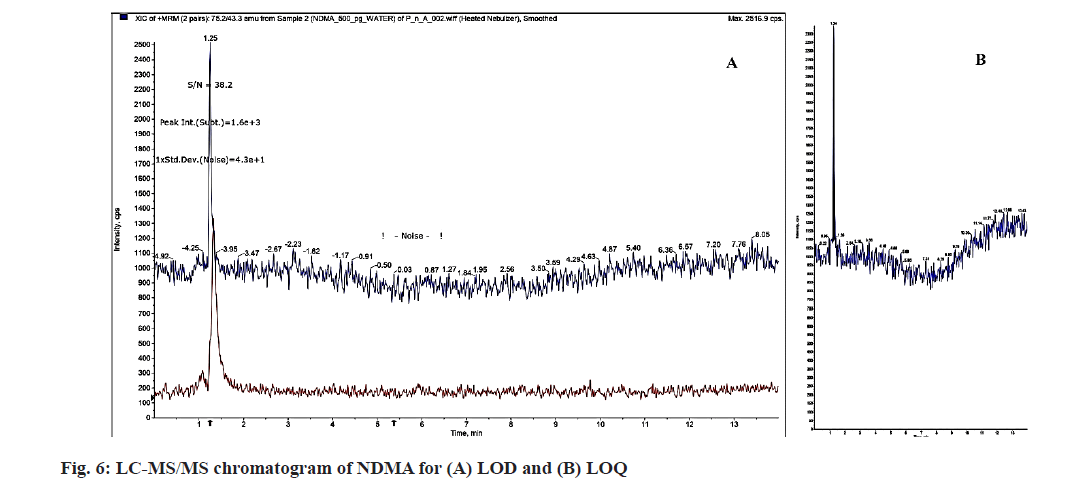
The effect of minute but deliberate modifications in optimum chromatographic conditions developed for NDMA was investigated using a robustness study. The change in flow rate (±10 %) and change in column temperature (±5°) was tested with mean of six samples replicates of LQC, MQC and HQC levels. Under this condition, all system suitability characteristics and changes in conditions were compared with the test samples. For the solution stability study, fresh solution, bench top solution (after 48 h) and auto sample solution (after 48 h) were used. The results of robustness and solution stability study are depicted in Table 5. The LOD and LOQ for NDMA with an S/N ratio of 38.2 were determined as 0.3 and 1 ng/ml, respectively as shown in fig. 6. The values for the detection and quantification limits show that the method has good sensitivity.
| Parameters | Conditions* | LQC (4 ng/ml) | MQC (8 ng/ml) | HQC (40 ng/ml) |
|---|---|---|---|---|
| Change in flow rate | 0.5 ml/min | 98.53±0.8419 | 100.41±1.4278 | 99.56±0.8143 |
| 0.6 ml/min | 99.15±0.7433 | 100.05±1.3594 | 99.70±0.4856 | |
| 0.7 ml/min | 98.74±0.8818 | 99.88±1.5761 | 99.97±0.8320 | |
| Change in column temperature | 35° | 98.84±2.0958 | 99.13±1.6362 | 100.31±0.5988 |
| 40° | 98.87±2.0364 | 99.77±1.6122 | 99.66±0.9553 | |
| 45° | 98.92±2.2703 | 99.47±1.1399 | 100.15±1.1991 | |
| Solution stability study | Fresh solution | 99.65±0.7898 | 100.91±0.8665 | 99.28±1.4873 |
| Bench top stability solutions (after 48 h) | 102.18±0.6235 | 102.27±0.8964 | 101.65±1.0383 | |
| Auto sampler stability solutions (after 48 h) | 101.65±1.0677 | 102.07±0.6045 | 101.02±1.130 |
Note: * replicates six sample analysis determinations
Table 5: Results for Robustness and Solution Stability Study for Ndma
In the present work, LC-MS/MS method for estimation of NDMA as an impurity in RAN tablets is developed and validated as per ICH guidelines. In most of reported methods, the main objective is assay of RAN along with other degradation products. Very few analytical methods like HSSPME- GC-MS are reported for quantitation of NDMA in RAN. The availability of HS-SPME-GCMS, highly sophisticated hyphenated technique limits the utility in routine analysis. Recently NDMA has been reported to be a common impurity found in food, water and many drug formulations during storage or process of manufacturing. Hence, it was thought worthwhile to develop and validate LC-MS/MS assay method for NDMA. This method can be applied to different brands of RAN formulations and used for estimation of NDMA as an impurity in RAN tablets. The retention time for NDMA and RAN was found to be 2.905±0.01 min and 10.544±0.01 min respectively. The chromatogram shows that there is no interference by the excipients likely to be present in formulation in analysis of NDMA. This indicates that method is simple, specific and less time consuming. The gradient mode of separation was selected using solvent A and solvent B as shown in chromatographic conditions. The linearity was established for NDMA with range of 1-50 ng/ml and validated as per ICH guidelines. The percent recovery was found to be in range of 98.07 to 100.22 % indicating that the method is accurate and can selectively determine NDMA in presence of the RAN formulations. As per literature study, the limit allowed per day in RAN tablets for the NDMA as an impurity is below 300 ng/ml intended for human use. LOD (0.3 ng/ml) and LOQ (1 ng/ml) is well below the reported limit which shows high sensitivity for the detection and quantification of NDMA in RAN. The standard deviation for intraday and inter-day precision was found in the range of (±0.5463 to ±1.2144) and (±0.9083 to ±1.7865) respectively, indicating the repeatability and reproducibility of the method for quantification of NDMA. The data obtained in ruggedness and solution stability studies expressed the lack of influence of operational and environmental variables on the test results obtained by proposed method. After the study in tablets, NDMA was not detected from RAN formulation in normal storage conditions as prescribed in pharmacopoeia. For the analysis of parent substance and tablet dosage forms, impurity of NDMA is unknown and it isn’t exceeded 0.10 % identification threshold. Hence, it was believed that this technique has the high potential for analyzing the estimation of NDMA in RAN pharmaceutical dosage form.
