- *Corresponding Author:
- Susan kurian
Department of Zoology, Mar Ivanios College (Autonomous), Thiruvananthapuram, Kerala 695015, India
E-mail: susankurianmic@gmail.com
| Date of Received | 02 February 2022 |
| Date of Revision | 04 May 2023 |
| Date of Acceptance | 02 January 2024 |
| Indian J Pharm Sci 2023;86(1):222-227 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Artocarpus heterophyllus Lam. is the common jackfruit tree belongs to family Moraceae. The protective role of leaf petiole part of the plant was investigated in experimental hepatotoxicity. Antioxidant enzyme activity was investigated in acetaminophen intoxicated rat liver treated with methanol extract of Artocarpus heterophyllus. Phytochemical analysis and the antioxidant property of successive hexane, ethyl acetate and methanol extracts of Artocarpus heterophyllus showed highest activity with methanol extract (Artocarpus heterophyllus). Superoxide dismutase, catalase (antioxidant enzymes) and glutathione showed significant increase in rats with oral administration of Artocarpus heterophyllus compared to the acetaminophen treated group. The degree of hepatoprotection was assessed by the activity of aspartate amino transferase, alanine aminotransferase, alkaline phosphatase, lactate dehydrogenase and gamma glutamyl transpeptidase. A dose dependant protective effect of Artocarpus heterophyllus (200 mg/kg body weight, 400 mg/kg body weight) against acetaminophen-induced hepatotoxicity in albino rats was identified. Histopathological evaluation of the liver confirmed the hepatoprotective function of methanol extract of Artocarpus heterophyllus as evident in biochemical evaluations. The results of the present study suggest the potential use of leaf petiole of Artocarpus heterophyllus in the treatment of liver disease due to its ability to protect as an antioxidant.
Keywords
Artocarpus heterophyllus Lam., hepatoprotection, antioxidant enzymes, acetaminophen
Reactive Oxygen Species (ROS) are spontaneously generated in cells during metabolism and are implicated in the etiology of different degenerative diseases[1]. Oxidative stress plays a fundamental role in the acute hepatoxicity of several drugs, including the world-widely used analgesic and antipyretic Acetaminophen (APAP)[2]. Increase in the use of synthetic drugs lead to many side effects and undesirable hazards[3]. This leads to the search of natural products which are economically viable and culturally acceptable.
The therapeutic use of many traditional medicines attributes the presence of antioxidant properties. Artocapus heterophyllus (A. heterophyllus) (Jackfruit), belonging to Moraceae is reported to have anti-inflammatory, antidiuretic, antioxidant, antifungal and immunomodulatory properties[4,5]. Various studies revealed the diverse secondary metabolites present in its fruits and seeds[6-9] . Hepatoprotective activity of aqueous extract of seed, leaf and fruit of A. heterophyllus was reported earlier with Carbon tetrachloride (CCl4) induced hepatotoxicity on Swiss albino mice[10-12]. Hot water decoction of the mature leaf petiole of Jackfruit is traditionally used for many ailments due to its anti-inflammatory effect[13].
Although many medicinal properties of the plant are reported, no investigations have been so far done to validate the hepatoprotective role of leaf petiole part of A. heterophyllus through its antioxidant protection. Hence the objective of the present study is to evaluate the hepatoprotective action of A. heterophyllus against APAP induced hepatotoxicity.
Materials and Methods
Plant material:
A. heterophyllus Lam. was collected locally from Thiruvananthapuram, Kerala, India. The plant materials were identified and the voucher specimen had been deposited in the Herbarium of Department of Botany, University of Kerala, Thiruvananthapuram, Kerala, with voucher number: KUBH-6027 for future reference.
Preparation of plant extracts:
The petioles of mature leaves of A. heterophyllus were collected, washed and shade dried. The dried plant material was powdered and was successively extracted in soxhlet apparatus with hexane (AHH), ethyl acetate (AHE) and methanol (AHM) in the order of their increasing polarity until it becomes colourless[14]. The solvents were removed from the extracts using rotary vacuum evaporator and stored in the refrigerator for further analysis. Phytochemical analysis and the antioxidant property of the extracts showed highest activity with AHM[6,7]. So AHM was selected for further investigations.
