- *Corresponding Author:
- P. P. Shivgunde
Department of Pharmacology, Maratha Vidya Prasarak Samaj's College of Pharmacy, MVP Campus, Nashik, Maharashtra 422002, India
E-mail: prashantshivgunde@gmail.com
| Date of Received | 12 March 2021 |
| Date of Revision | 21 May 2022 |
| Date of Acceptance | 19 July 2023 |
| Indian J Pharm Sci 2023;85(4):997-1009 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
L-citrulline, a natural antioxidant and nitric oxide donor, has beneficial cardiovascular properties. The present study investigated the differences in the outcomes between L-citrulline monotherapy and combination therapy with spironolactone as initial treatments for test animals with deoxycorticosterone acetate saltinduced hypertension. Deoxycorticosterone acetate salt (20 mg/kg, s.c.) was administered twice a week in male Wistar rats for 4 w with daily drinking water containing sodium chloride (1 %) and potassium chloride (0.2 %) to develop hypertension. In the development period, treatment groups received a daily dose of L-citrulline (50 or 100 mg/kg, p.o.) alone or in spironolactone (50 or 100 mg/kg, p.o.) combination. Treatment effects assessed by measuring non-invasive and invasive blood pressure, body and heart weight, vascular reactivity to catecholamines, acetylcholine-induced vasorelaxation in the isolated artery, and oxidative stress in heart specimens. Compared to the deoxycorticosterone acetate group, the L-citrulline and spironolactone combination therapy dose-dependently ameliorated the elevated levels of different blood pressure parameters, improved cardiac hypertrophy, vascular reactivity, aorta relaxant responses and attenuated oxidative stress. In addition, L-citrulline low and high-dose monotherapy demonstrated significant improvements in all evaluated parameters except vascular reactivity. However, compared to combination therapy, the performance of L-citrulline or spironolactone alone treatment is slightly inferior. Our results conclude that L-citrulline is a promising treatment option for treating hypertension, and L-citrulline can be combined with spironolactone to get a synergistic effect in treating hypertension.
Keywords
Combination therapy, antioxidants, blood pressure, citrulline, nitric oxide, spironolactone
In the 'HEARTS technical package treatment protocols', World Health Organization recommends polytherapy when monotherapy cannot control hypertension. This protocol is now accepted and implemented by about fifteen countries globally[1]. Until now, there has been scant evidence of polytherapy-related side effects. However, few components of polytherapy have been recognized as ineffective, for example, concurrent use of Angiotensin-Converting Enzyme-Inhibitors (ACE-Is) and Angiotensin II Receptor Blockers (ARBs)[2]. The need for novel, effective, and safe combination therapies for patients with hypertension who are not responding to monotherapy is well acknowledged.
L-citrulline (CIT), a natural precursor to L-arginine, is receiving attention due to many pieces of data from preclinical and clinical trials. In preclinical studies, CIT enhanced the L-arginine/asymmetrical dimethylarginine ratio, boosted Nitric Oxide (NO) levels, protected endothelial function by lowering superoxide generation, and the accompanying ELK- 1 and p-CREB, two oxygen-sensitive proteins[3-5]. Preclinical investigations support the evidence for managing Hypertension following CIT introduction. In clinical trials on healthy, young subjects, CIT supplementation increases L-arginine, NO-dependent signaling, vascular conductance and has the potential to upsurge peripheral tissue oxygenation and dwindle systemic BP[6-8]. However, CIT supplementation showed no apparent side effects[9,10].
Evidence is growing to suggest the therapeutic efficacy and safety of CIT supplementation in hypertension. However, no previous research evaluated the impact of CIT alone or in conjunction with marketed anti- hypertensive medications in a Deoxycorticosterone Acetate salt (DOCA salt)-induced hypertension model. The current model chosen as oxidative stress is a significant factor in the DOCA salt hypertension model and closely reflects the clinical condition of aberrant aldosterone secretion[11]. Furthermore, smaller doses of drugs with distinct modes of action can generate synergistic or additive benefits in BP while avoiding dose-dependent side effects[12]. Therefore, the present study examined CIT effects with or without Spironolactone (SPRL) in DOCA salt-induced hypertension model.
Materials and Methods
Animals:
The National Institute of Biosciences, Pune, India, supplied the healthy young adult male Wistar rats weighing 220 g (±10 %). Previous studies showed that upregulation of Endothelin Receptors (ETB) receptors in males, more remarkable T regulatory cells in females, and adrenal medullae role in the maintenance of hypertension in males causes females not become as hypertensive as male rats after DOCA salt administration; hence male rats were preferred in this study[13-15]. As other researchers successfully developed hypertension at the selected ages, young Wistar rats were considered in the study[16,17]. During this study, the experimental animal room was kept at a temperature of 22° (±3°) and relative humidity of 50-60 %. An artificial 12 h light and a dark cycle were provided throughout the experiment. Standard laboratory food (protein~23 %) was used with an unlimited supply of water for feeding. According to dose, animals were housed in groups of three or fewer to ensure that each animal was observed clearly. However, no animal was individualized in the cage. Care was taken to keep bedding dry throughout the experiment as some groups of animals frequently urinated. Also, animals were trained for restraining procedures to measure blood pressure and placed in cages having paper strips and pipes cuttings to hide. The welfare of animals was maintained by carrying out the research in a conventional housing facility according to the standards set forth by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), New Delhi (India). In addition, the study methodology was approved by the Maratha Vidya Prasarak Samaj's College of Pharmacy's Institutional Animal Ethics Committee (IAEC/2017/08), located in Nashik, Maharashtra, India.
Materials:
Pharmaceutical grade CIT from Nutrija Lifesciences, India, and DOCA salt from Sigma-Aldrich (USA) was purchased. In addition, SPRL (Aldactone 100) was secured from RPG Life Sciences, Ankleshwar, India. The remainder of the compounds were of analytical quality and were obtained from Sigma Aldrich (USA).
Sample size calculation:
The sample size was calculated from previous studies' data performed at our laboratory and using GPower 3.1 software. F test-Analysis of Varience (ANOVA); repeated measures, within-between interaction was chosen with effect size f=0.30, α error probability=0.05, power (1-β error probability)=0.95, number of groups=11, number of measurements=5, nonsphericity correction ε=1 as input parameters. Inputs yielded a total sample size of 55 with a 1.46 critical F value. However, one additional animal was used in each group over the calculated sample size to manage attrition. Likewise, the total number of animals used in the study were 66.
Dose selection:
The two CIT doses, 50 and 100 mg/kg, were selected based upon reported arginase inhibiting activity in a fructose-induced hypertension study by El-Bassossy et al.[18].
Experimental design:
Before being used in this investigation, rats were given 14 d to acclimate to laboratory settings. In addition, a week before the commencement of the experiment, animals were trained twice weekly for restraining procedures to monitor BP using the tail- cuff method. On the 1st d, animals with a BP of 110 mm Hg (±10 %) were randomized into eleven groups (n=6). Then hypertension was induced in rats as reported previously by administering DOCA salt (20 mg/kg, s.c.) in olive oil twice weekly with an uninterrupted supply of drinking water containing 1 % sodium chloride and 0.2 % potassium chloride for 4 w[16,17].