Acknowledgments:
The sample of RAN and NDMA was provided by "Clearsynth Lab" (Mumbai, India), for which the authors are appreciative. For their assistance in completing this work, the authors thank to Bioanalytical Technologies (India) Pvt. Ltd., Pune, India.
Conflict of interests:
The authors declared no conflict of interests.
References
- Abe Y, Yamamoto E, Yoshida H, Usui A, Tomita N, Kanno H, et al. Temperature-dependent formation of N-nitrosodimethylamine during the storage of ranitidine reagent powders and tablets. Chem Pharm Bull 2020;68(10):1008-12.
[Crossref] [Google Scholar] [PubMed]
- White CM. Understanding and preventing (N-nitrosodimethylamine) NDMA contamination of medications. Ann Pharmacother 2020;54(6):611-4.
[Crossref] [Google Scholar] [PubMed]
- United States Food and Drug Administration. Statement alerting patients and health care professionals of NDMA found in samples of ranitidine; 2019.
- Zeng T, Mitch WA. Oral intake of ranitidine increases urinary excretion of N-nitrosodimethylamine. Carcinogenesis 2016;37(6):625-34.
[Crossref] [Google Scholar] [PubMed]
- Aldawsari FS, Alshehry YM, Alghamdi TS. N-nitrosodimethylamine (NDMA) contamination of ranitidine products: A review of recent findings. J Food Drug Anal 2021;29(1):39.
[Crossref] [Google Scholar] [PubMed]
- Pottegård A, Kristensen KB, Ernst MT, Johansen NB, Quartarolo P, Hallas J. Use of N-nitrosodimethylamine (NDMA) contaminated valsartan products and risk of cancer: Danish nationwide cohort study. BMJ 2018;362.
- United States Department of Health and Human Services. Food and Drug Administration Center for Drug Evaluation and Research (CDER). Guidance for industry, control of nitrosamine impurities in human drugs, pharmaceutical quality/manufacturing standards/current good manufacturing practice (CGMP); 2021.
- United States Food and drug administration. Statement on new testing results, including low levels of impurities in ranitidine drugs; 2019.
- United States Food and drug administration. FDA Requests removal of all ranitidine products (Zantac) from the market; 2020.
- Tummala SR, Pawar KM. Drug Recalled. Spinco Biotech Cutting Edge2021;11(5): 26-31.
- United States of Food and Drug Administration. Food laboratory tests/ranitidine, laboratory analysis of ranitidine and nizatidine products; 2019.
- Tricker AR, Preussmann R. Carcinogenic N-nitrosamines in the diet: occurrence, formation, mechanisms and carcinogenic potential. Mutat Res 1991;259(3-4):277-89.
[Crossref] [Google Scholar] [PubMed]
- Fristachi A, Rice G. Estimation of the total daily oral intake of NDMA attributable to drinking water. J Water Health 2007;5(3):341-55.
[Crossref] [Google Scholar] [PubMed]
- Therapeutic Goods Administration. Laboratories testing of ranitidine medicines: Contamination of ranitidine medicines with the nitrosamine NDMA; 2019.
- O'Neil MJ, editor. The Merck index: An encyclopedia of chemicals, drugs, and biologicals. RSC Publishing; 2013.
- Sweetman SC, Blake PS. Martindale. The complete drug reference. 2011;1389.
- The United States Pharmacopeia, USP 34: The National Formulary 29. The United States Pharmacopoeial Convention: Rockville; 2011:4120.
- Alshehri YM, Alghamdi TS, Aldawsari FS. HS-SPME-GC-MS as an alternative method for NDMA analysis in ranitidine products. J Pharm Biomed Anal 2020;191:113582.
[Crossref] [Google Scholar] [PubMed]
- Yokoo H, Yamamoto E, Masada S, Uchiyama N, Tsuji G, Hakamatsuka T, et al. N-Nitrosodimethylamine (NDMA) formation from ranitidine impurities: possible root causes of the presence of NDMA in ranitidine hydrochloride. Chem Pharm Bull 2021;69(9):872-6.
[Crossref] [Google Scholar] [PubMed]
- United States Food Drug Administration, Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) Method for the determination of NDMA in ranitidine drug substance and solid dosage drug product; 2019.
- Angrish P, Mani C, Banerjee S. Development of a validated method of testing for NDMA in ranitidine. Pharm Technol 2020;44(10):42-7.
[Crossref] [Google Scholar] [PubMed]
- Yamamoto E, Kan-No H, Tomita N, Ando D, Miyazaki T, Izutsu KI. Isolation of N-nitrosodimethylamine from drug substances using solid-phase extraction-liquid chromatography–tandem mass spectrometry. J Pharm Biomed Anal 2022;210:114561.
[Crossref] [Google Scholar] [PubMed]