Experimental animals:
Male and female albino rats (Rattus norvegicus) of Wistar strain (150-200 g) were used for the experiment. The animals were maintained in animal house as per Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) regulations. Food and water was provided ad libitum. The animals were acclimatized with the laboratory conditions before doing the experiments. Ethical clearances were obtained prior to the experiments, from the Institutional Animal Ethical Committee for the studies with the leaf petiole extracts of A. heterophyllus Lam. (B1/3/2015/1396/PO/E/S/10/ CPCSEA).
Acute toxicity study:
Wistar albino rats (200 g body weight) were used for the acute toxicity studies following Organisation for Economic Co-operation and Development guideline No. 423[15]. The methanol extract of A. heterophyllus Lam. was administered orally to the fasted (overnight) animals at a dose 2000 mg/kg body weight. The animals were monitored constantly for 3 h and extended up to 24 h. The animals were furthered observed for next 14 d for mortality and behavioural changes.
APAP induced hepatotoxicity:
Hepatoprotective activity of the AHM of the plant, particularly its protective role was determined by the method of Kumar et al.[16]. One-tenth and twotenth of the minimum lethal dose, i.e., 200 mg/ kg body weight and 400 mg/kg body weight were selected as the therapeutic dose for the evaluation of hepatoprotective activity. Male Wistar strain of albino rats (130-200 g body weight) were divided into five groups of 6 animals each and treated as follows.
Animals in Group I served as normal and was given normal saline for 10 d in succession. Group II animals served as negative control, administered with normal saline for 10 d and were given APAP at 3 g/kg body weight on the 10th d. The group III and IV animals served as experimental and were treated orally with methanol extract of 200 mg/kg body weight and 400 mg/body weight respectively for 10 d followed by a single oral administration of APAP at 3g/kg body weight, 1 h after last day of extract administration. Group V served as the standard and were treated with Silymarin at 50 mg/ kg body weight for 10 d followed by a single oral administration of APAP at 3 g/kg body weight, 1 h after last day of extract administration. Blood samples and liver tissues were collected for the estimations and histopathological observations.
Biochemical estimations:
Serum was obtained from clotted blood samples by centrifugation at 1500 g for 10 min. The Liver marker enzymes such as Aspartate Aminotransferase (AST) Alanine Aminotransferase (ALT) Alkaline Phosphatase (ALP), Lactate Dehydrogenase (LDH) and Gamma Glutamyl Transpeptidase (GGT) were evaluated following standard protocol using the kit from AGAPPE Diagnostics, India.
A part of the rat’s liver as 10 % homogenate in ice-cold sucrose and supernatant fraction after centrifugation at 600 ×g in a cold centrifuge was used for assessing the biochemical estimations while remaining part of the liver was used for histopathological studies. Antioxidant status of liver tissue was assessed from reduced Glutathione (GSH) level and enzyme activities of Superoxide Dismutase (SOD), Catalase (CAT), Glutathione- S-Transferase (GST), and Glutathione Peroxidase (GPx) estimations[17-21]. The protein content in tissue homogenates was determined by the method of Lowry et al.[22] using bovine serum albumin as a standard. Lipid peroxidation was determined by measuring the Thiobarbituric Acid Reactive Substances (TBARS)[23] and the Conjugated Dienes (CD)[24] concentrations.
Histopathological examination:
Immediately after sacrifice, excised liver tissue was fixed in 10 % formalin, then washed, dehydrated in descending grades of isopropanol and finally rinsed with xylene. The tissues were then embedded in molten paraffin wax. Sections were cut at 5 mm thickness, stained with haematoxylin-eosin and observed microscopically for histopathological changes.