Vehicle control belonged to Group I; negative controls belonged to Groups II and III. In contrast, hypertension was induced in only Groups IV-XI. Groups I, II, and III were given vehicle (10 ml/ kg/d) corresponded to 0.5 % carboxymethylcellulose where SPRL was dissolved, CIT (50 mg/kg/d), and CIT (100 mg/kg/d), respectively, along with olive oil (10 ml/kg, s.c.) twice weekly. Group IV served as a positive control (DOCA Group). Groups V to X were experimental treatment groups. Group V received CIT (50 mg/kg/d), Group VI received CIT (50 mg/kg/ d)+SPRL (50 mg/kg/d), Group VII received CIT (50 mg/kg/d)+SPRL (100 mg/kg/d), Group VIII received CIT (100 mg/kg/d), Group IX received CIT (100 mg/kg/d)+SPRL (50 mg/kg/d), Group X received CIT (100 mg/kg/d)+SPRL (100 mg/kg/d). Group XI served as a comparative control and only received SPRL (100 mg/kg/d). CIT dissolved in water and SPRL suspended in 0.5 % carboxymethylcellulose to prepare formulations. Once-daily for 4 w, CIT and SPRL were given orally (10 ml/kg, time-8:30 am to 9:30 am) using an intragastric tube.
Data collection:
Hemodynamic Assessment: On 0, 07, 14, 21, and 28 d all rats' non-invasive Blood Pressure (BP) levels (NIBP) were measured using the tail-cuff method. On 29th d, the animal was anesthetized with pentobarbitone sodium (70 mg/kg, i.p.). The right carotid artery was cannulated with a cannula filled with heparinized saline, and the cannula was connected to a pressure transducer to record Heart Rate (HR), and Invasive BP (IBP) parameters viz. Systolic BP (SBP), Diastolic BP (DBP), and Mean Arterial BP (MAP). Thereafter, the right jugular vein was cannulated with a catheter to record vascular reactivity to Adrenaline (ADR) (1 µg/kg/ml, i.v.), Nor-ADR (1 µg/kg/ml, i.v.) and Phenylephrine (PE) (1 µg/kg/ml, i.v.). These hemodynamic parameters were measured with an eight-channel PowerLab instrument equipped with LabChart Pro 6 software (ADInstruments).
Sample collection:
Following hemodynamic measurements, animals were sacrificed through a midline abdominal incision to collect heart tissue and the whole thoracic aortic arch to the diaphragm. Individual Heart Weight (HW) was measured after sacrifice. Heart tissues were isolated from the aorta and fat tissue after being purged and cleaned with a cold Phosphate Buffer Saline solution. To arrive at a HW Index (HWI, mg/g), we weighed the animal and measured the HW (mg) against its body weight (g).
Connective tissue and adhering fat were removed from the aorta to study Acetylcholine (ACh)-induced vasorelaxation. The heart tissues were excised and quickly ice-cold saline washed. Using a Tris-HCl/ Phosphate buffer (0.1 M/pH 7.4), each heart was homogenized to a 10 % w/v concentration before being placed in the Krebs solution at 37° and aerated with 95 % oxygen and 5 % carbon dioxide.
ACh-induced vasorelaxation:
ACh-induced vasorelaxation was measured using an isolated artery technique, as reported previously[19]. In brief, we made and mounted 3 mm rings in an organ bath filled with Krebs solution. Thereafter, used a force transducer to record contraction data, including two stainless steel hooks attached to a bathing hose (PowerLab, ADInstruments). An immersion period of 90 to 120 min, followed by 15 ml bathing solution changes every 15 min, was provided before the experiment started. Then rings were subjected to 10-6 M PE after equilibration. An ACh doses (10-9 M to 10-4 M) vasorelaxant effect was observed when PE induced a peak contraction response.
Biochemical analyses:
The antioxidant activity was measured in supernatant samples from the heart tissue. To assay the catalase by Luck et al. method, we used the post- nuclear fraction yielded after centrifugation of the homogeneous mixture at 1000 g for 20 min at 4° (Remi C30, Remi Industries Ltd. Mumbai, India) [20]. Centrifugation at 12 000 g for 60 minutes at 4° was used for other enzyme assays. Follow-up assays were performed with the help of an ultra-fast ultra- violet spectrometer (SPECTROstar® Nano from BMG LABTECH). The technique used to measure reduced glutathione (GSH) was Ellman et al.[21], and Superoxide Dismutase (SOD) activity was Kono et al.[22]. The Malondialdehyde (MDA) level was determined using the Wills method[23]. Finally, the NO activity was determined by the method laid down by Titheradge et al.[24].
Statistical analysis:
GraphPad Prism software was used (Ver. 6) for the statistical analysis. To statistically compare the influence of time and the treatment groups on the baseline and subsequent NIBP values a two-way ANOVA followed by Tuckey's post hoc test was performed. A one-way ANOVA was used for other parameters, followed by Tuckey's post hoc test. All the data were presented as the mean±Standard Error of the Mean (SEM). Groups were considered to be significantly different when they met the 95 % confidence level.
Results and Discussion
Table 1 shows the weekly change in NIBP in different groups of DOCA salt-induced hypertensive rat model. For all groups, the mean pretreatment NIBP was 109±0.85 mm Hg. NIBP measurements taken by the tail-cuff method showed no statistical difference between 0th d and the end of 28th d for the vehicle and negative control groups. However, at the end of 28th d, NIBP readings in all remaining groups were significantly higher (p<0.0001) than their 0th d results. DOCA salt administration was associated with significant elevations (60.95 %) in the NIBP levels compared to vehicle control (111.82±1.61 mm Hg). The elevation of the NIBP was significantly inhibited (p<0.0001) by all the treatment groups. However, this improvement was statistically higher (p<0.001) in the CIT (100)+SPRL (50) and CIT (100)+SPRL (100) treatment groups when compared to the only SPRL (100) treatment group.