Statistical analysis:
The data was expressed as mean±standard error of mean, (n=6). One-way Analysis of Variance (ANOVA) followed by Duncan’s Multiple Range Test (DMRT) (Statistical Package for Social Sciences (SPSS)-version 19) was used for analysis. Values of p<0.05 were considered statistically significant.
Results and Discussion
Acute toxicity studies on AHM specified the absence of any toxic changes at a dose level of 2000 mg/kg, indicating the extract could be considered safe to rats. Based on this observation the doses of 200 mg/kg (one tenth of the maximum dose) and 400 mg/kg (twice that of one tenth dose), were justified for the rest of the investigations.
Oral administration of APAP to rats produced significant rise (p<0.01) in enzyme levels such as AST, ALT, ALP, LDH and GGT. Treatment with AHM significantly (p<0.05) prevented the increase in the level of these enzymes in a dose dependent manner (Table 1). The level of the antioxidant enzymes like SOD, CAT, GPx and GST were also found significantly (p<0.05) decreased by APAP administration when compared to the control group. Treatment with the methanol extract (200 mg/kg and 400 mg/kg) resulted in significant increase in antioxidant enzymes that is comparable with that of Silymarin treated groups (Table 2).
| Group | AST (U/l) | ALT (U/l) | ALP (U/l) | GGT (U/l) | LDH (U/l) |
|---|---|---|---|---|---|
| Normal | 73.66±1.11 | 147.16±0.8 | 133.6±0.7 | 3.45±0.05 | 181.09± 0.83 |
| APAP control (3 g/kg body weight) | 185.33±0.98# | 551.33±2.2# | 258±2.3# | 8.53±0.08# | 577.88± 1.32# |
| APAP+AHM (200 mg/kg body weight) | 134.16±0.74#* | 245.33±1.2#* | 238.1±1.5#* | 7.48±0.07#* | 475.08± 1.01#* |
| APAP+AHM (400 mg/kg body weight) | 78.66±1.14#* | 151.33±1.0* | 148.3±0.6#* | 5.78±0.03#* | 191.35± 1.53#* |
| APAP+Silymarin (50 mg/kg body weight) | 74.83±1.3* | 147.50±1.56* | 142.8±1.5#* | 5.3±0.03#* | 189.05± 2.4#* |
| p | .000** | .000** | .000** | .000** | .000** |
Note: **Significant at 1 % level (p<0.01); Values are mean±standard error (n=6); #p<0.05 significantly different when compared with normal
Table 1: Effect of methanol extract of A. Heterophyllus (AHM) on the hepatic function marker enzymes (AST, ALT, ALP, GGT and LDH) in serum of acetaminophen intoxicated rats
| Group | SOD | CAT | GPx | GST |
|---|---|---|---|---|
| Normal | 1.89±0.01 | 72.5±0.62 | 1.75±0.03 | 3.68±0.13 |
| APAP control (3 g/kg body weight) | 1.14±0.02# | 29.7±0.39# | 0.4±0.07# | 1.01±0.02# |
| APAP+AHM (200 mg/kg body weight) | 1.31±0.02#* | 41.1±0.41#* | 0.41±0.009# | 1.13±0.02# |
| APAP+AHM (400 mg/kg body weight) | 1.55±0.04#* | 48.02±0.73#* | 0.86±0.07#* | 1.68±0.05#* |
| APAP+Silymarin (50 mg/kg body weight) | 1.8±0.02* | 61.9±4.9#* | 0.97±0.03#* | 2.3±0.07#* |
| p | .000** | .000** | .000** | .000** |
Note: **Significant at 1 % level (p<0.01); values are mean±standard error of mean (n=6); #p<0.05 significantly different when compared with normal; *p<0.05 significantly different when compared with control; SOD Units/min/mg protein, CAT µmol of H2O2 decomposed/min/mg protein, GPx µmol/min/mg protein and GST µmol of CDNB/mg protein
Table 2: Effect of methanol extract of A. Heterophyllus (AHM) on the activity of antioxidant enzymes (SOD, CATALASE, GPX and GST) in liver of acetaminophen intoxicated rats
The TBARS and CD values were increased from normal level (TBARS: 0.01±0.009 μmol/g of tissue and CD: 4.35±0.09 μmol/g of tissue) to 0.07±0.06 μmol/g of tissue and 10.2±0.06 μmol/g of tissue respectively in APAP intoxication. Administration of AHM (400 mg/kg body weight) extract significantly (p<0.05) reduced these levels to 0.02±0.03 μmol/g of tissue (TBARS) and 7.85±0.12 μmol/g of tissue (CD) respectively. The GSH level was decreased significantly (p<0.05) in the APAP treated rat liver, while GSH level was increased significantly (p<0.05) to the normal level in the AHM pre-treated animals.