| Group no. | Non-invasive blood pressure (mm Hg) at the end of | ||||
|---|---|---|---|---|---|
| 0 d | 7th d | 14th d | 21st d | 28th d | |
| Group I-Vehicle Control | 108.20±2.93 | 108.69±3.91d,z | 113.04±2.63d,z | 113.79±1.82d,z | 111.82±1.61d,z |
| Group II-CIT (50) | 107.90±2.48 | 108.15±2.55d,z | 111.28±1.45d,z | 110.53±2.57d,z | 110.82±1.89d,z |
| Group III-CIT (100) | 112.59±2.58 | 112.64±0.65d,x | 112.06±1.66d,z | 111.58±1.57d,z | 109.68±1.53d,z |
| Group IV-DOCA | 111.79±2.91 | 126.29±3.46u,* | 142.39±3.68u,*,x | 157.23±3.94u,*,z | 172.78±3.81u,*,z |
| Group V-DOCA+CIT (50) | 114.35±2.04w | 127.94±2.01u,*,w | 141.25±2.23u,*,w | 152.82±2.13u,*,z | 155.05±2.07u,*,d,z |
| Group VI-DOCA+CIT (50)+SPRL (50) | 110.69±2.39 | 125.61±2.29u,* | 138.79±2.81u,* | 150.19±3.55u,*,a,z | 149.14±3.01u,*,d,z |
| Group VII-DOCA+CIT (50)+SPRL (100) | 108.46±2.19 | 122.34±2.79u,* | 133.39±3.67u,*,c | 137.69±4.40u,*,d | 137.57±2.54u,*,d |
| Group VIII-DOCA+CIT (100) | 108.06±3.62 | 120.56±3.84u,* | 134.91±3.78u,*,b | 139.00±3.98u,*,d | 138.95±3.32u,*,d |
| Group IX-DOCA+CIT (100)+SPRL (50) | 111.57±3.19 | 123.99±4.43u,* | 137.49±3.69u,* | 136.77±5.24u,*,d | 128.66±4.09u,*,d,z |
| Group X-DOCA+CIT (100)+SPRL (100) | 106.54±3.34 | 119.09±3.99u,*,a | 129.44±4.07u,*,d | 131.04±4.90u,*,d,y | 122.81±3.49u,*,d,z |
| Group XI-DOCA+SPRL (100) | 107.51±3.57 | 121.02±3.00u,* | 134.44±3.16u,*,b | 139.59±3.69u,*,d | 137.79±3.10u,*,d |
Note: Data are shown as mean±SEM (n=6 per Group, Two-way ANOVA followed by Tukey's multiple comparisons test). Significant differences of respective groups for 1-4 w compared to 0 w indicated by up<0.0001. Significant differences from the Group I compared to the remaining groups at 0th and 28th d are indicated by *p<0.0001. Significant differences from Group IV is indicated by ap<0.05, bp<0.01, cp<0.001, and dp<0.0001. Significant differences from the Group XI at 28th d are indicated by wp<0.05, xp<0.01, yp<0.001, and zp<0.0001
Table 1: Effect of CIT (50 or 100 mg/kg) Administration With or Without SPRL (50 or 100 mg/kg) Combination on Non-Invasive Blood Pressure in Different Groups of DOCA Salt-Induced Hypertensive Rat Model
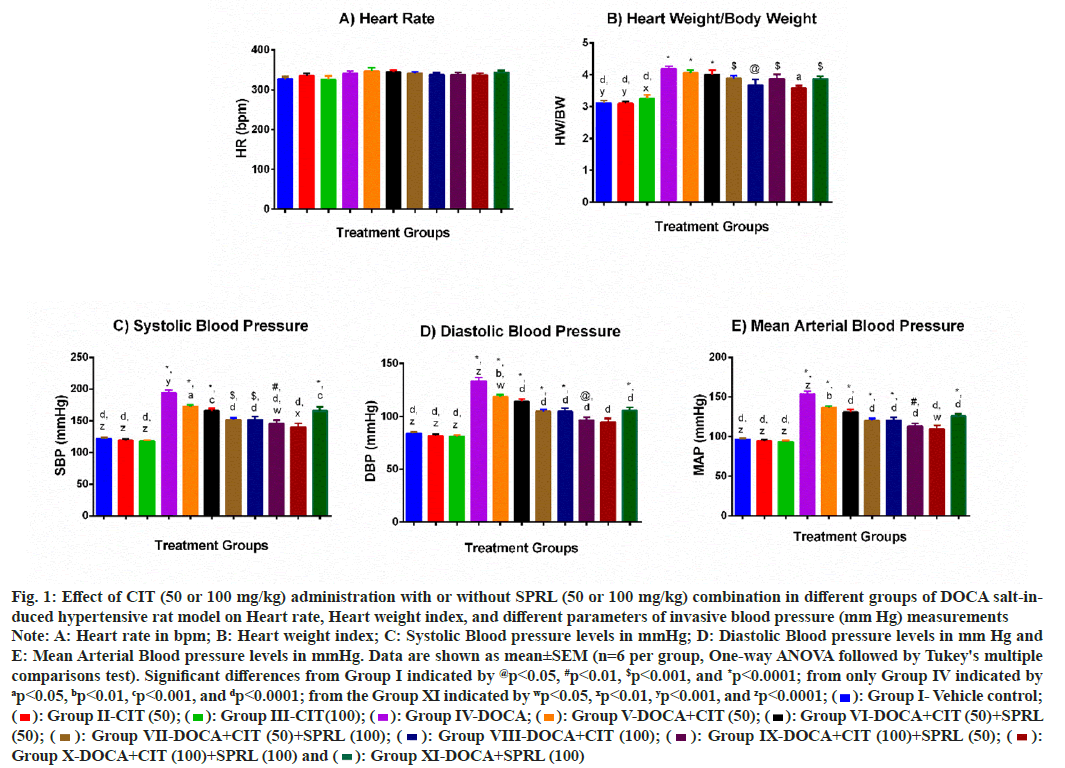
Even though there was no statistically significant difference between all groups and the vehicle control group, DOCA salt received by all groups exhibited a slightly higher mean HR than the vehicle control group (fig. 1A). Next, we found that the HWI was more than 34 % elevated after 28 d in the positive control group than in the vehicle control group. Only treatment with the CIT (100)+SPRL (100) group (p<0.05) significantly reduced this high index in the positive control group (fig. 1B).
At the end of 28th d, the SBP (fig. 1C), DBP (fig. 1D), and MAP (fig. 1E) of the DOCA group were significantly higher than those of the vehicle control group. All mono and combination therapy significantly prevented increases in these three IBP parameters by the DOCA. This significant prevention is greater for SBP (p<0.01) and MAP (p<0.05) by the CIT (100)+SPRL (100) group, and for SBP (p<0.05) by the CIT (100)+SPRL (50) group compared with the comparator group. To note, the CIT (100)+SPRL (100) combination group showed an insignificant difference in the IBP parameters measured in comparison to the vehicle control group.