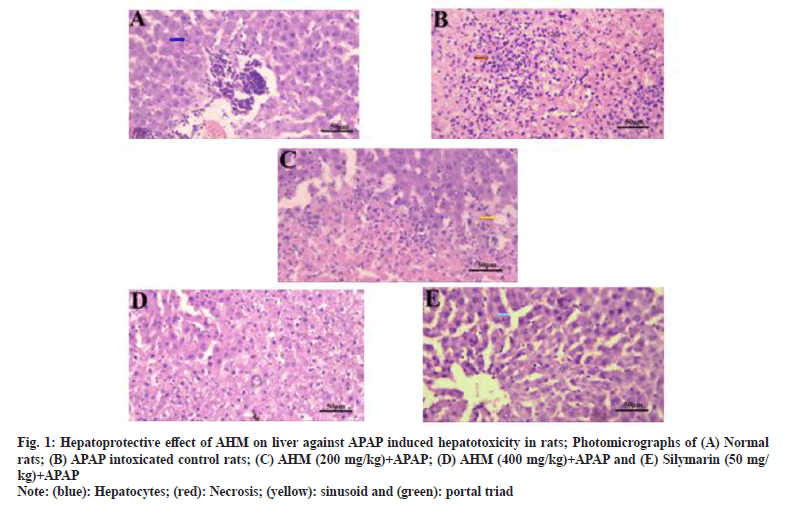
Hepatoprotective effect of the methanol extracts of the petiole of mature leaves of A. heterophyllus Lam. were evident and confirmed upon histopathological examinations. The liver histology of the normal control rats is shown in fig. 1A. The liver histology of APAP treated animals showed high necrosis (fig. 1B). The hepatic cells showed severe toxicity which is characterized by centrilobular necrosis, dilated sinusoidal spaces and nodule formation.
AHM (400 mg/kg body weight) administered rats showed liver pattern with fewer necrotic zones (fig. 1D). The liver histology of the rats pretreated with silymarin showed normal architecture with few fatty changes (fig. 1E). Histological observations supported the results obtained from biochemical investigations. Thus the methanol extract of A. heterophyllus evoke hepatoprotective activity against APAP induced cellular damage in liver at doses 200 mg/kg body weight and more significantly at 400 mg/kg body weight.
Fig. 1:Hepatoprotective effect of AHM on liver against APAP induced hepatotoxicity in rats; Photomicrographs of (A) Normal rats; (B) APAP intoxicated control rats; (C) AHM (200 mg/kg)+APAP; (D) AHM (400 mg/kg)+APAP and (E) Silymarin (50 mg/kg)+APAP
Note: (blue): Hepatocytes; (red): Necrosis; (yellow): sinusoid and (green): portal triad
Phytochemical screening of the hexane, ethyl acetate and methanol extracts of leaf petiole of A. heterophyllus showed a higher concentration of saponins, alkaloids, tannins, phenols, flavanoids, quinones, steroids and terpenoids in methanol extract than other two extracts[6,7]. Methanolic extract of A. heterophyllus was demonstrated to be hepatoprotective in action through its antioxidant property in APAP intoxicated experimental rats. APAP, a common antipyretic agent, though safe in therapeutic doses but cause fatal hepatic necrosis with toxic dose[25]. The APAP toxicity is evident with increased cytoplasmic enzymes (AST, ALT, ALP, LDH and GGT) showing the hepatic damage[26]. The damage of plasma membrane of liver cell causes the release of enzymes into blood stream. Restoring this enzyme activity to normal level by methanolic extract of A. heterophyllus proves the hepatoprotective role of these plant extract. The pre-treatment of AHM (400 mg/kg) has significantly improved LDH activity to normal in hepatic challenged rats. Increase in LDH level of serum is met with hepatic dysfunctions[27].
AHM treatment elevated the antioxidant enzymes in hepatic-challenged rats. The antioxidant enzymes, SOD and CAT have been found to be of great importance in assessment of liver damage which serves as biomarker of hepatocellular injury due to alcohol and drug toxicity[28]. The decline in the level of catalase may result in deleterious effect since it may lead to the accumulation of superoxide radicals and hydrogen peroxide[29]. GSH, plays a central role in coordinating the body’s antioxidant defence processes[30]. The GSH content in the liver of APAP intoxicated rats was lowered that returned towards a near normalcy in groups administered with extracts (AHM) prior to APAP indicating the prevention of lipid peroxidation by the phyto-compounds present in the extract. The decreased level of GSH is always associated with enhanced level of lipid peroxidation in APAP intoxicated groups of rats. Elevated level of GSH in AHM treatment conclusively prove the antioxidant property of this plant product. The hepatoprotective activity of the extracts was also evaluated by estimating the lipid profile (Cholesterol, Triglycerides and Total lipids) in liver tissues and blood serum. The lipid profile significantly elevated in APAP intoxicated animal groups than the normal ones[31]. The pretreatment of AHM showed a significant decrease in the lipid profile (Table 3).
| Group | TBARS (µmol/g of tissue) | CD (µmol/g of tissue) | GSH (µmol/g of tissue) |
|---|---|---|---|
| Normal | 0.01±0.0009 | 4.35±0.09 | 2.63± 0.06 |
| APAP control (3 g/kg body weight) | 0.07±0.008# | 10.2±0.06# | 1.23±0.02# |
| APAP+AHM (200 mg/kg body weight) | 0.06±0.008# | 8.06±0.11#* | 1.6±0.03# |
| APAP +AHM (400 mg/kg body weight) | 0.02±0.003* | 7.85±0.12#* | 1.6±0.1#* |
| APAP+Silymarin (50 mg/kg body weight) | 0.02±0.07* | 6.09±0.1#* | 1.64±0.07#* |
| p | .018** | .000** | .000** |
Note: **Significant at 1 % level (p<0.01); Values are mean±standard error (n=6); #p<0.05 significantly different when compared with normal; *p< 0.05 significantly different when compared with control
Table 3: Effect of methanol extract of A. Heterophyllus (AHM) on lipid peroxidation (TBARS and CD) and gsh in liver of acetaminophen intoxicated rats
The present study showed that the extract, AHM was effective in regaining the normal serum values of liver function marker enzymes from the APAP intoxicated conditions. This was comparable to the effect of using standard drug, silymarin[32]. Histopathological evaluation of the liver confirmed the hepatoprotective functions of methanol extract of A. heterophyllus as evident in biochemical evaluations.
AHM plays a significant role in peroxidation by inhibiting the free radical attack on bio membrane. The methanol extract retained the normal architecture of liver. The maintenance of tissue architecture by the extract helped to validate the results obtained in biochemical estimations of serum and tissue markers of toxicity.
In conclusion, the AHM of the plant showed a significant hepatoprotection of APAP induced liver injury. Antioxidant status in the liver tissue (SOD, CAT, GPx, and GST) as well as lipid peroxidation (TBARS and CD) substantiated the protective effect of the plant extract. The tissue architecture was maintained by the plant extract in histological examination. The mechanism of the hepatoprotective effect of AHM may be due to its antioxidant effect. These properties show the high medicinal use and effectiveness of the plant in treatment of different diseases. Further study is needed to identify and isolate the active compounds present in the plant extract which can have antioxidant and hepatoprotective properties.