Fig. 1: Effect of CIT (50 or 100 mg/kg) administration with or without SPRL (50 or 100 mg/kg) combination in different groups of DOCA salt-induced
hypertensive rat model on Heart rate, Heart weight index, and different parameters of invasive blood pressure (mm Hg) measurements
Note: A: Heart rate in bpm; B: Heart weight index; C: Systolic Blood pressure levels in mmHg; D: Diastolic Blood pressure levels in mm Hg and
E: Mean Arterial Blood pressure levels in mmHg. Data are shown as mean±SEM (n=6 per group, One-way ANOVA followed by Tukey's multiple
comparisons test). Significant differences from Group I indicated by @p<0.05, #p<0.01, $p<0.001, and *p<0.0001; from only Group IV indicated by ap<0.05, bp<0.01, cp<0.001, and dp<0.0001; from the Group XI indicated by wp<0.05, xp<0.01, yp<0.001, and zp<0.0001;  Group I- Vehicle control;
Group I- Vehicle control;  Group II-CIT (50);
Group II-CIT (50);  Group III-CIT(100);
Group III-CIT(100);  Group IV-DOCA;
Group IV-DOCA;  Group V-DOCA+CIT (50);
Group V-DOCA+CIT (50);  Group VI-DOCA+CIT (50)+SPRL
(50);
Group VI-DOCA+CIT (50)+SPRL
(50);  Group VII-DOCA+CIT (50)+SPRL (100);
Group VII-DOCA+CIT (50)+SPRL (100);  Group VIII-DOCA+CIT (100);
Group VIII-DOCA+CIT (100);  Group IX-DOCA+CIT (100)+SPRL (50);
Group IX-DOCA+CIT (100)+SPRL (50);  Group X-DOCA+CIT (100)+SPRL (100) and
Group X-DOCA+CIT (100)+SPRL (100) and  Group XI-DOCA+SPRL (100)
Group XI-DOCA+SPRL (100)
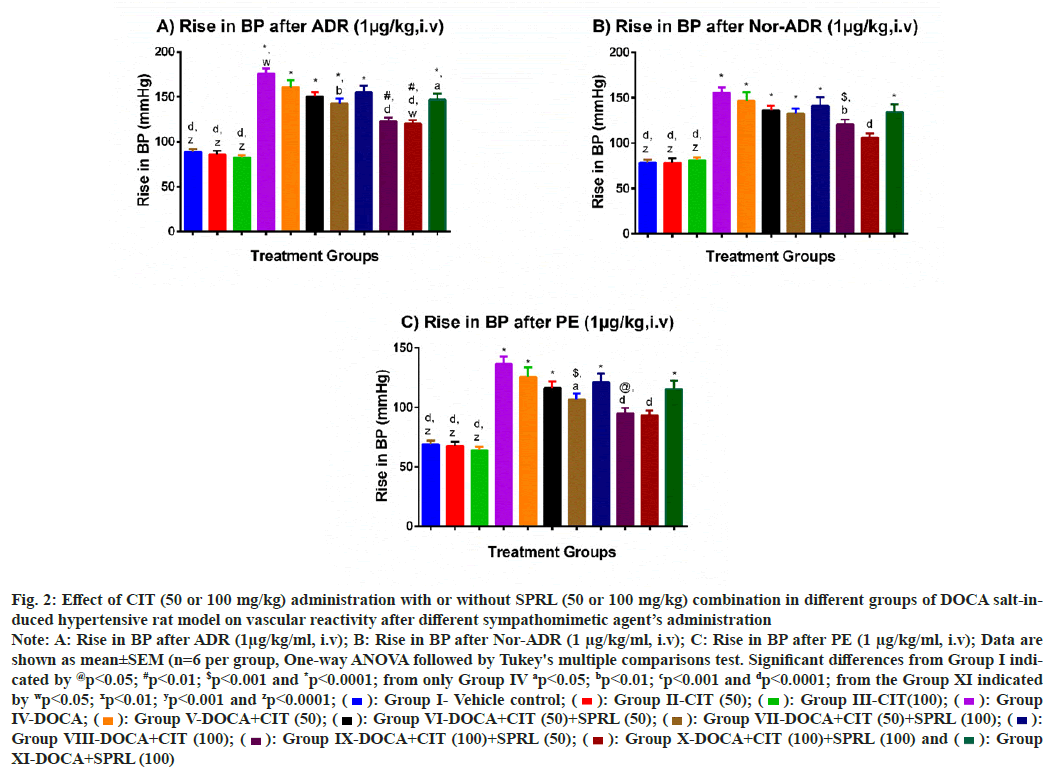
In the vehicle control group, all three catecholamines (ADR, Nor-ADR, and PE) had an expected effect on BP at the end of 28th d. However, the DOCA salt- treated group had an exaggerated response to all three catecholamines as compared to the vehicle control group (p<0.001) (fig. 2). When compared to the DOCA group, only the CIT (50)+SPRL (100), CIT (100)+SPRL (50), and CIT (100)+SPRL (100) groups showed a significant fall in the mean rise in BP after the ADR and PE administrations, and the latter two groups also for Nor-ADR administration with p<0.01. Although there was also a trend towards a fall in the mean rise in BP for all the three catecholamine responses in other treated groups, this did not reach statistical significance. However, the fall in the increase in BP in the CIT (100)+SPRL (100) treatment group was more significant (p<0.05) than in the comparative control group after ADR (1 μg/kg) administration. To note, the mean rise in BP in the CIT (100)+SPRL (100) group after Nor-ADR and PE is not statistically different compared to the vehicle control group.
Fig. 2: Effect of CIT (50 or 100 mg/kg) administration with or without SPRL (50 or 100 mg/kg) combination in different groups of DOCA salt-induced
hypertensive rat model on vascular reactivity after different sympathomimetic agent’s administration
Note: A: Rise in BP after ADR (1μg/kg/ml, i.v); B: Rise in BP after Nor-ADR (1 μg/kg/ml, i.v); C: Rise in BP after PE (1 μg/kg/ml, i.v); Data are
shown as mean±SEM (n=6 per group, One-way ANOVA followed by Tukey's multiple comparisons test. Significant differences from Group I indicated
by @p<0.05; #p<0.01; $p<0.001 and *p<0.0001; from only Group IV ap<0.05; bp<0.01; cp<0.001 and dp<0.0001; from the Group XI indicated
by wp<0.05; xp<0.01; yp<0.001 and zp<0.0001;  Group I- Vehicle control;
Group I- Vehicle control;  Group II-CIT (50);
Group II-CIT (50); Group III-CIT(100);
Group III-CIT(100); Group
IV-DOCA;
Group
IV-DOCA;  Group V-DOCA+CIT (50);
Group V-DOCA+CIT (50); Group VI-DOCA+CIT (50)+SPRL (50);
Group VI-DOCA+CIT (50)+SPRL (50);  Group VII-DOCA+CIT (50)+SPRL (100);