Acknowledgement:
The authors thank the Principal, Mar Ivanios College, Thiruvananthapuram, Kerala, for facilitating with laboratory support during this study. The financial assistance from the UGC to the first author is gratefully acknowledged.
Conflict of interests:
The authors declared no conflict of interests.
References
- Halliwel B. Free radicals, antioxidants and human disease. Where are we now? J Lab Clin Med 1992;119:598-620.
[Google Scholar] [PubMed]
- Reid AB, Kurten RC, McCullough SS, Brock RW, Hinson JA. Mechanisms of acetaminophen-induced hepatotoxicity: Role of oxidative stress and mitochondrial permeability transition in freshly isolated mouse hepatocytes. J Pharmacol Exp Ther 2005;312(2):509-16.
[Crossref] [Google Scholar] [PubMed]
- Kuriakose GC, Kurup MG. Antioxidant and hepatoprotective activity of Aphanizomenon flos-aquae Linn against paracetamol intoxication in rats. Indian J Exp Biol 2010;48(11):1123-30.
[Google Scholar] [PubMed]
- Fernando MR, Wickramasinghe SN, Thabrew MI, Ariyananda PL, Karunanayake EH. Effect of Artocarpus heterophyllus and Asteracanthus longifolia on glucose tolerance in normal human subjects and in maturity-onset diabetic patients. J Ethnopharmacol 1991;31(3):277-82.
[Crossref] [Google Scholar] [PubMed]
- Li Z, Lan Y, Miao J, Chen X, Chen B, Liu G, et al. Phytochemicals, antioxidant capacity and cytoprotective effects of jackfruit (Artocarpus heterophyllus Lam.) axis extracts on HepG2 cells. Food Biosci 2021;41:100933.
- Kurian S, Joseph L, Josekumar VS. Phytochemical evaluation, cytotoxicity screening, and in Family: Moraceae). J Pharm Res 2018;12(4):455-60.
- Kurian S, Joseph L, Josekumar VS. Phytochemical evaluation, GC-MS analysis and antimicrobial activity of the leaves petiole of Lam Artocarpus heterophyllus. Asian J Pharm Pharmacol 2018;4(3):280-7.
- vitro free radical scavenging activity of the leaf petiole of Artocarpus heterophyllus Lam. (
- Sreeja Devi PS, Kumar NS, Sabu KK. Phytochemical profiling and antioxidant activities of different parts of Artocarpus heterophyllus Lam. (Moraceae): A review on current status of knowledge. Future J Pharm Sci 2021;7:1-7.
- Sreeja Devi PS, Kumar NS, Sabu KK. Phytochemical profiling and antioxidant activities of different parts of Artocarpus heterophyllus Lam.(Moraceae): A review on current status of knowledge. Future Journal of Pharmaceutical Sciences. 2021 Dec;7:1-7.
- Prakash O, Srivastava R, Kumar R, Kumar R. Hepatoprotective activity of Artocarpus heterophyllus lam. Leaves against thioacetamide induced hepatotoxicity on Wistar albino rats. Int Res J Pharm 2016;7(4):24?9.
- Phukan H, Singha LI, Mitra PK. Hepato-protective effect of aqueous extract of seed, leaf and fruit of jackfruit (Artocarpus heterophyllus lam.) against ccl4 induced hepatotoxicity on Swiss albino mice. World J Pharm Res 2018;7(9):766?79.
- Sreeletha AS, Lini JJ, Dhanyalekshmi CS, Sabu KR, Pratap Chandran R Phytochemical analysis, antimicrobial and antioxidant activity evaluations of fruit of Artocarpus heterophyllus Lam. integrative food. Nutr Metab 2018;5(6):1-7.