Group VII-DOCA+CIT (50)+SPRL (100);  Group VIII-DOCA+CIT (100);
Group VIII-DOCA+CIT (100);  Group IX-DOCA+CIT (100)+SPRL (50);
Group IX-DOCA+CIT (100)+SPRL (50);  Group X-DOCA+CIT (100)+SPRL (100) and
Group X-DOCA+CIT (100)+SPRL (100) and  Group
XI-DOCA+SPRL (100)
Group
XI-DOCA+SPRL (100)
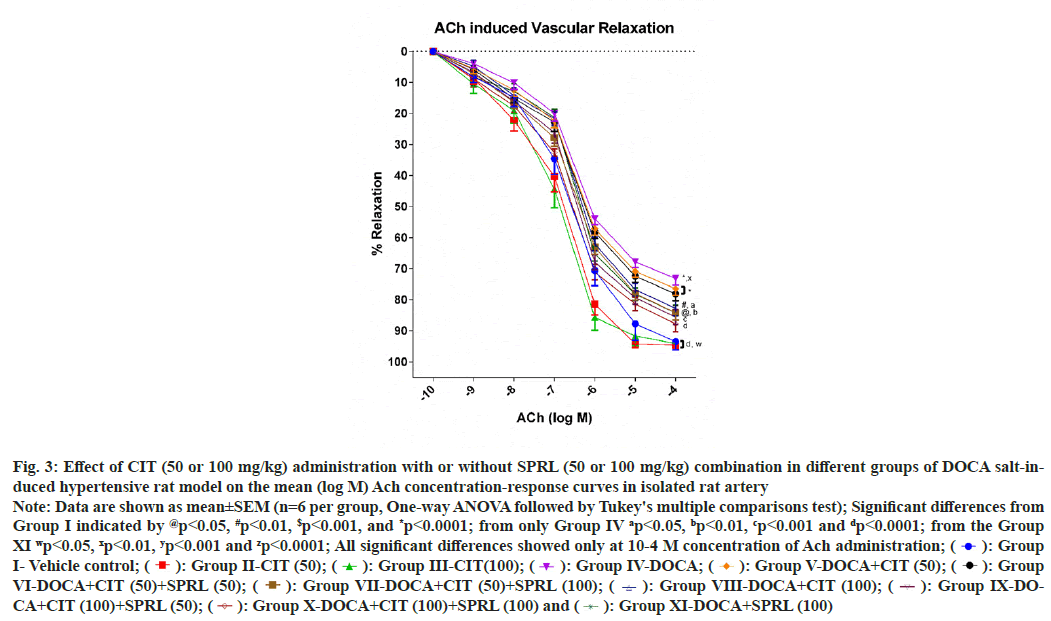
The aorta's final relaxant response to ACh doses was considered 100 % in the vehicle group. This response was thought to be a typical endothelial response. When compared to the vehicle control group, the positive control group showed a significant (p<0.001) impairment of relaxation at the ACh (10- 4 M) dose. Aortas from groups treated with CIT (50)+SPRL (100), CIT (100), CIT (100)+SPRL (50), CIT (100)+SPRL (100) and SPRL (100) showed significant improvement in relaxant response with p<0.01, p<0.05, p<0.001, p<0.0001 and p<0.01, respectively, as compared to the positive control group. Nevertheless, this study did not demonstrate significant differences in relaxant responses by the aortas from groups treated with CIT (50) and CIT (50)+SPRL (50) compared to the positive control group. There were no substantial variations in improving relaxant responses between the treatment groups and the comparative control (fig. 3).
Fig. 3: Effect of CIT (50 or 100 mg/kg) administration with or without SPRL (50 or 100 mg/kg) combination in different groups of DOCA salt-induced
hypertensive rat model on the mean (log M) Ach concentration-response curves in isolated rat artery
Note: Data are shown as mean±SEM (n=6 per group, One-way ANOVA followed by Tukey's multiple comparisons test); Significant differences from
Group I indicated by @p<0.05, #p<0.01, $p<0.001, and *p<0.0001; from only Group IV ap<0.05, bp<0.01, cp<0.001 and dp<0.0001; from the Group
XI wp<0.05, xp<0.01, yp<0.001 and zp<0.0001; All significant differences showed only at 10-4 M concentration of Ach administration;  Group
I- Vehicle control;
Group
I- Vehicle control;  Group II-CIT (50);
Group II-CIT (50);  Group III-CIT(100);
Group III-CIT(100);  Group IV-DOCA;
Group IV-DOCA;  Group V-DOCA+CIT (50);
Group V-DOCA+CIT (50);  Group
VI-DOCA+CIT (50)+SPRL (50);
Group
VI-DOCA+CIT (50)+SPRL (50);  Group VII-DOCA+CIT (50)+SPRL (100);
Group VII-DOCA+CIT (50)+SPRL (100);  Group VIII-DOCA+CIT (100);
Group VIII-DOCA+CIT (100);  Group IX-DOCA+
CIT (100)+SPRL (50);
Group IX-DOCA+
CIT (100)+SPRL (50);  Group X-DOCA+CIT (100)+SPRL (100) and
Group X-DOCA+CIT (100)+SPRL (100) and  Group XI-DOCA+SPRL (100)
Group XI-DOCA+SPRL (100)
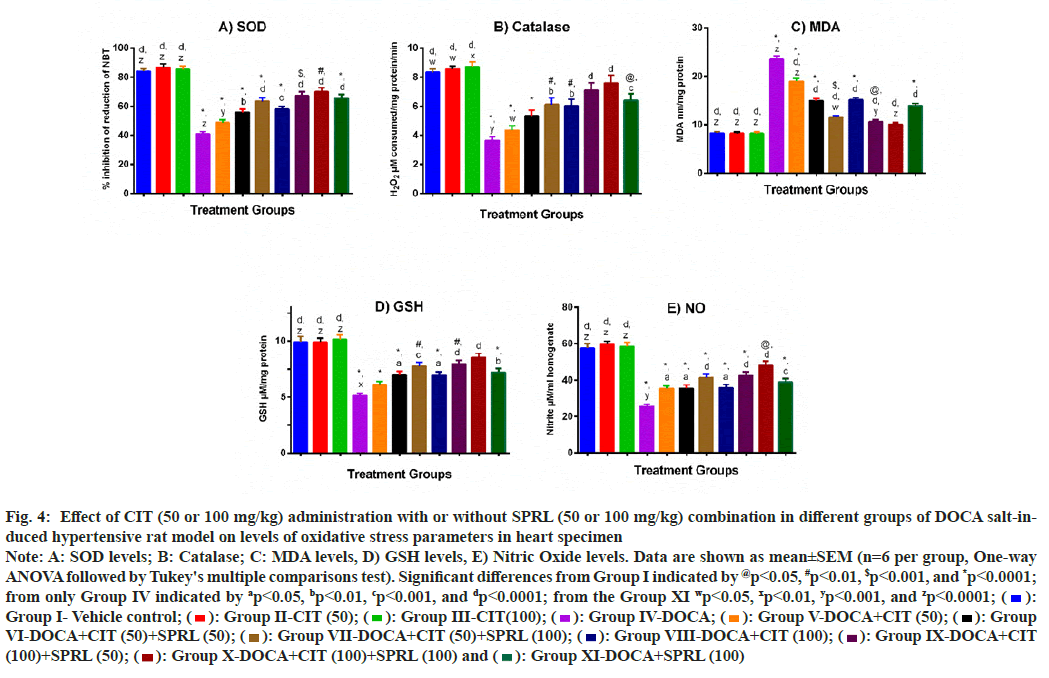
Percentage inhibition of reduction of Nitrotetrazolium Blue Chloride (NBT) was used to calculate SOD levels in heart homogenates. Statistical analysis revealed that mean SOD levels in the DOCA group were 51.31 % lower than in the vehicle- treated group. The treatment groups significantly attenuated this decrease in the DOCA group except for the DOCA+CIT (50) group. Compared to the comparative control, the significant attenuation of SOD level lowering by any treatment groups is not significantly higher (fig. 4A).
Fig. 4B shows catalase activity in rat heart samples. The positive control group's antioxidant activity was significantly reduced (p<0.001) compared to the vehicle control. All the treatment groups significantly restored the activity of catalase except the DOCA+CIT (50) and DOCA+CIT (50)+SPRL (50) treatment groups. The dose combination of CIT 100 mg/kg plus SPRL 100 mg/kg showed the most significant effect (p<0.0001). However, none of the treatment groups restored function better than the comparator group.
DOCA hypertensive rats had significantly higher MDA levels in their heart specimens (p<0.0001). After administration of all treatment groups, the unwanted rise in this lipid peroxidation marker was drastically decreased (p<0.0001). CIT (50)+SPRL (100), CIT (100)+SPRL (50), and CIT (100)+SPRL (100) treatments significantly reduced elevated MDA levels more than the SPRL (100) treatment group alone (fig. 4C).
The GSH concentration in the hearts of DOCA hypertensive rats was nearly 49 % lower than in the vehicle control group (p<0.0001). Treatments with CIT 100 mg/kg and all the combination groups notably improved (p<0.05) the level of this non- enzymatic antioxidant. However, CIT (50 mg/kg) monotherapy did not yield expected improvements in GSH concentration. The upgrades in decreased GSH levels by any treatment groups were not significantly more prominent than the comparative control (fig. 4D).
Fig. 4E shows the effect of CIT alone or in combination with other therapies on NO level. The positive control group had a lower status (more than 55 %) of this oxidative stress indicator than the vehicle control group. All the CIT-treated animals alone or combined with SPRL exhibited a significant increment in NO concentration (p<0.05). Though all experimental treatment groups showed a dose- dependent increment in NO levels, no treatment group brought back this indicator significantly better when compared with SPRL (100 mg/kg) alone treatment.
Fig. 4: Effect of CIT (50 or 100 mg/kg) administration with or without SPRL (50 or 100 mg/kg) combination in different groups of DOCA salt-induced
hypertensive rat model on levels of oxidative stress parameters in heart specimen
Note: A: SOD levels; B: Catalase; C: MDA levels, D) GSH levels, E) Nitric Oxide levels. Data are shown as mean±SEM (n=6 per group, One-way
ANOVA followed by Tukey's multiple comparisons test). Significant differences from Group I indicated by @p<0.05, #p<0.01, $p<0.001, and *p<0.0001;
from only Group IV indicated by ap<0.05, bp<0.01, cp<0.001, and dp<0.0001; from the Group XI wp<0.05, xp<0.01, yp<0.001, and zp<0.0001;  Group I- Vehicle control;
Group I- Vehicle control;  : Group II-CIT (50);
: Group II-CIT (50);  Group III-CIT(100);
Group III-CIT(100);  Group IV-DOCA;
Group IV-DOCA;  Group V-DOCA+CIT (50);
Group V-DOCA+CIT (50);  Group
VI-DOCA+CIT (50)+SPRL (50);
Group
VI-DOCA+CIT (50)+SPRL (50);  Group VII-DOCA+CIT (50)+SPRL (100);
Group VII-DOCA+CIT (50)+SPRL (100);  Group VIII-DOCA+CIT (100);
Group VIII-DOCA+CIT (100);  Group IX-DOCA+CIT
(100)+SPRL (50);
Group IX-DOCA+CIT
(100)+SPRL (50);  Group X-DOCA+CIT (100)+SPRL (100) and (
Group X-DOCA+CIT (100)+SPRL (100) and ( Group XI-DOCA+SPRL (100)
Group XI-DOCA+SPRL (100)
The study's principal findings are that in Wistar rats, 1) CIT alone once daily contributes to anti-hypertensive effects, and 2) CIT and SPRL combination therapy profits synergistic anti-hypertensive effects. The rigorous examination discovered that the combination intervention attenuated hemodynamic parameters, improved HWI, regulated the vascular reactivity and aorta relaxant responses towards expected values, and increased antioxidant defense in DOCA salt-induced hypertensive rats. However, CIT or SPRL alone treatment performs slightly inferior compared to combination therapy, and the results are more mixed in terms of statistical significance. Although the CIT low and high-dose monotherapy showed significant improvements in all parameters assessed except vascular reactivity, a higher dose was statistically more effective than a low dose in the overall parameters measured.
Several preclinical models and human trials have demonstrated CIT's anti-hypertensive effects[4,7,18,25–28]. However, current efforts to treat high BP have included a combination of medications. In the DOCA salt experimental paradigm, which is highly dependent on sodium and enhanced oxidative stress, no previous study has established the anti- hypertensive effects of CIT in combination with other anti-hypertensive medications.
The DOCA salt model assesses the drug's anti- hypertensive effects in test animals and represents human's primary aldosteronism and volume- dependent hypertension[29,30]. There are no reliable animal models to compare it to because human hypertension has many underlying causes. Though this study selected the non-uninephrectomized DOCA salt hypertension model, the main benefit is salt sensitivity[30,31]. This studies comparator drug SPRL non-selectively blocks mineralocorticoid receptors causing no sodium reabsorption and water retention. SPRL also inhibits mineralocorticoid receptors mediated pro-inflammatory and other cellular pathways activation[32,33].
According to previous studies, a 28 d course of DOCA salt administration results in a BP increase of 30 to 40 mm Hg in non-uninephrectomized animals, and consequently, cardiac hypertrophy[16,17,34–37]. Our study also showed progressive increases in NIBP, IBP, and HWI levels after DOCA administration. We also got a similar outcome for non-significant changes in HR after DOCA administration, as per Maurya et al.[38]. However, a study by Kandlikar et al.[37] showed HR lowering after treatment with DOCA salt.
The combination therapy had significant dose- dependent improvement for investigations on the effects of various catecholamines on vascular reactivity. Similarly, pre-contracted isolated rat arteries relaxed dose-dependently after ACh administration for mono-and combination therapy. In this study, the CIT may aid the cardiovascular system by raising NO synthesis and suppressing arginase expression, thereby giving even more L-arginine to the nitric oxide synthase (NOS) pathway, further enhancing NO levels.
Improved vascular reactivity and rat artery relaxation responses by treatment groups suggest preservation of vascular endothelial functioning. In the study of arginase knockout diabetic mice, Romero et al. proved that reduced arginase expression improves the endothelium-dependent vasorelaxation in diabetes[39]. In another study, CIT (50 and 100 mg/kg) has shown inhibitory effects on arginase expression, thus maintaining the normal endothelial vasculature in a metabolic syndrome- induced vascular dysfunction[18]. Others have also demonstrated that arginase inhibitors benefit by restoring normal vascular function in hypertension and diabetes[7]. Multiple studies have shown that CIT supplementation inhibits arginase expression and increases arginine and NO availability[7,27,40–43].
Ours and others' results direct that CIT regulates endothelial senescence as an efficient source of L-arginine and NO.
Hypertension is thought to be primarily caused by oxidative stress after DOCA salt intake. It is possible that Nicotinamide Adenine Dinucleotide (NADPH) oxidase and perhaps Cyclooxygenase (COX)-pathways are elevated when aldosterone concentrations are increased, resulting in decreased antioxidant defense and an increase in Reactive Oxygen Species (ROS). Using superoxide to generate the highly reactive free radical peroxynitrite, ROS deactivates or rapidly removes NO, resulting in endothelial dysfunction and vasoconstriction[44–46].