- Chandrika UG, Wedage WS, Wickramasinghe SM, Fernando WS. Hypoglycaemic action of the flavonoid fraction of Artocarpus heterophyllus leaf. Afr J Tradit Complemen Altern Med 2006;3(2):42-50.
- Harborne JB, Phytochemical methods. Chapman and Hall publications. 2nd ed. London; New York;1984. p. 288.
- OECD (Organization for Economic Cooperation and Development). Guideline for testing of chemicals: Acute oral toxicity?acute toxic class method. Guideline 423; 2001.
- Kumar G, Banu GS, Kannan V, Pandian MR. Antihepatotoxic effect of ß-carotene on paracetamol induced hepatic damage in rats. Indian J Exp Biol 2005;43(4):351-5.
[Google Scholar] [PubMed]
- Ellman M. A spectrophotometric method for determination of reduced glutathione in tissues. Anal Biochem 1959;74(1):214-6.
[Crossref] [Google Scholar] [PubMed]
- Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys 1984;21(2):130-2.
[Google Scholar] [PubMed]
- Marklund S. Handbook of methods for oxygen radical research. Boca Raton: FL; 1985. p. 243-7.
- Jakoby WB, Habig WH. Glutathione transferases. Enzymatic Basis Detoxication 1980;2:63-94.
- Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra W. Biochemical role as a component of glutathione peroxidase. Science 1973;179(4073):588-90.
[Crossref] [Google Scholar] [PubMed]
- Lowry OH, Rosebrough NJ, Farr AL, Randall R J. Protein estimation by Lowry?s method. J Biol Chem 1951;193-265.
- Niehaus Jr WG, Samuelsson B. Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur J Biochem 1968;6(1):126-30.
[Crossref] [Google Scholar] [PubMed]
- Beuje JA, Aust SD. In Methods in enzymology, edited by Clowick S P and Kaplan NO, 1978.
- Hinson JA, Roberts DW, James LP. Mechanisms of acetaminophen-induced liver necrosis. Handb Exp Pharmacol 2010:(196):369-405.
[Crossref] [Google Scholar] [PubMed]
- Plaa GL, Hewitt WR. Quantitative evaluation of indices of hepatotoxicity. Toxicol Liver 1982;103-20.
- Sherlock S, Dooley J. The liver in infancy and childhood. Diseases of the liver and biliary system. Blackwell Sci Publications. Oxford 1993; 434-51.
- Kuriakose GC, Kurup MG. Antioxidant and hepatoprotective activity of Aphanizomenon flos-aquae Linn against paracetamol intoxication in rats. Indian J of Exp Biol 2010; 48. 1123-30.
[Google Scholar] [PubMed]
- Gupta M, Mazumder UK, Siva KT, Gomathi P, SAMBATH KR. Antioxidant and hepatoprotective effects of bauhinia racemosa against paracetamol and carbon tetrachloride induced liver damagein rats. Irania J Pharmacol Ther 2004;3(1):12-20.
- Colvin OM, Friedman HS, Gamcsik MP, Fenselau C, Hilton J. Role of glutathione in cellular resistance to alkylating agents. Adv Enzyme Regul 1993;33:19-26.
[Crossref] [Google Scholar] [PubMed]
- Sharifudin SA, Fakurazi S, Hidayat MT, Hairuszah I, Aris Mohd Moklas M, Arulselvan P. Therapeutic potential of Moringa oleifera extracts against acetaminophen-induced hepatotoxicity in rats. Pharm Biol 2013;51(3):279-88.
[Crossref] [Google Scholar] [PubMed]
- Ahmed AA, Rahman MA, Hossen MA, Reza AA, Islam MS, Rashid MM, et al. Epiphytic Acampe ochracea orchid relieves paracetamol-induced hepatotoxicity by inhibiting oxidative stress and upregulating antioxidant genes in in vivo and virtual screening. Biomed Pharmacother 2021;143:112215.
[Crossref] [Google Scholar] [PubMed]