CIT has been shown to have antioxidant properties that may lower BP in hypertension[18,47,48]. NO- dependent and NO-independent routes explain the antioxidant action of CIT. In the NO-dependent route, CIT drives the action by increasing endothelial NOS, which shrinks ROS formation[49]. A reduction in the formation of hydroxyl radicals via alpha- amino acids in their protonated NH3 state is achieved independently of NO in the second pathway, resulting in water formation[47,50].
The CIT and SPRL combination therapy offers simultaneous effects advantages such as aldosterone receptor antagonism, smooth muscle vasodilation, enhanced antioxidant defense, and inflammation reduction. Moreover, considering the clinical safety profile of CIT, the SPRL dose can be reduced after the addition of CIT to achieve the alone SPRL equivalent anti-hypertensive effect and minimize SPRL dose-dependent adverse effects.
Though the study used the non-uninephroctomized model, we utilized the method that gives a gradual increase in the BP, mimicking the same response as seen in humans. We measured the combination therapy effects but not the drug interaction measurements. Also, we used only male Wistar rats in our study, so this also offers a future direction that requires exploration.
In conclusion, concurrent treatment of CIT and SPRL may have averted the development of DOCA salt- induced hypertension by lowering BP, alleviating oxidative stress, and raising NO levels. In addition, their combination strategy may provide a synergistic anti-hypertensive effect against DOCA salt-induced hypertension. Therefore, further research is warranted demonstrating the rationale for the combination of CIT and SPRL in preventing hypertension.
Conflict of interest:
The authors declared no conflict of interests.
References
- World Health Organization. Improving hypertension control in 3 million people: country experiences of programme development and implementation. 2020.
- Falster MO, Buckley NA, Brieger D, Pearson SA. Antihypertensive polytherapy in Australia: prevalence of inappropriate combinations, 2013–2018. J Hypertens 2020;38(8):1586-92.
[Crossref] [Google Scholar] [PubMed]
- Tain YL, Lee CT, Huang LT. Long-term effects of maternal citrulline supplementation on renal transcriptome prevention of nitric oxide depletion-related programmed hypertension: The impact of gene-nutrient interactions. Int J Mol Sci 2014;15(12):23255-68.
[Crossref] [Google Scholar] [PubMed]
- Chien SJ, Lin KM, Kuo HC, Huang CF, Lin YJ, Huang LT, et al. Two different approaches to restore renal nitric oxide and prevent hypertension in young spontaneously hypertensive rats: l-citrulline and nitrate. Transl Res 2014;163(1):43-52.
[Crossref] [Google Scholar] [PubMed]
- Hayashi T, Juliet PA, Matsui-Hirai H, Miyazaki A, Fukatsu A, Funami J, et al. l-Citrulline and l-arginine supplementation retards the progression of high-cholesterol-diet-induced atherosclerosis in rabbits. Proc Natl Acad Sci 2005;102(38):13681-6.
[Crossref] [Google Scholar] [PubMed]
- Moinard C, Nicolis I, Neveux N, Darquy S, Bénazeth S, Cynober L. Dose-ranging effects of citrulline administration on plasma amino acids and hormonal patterns in healthy subjects: the Citrudose pharmacokinetic study. Br J Nutr 2008;99(4):855-62.
[Crossref] [Google Scholar] [PubMed]
- Alsop P, Hauton D. Oral nitrate and citrulline decrease blood pressure and increase vascular conductance in young adults: A potential therapy for heart failure. Eur Appl Physiol 2016;116:1651-61.
[Crossref] [Google Scholar] [PubMed]
- Schwedhelm E, Maas R, Freese R, Jung D, Lukacs Z, Jambrecina A, et al. Pharmacokinetic and pharmacodynamic properties of oral L‐citrulline and L‐arginine: impact on nitric oxide metabolism. Br J Clin Pharmacol 2008;65(1):51-9.
- Morita M, Sakurada M, Watanabe F, Yamasaki T, Ezaki H, Morishita K, et al. Effects of oral L-citrulline supplementation on lipoprotein oxidation and endothelial dysfunction in humans with vasospastic angina. Immunol Endocr Metab Agents Med Chem 2013;13(3):214-20.
[Crossref] [Google Scholar] [PubMed]
- Kaore SN, Kaore NM. Arginine and citrulline as nutraceuticals: Efficacy and safety in diseases. In: Nutraceuticals 2021. p. 925-44.
- Amaral JH, Ferreira GC, Pinheiro LC, Montenegro MF, Tanus-Santos JE. Consistent antioxidant and antihypertensive effects of oral sodium nitrite in DOCA-salt hypertension. Redox Biol 2015;5:340-6.
[Crossref] [Google Scholar] [PubMed]
- Moser M, Black HR. The role of combination therapy in the treatment of hypertension. Am J Hypertens 1998;11(S5):73S-8S.
[Crossref] [Google Scholar] [PubMed]
- Passaglia RC, David FL, Fortes ZB, Nigro D, Scivoletto R, Catelli de Carvalho MH. Deoxycorticosterone acetate‐salt hypertensive rats display gender‐related differences in ETB receptor‐mediated vascular responses. Br J Pharmacol 2000;130(5):1092-8.
[Crossref] [Google Scholar] [PubMed]
- Belanger KM, Crislip GR, Gillis EE, Abdelbary M, Musall JB, Mohamed R, et al. Greater t regulatory cells in females attenuate doca-salt–induced increases in blood pressure versus males. Hypertension 2020;75(6):1615-23.
[Crossref] [Google Scholar] [PubMed]
- Lange DL, Haywood JR, Hinojosa-Laborde C. Role of the adrenal medullae in male and female DOCA-salt hypertensive rats. Hypertension 1998;31(1):403-8.
[Crossref] [Google Scholar] [PubMed]
- Bockman CS, Jeffries WB, Pettinger WA, Abel PW. Enhanced release of endothelium-derived relaxing factor in mineralocorticoid hypertension. Hypertension 1992;20(3):304-13.
[Crossref] [Google Scholar] [PubMed]
- Safaeian L, Emami R, Hajhashemi V, Haghighatian Z. Antihypertensive and antioxidant effects of protocatechuic acid in deoxycorticosterone acetate-salt hypertensive rats. Biomed Pharmacother 2018;100:147-55.
[Crossref] [Google Scholar] [PubMed]
- El‐Bassossy HM, El‐Fawal R, Fahmy A, Watson ML. Arginase inhibition alleviates hypertension in the metabolic syndrome. Br J Pharmacol 2013;169(3):693-703.
- Honda H, Ushijima D, Ishihara H, Yanase M, Kogo H. A regional variation of acetylcholine-induced relaxation in different segments of rat aorta. Physiol Behav 1997;63(1):55-8.
[Crossref] [Google Scholar] [PubMed]
- Luck H. Catalases. Section C: Methods for determination of enzyme activity. Methods in enzymatic analysis. 2nd ed. New York: Academic Press; 1971. p. 885-8.
- Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys 1959;82(1):70-7.
[Crossref] [Google Scholar] [PubMed]
- Kono Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys 1978;186(1):189-95.
[Crossref] [Google Scholar] [PubMed]
- Wills E. Mechanisms of lipid peroxide formation in animal tissues. Biochem J 1966;99(3):667.
[Crossref] [Google Scholar] [PubMed]
- Titheradge MA. The enzymatic measurement of nitrate and nitrite. Methods Mol Biol 1998:83-91.
[Crossref] [Google Scholar] [PubMed]
- Tain YL, Sheen JM, Chen CC, Yu HR, Tiao MM, Kuo HC, et al. Maternal citrulline supplementation prevents prenatal dexamethasone-induced programmed hypertension. Free Radical Res 2014;48(5):580-6.
[Crossref] [Google Scholar] [PubMed]
- Ishikura F, Egawa M, Takano Y, Kumagai K, Suzuki T. Effects of Citrulline combined with Tadalafil on Monocrotaline-induced pulmonary hypertension in rats compared with arginine. J Nov Physiother 2015;5(269):1-5.
- Tsuboi T, Maeda M, Hayashi T. Administration of L-arginine plus L-citrulline or L-citrulline alone successfully retarded endothelial senescence. PLoS One 2018;13(2):e0192252.
[Crossref] [Google Scholar] [PubMed]
- Figueroa A, Alvarez-Alvarado S, Jaime SJ, Kalfon R. l-Citrulline supplementation attenuates blood pressure, wave reflection and arterial stiffness responses to metaboreflex and cold stress in overweight men. Br J Nutr 2016;116(2):279-85.
[Crossref] [Google Scholar] [PubMed]
- Badyal DK, Lata H, Dadhich AP. Animal models of hypertension and effect of drugs. Indian J Pharmacol 2003;35(6):349-62.
- Pestana-Oliveira N, Nahey DB, Johnson T, Collister JP. Development of the deoxycorticosterone acetate (DOCA)-salt hypertensive rat model. Bio Protoc 2020;10(15):e3708.
[Crossref] [Google Scholar] [PubMed]
- Basting T, Lazartigues E. DOCA-salt hypertension: An update. Curr Hypertens Rep 2017;19:1-8.
[Crossref] [Google Scholar] [PubMed]
- Patibandla S, Heaton J KH. Spironolactone. In: StatPearls. NCBI Bookshelf. Treasure Island (FL): StatPearls Publishing 2021.
- Carone L, Oxberry SG, Twycross R, Charlesworth S, Mihalyo M, Wilcock A. Therapeutic reviews: Spironolactone. J Pain Symptom Manag 2017;53(2):288–92.
- Chen YY, Ji W, Du JR, Yu DK, He Y, Yu CX, et al. Preventive effects of low molecular mass potassium alginate extracted from brown algae on DOCA salt-induced hypertension in rats. Biomed Pharmacother 2010;64(4):291-5.
[Crossref] [Google Scholar] [PubMed]
- Huang LL, Pan C, Wang L, Ding L, Guo K, Wang HZ, et al. Protective effects of grape seed proanthocyanidins on cardiovascular remodeling in DOCA-salt hypertension rats. J Nutr Biochem 2015;26(8):841-9.
[Crossref] [Google Scholar] [PubMed]
- Jackson D, Connolly K, Batacan R, Ryan K, Vella R, Fenning A. (−)-Epicatechin reduces blood pressure and improves left ventricular function and compliance in deoxycorticosterone acetate-salt hypertensive rats. Molecules 2018;23(7):1511.
[Crossref] [Google Scholar] [PubMed]
- Kandlikar SS, Fink GD. Splanchnic sympathetic nerves in the development of mild DOCA-salt hypertension. Am J Physiol Heart Circ Physiol 2011;301(5):H1965-73.
[Crossref] [Google Scholar] [PubMed]
- Syed AA, Lahiri S, Mohan D, Valicherla GR, Gupta AP, Riyazuddin M, et al. Evaluation of anti-hypertensive activity of Ulmus wallichiana extract and fraction in SHR, DOCA-salt-and L-NAME-induced hypertensive rats. J Ethnopharmacol 2016;193:555-65.
[Crossref] [Google Scholar] [PubMed]
- Romero MJ, Platt DH, Tawfik HE, Labazi M, El-Remessy AB, Bartoli M, et al. Diabetes-induced coronary vascular dysfunction involves increased arginase activity. Circ Res 2008;102(1):95-102.
[Crossref] [Google Scholar] [PubMed]
- Morita M, Hayashi T, Ochiai M, Maeda M, Yamaguchi T, Ina K, et al. Oral supplementation with a combination of L-citrulline and L-arginine rapidly increases plasma L-arginine concentration and enhances NO bioavailability. Biochem Biophys Res Commun 2014;454(1):53-7.
[Crossref] [Google Scholar] [PubMed]
- Figueroa A, Wong A, Jaime SJ, Gonzales JU. Influence of L-citrulline and watermelon supplementation on vascular function and exercise performance. Curr Opin Clin Nutr Metab Care 2017;20(1):92-8.
[Crossref] [Google Scholar] [PubMed]
- Romero MJ, Yao L, Sridhar S, Bhatta A, Dou H, Ramesh G, et al. L-citrulline protects from kidney damage in type 1 diabetic mice. Front Immunol 2013;4:480.
[Crossref] [Google Scholar] [PubMed]
- Ochiai M, Hayashi T, Morita M, Ina K, Maeda M, Watanabe F, et al. Short-term effects of L-citrulline supplementation on arterial stiffness in middle-aged men. Int J Cardiol 2012;155(2):257-61.
[Crossref] [Google Scholar] [PubMed]
- Beswick RA, Dorrance AM, Leite R, Webb RC. NADH/NADPH oxidase and enhanced superoxide production in the mineralocorticoid hypertensive rat. Hypertension 2001;38(5):1107-11.
[Crossref] [Google Scholar] [PubMed]
- Fenning A, Harrison G, Rose’meyer R, Hoey A, Brown L. L-Arginine attenuates cardiovascular impairment in DOCA-salt hypertensive rats. Am J Physiol Heart Circ Physiol 2005;289(4):1408-16.
[Crossref] [Google Scholar] [PubMed]
- Silambarasan T, Raja B. Diosmin, a bioflavonoid reverses alterations in blood pressure, nitric oxide, lipid peroxides and antioxidant status in DOCA-salt induced hypertensive rats. Eur J Pharmacol 2012;679(1-3):81-9.
[Crossref] [Google Scholar] [PubMed]
- Coles KE. Investigation into the antioxidant capacity of L-arginine and L-citrulline in relation to their vascular protective properties. Cardiff University (United Kingdom); 2007. p. 1-308.
- Akashi K, Miyake C, Yokota A. Citrulline, a novel compatible solute in drought-tolerant wild watermelon leaves, is an efficient hydroxyl radical scavenger. FEBS Lett 2001;508(3):438-42.
[Crossref] [Google Scholar] [PubMed]
- Förstermann U, Li H. Therapeutic effect of enhancing endothelial nitric oxide synthase (eNOS) expression and preventing eNOS uncoupling. Br J Pharmacol 2011;164(2):213-23.
[Crossref] [Google Scholar] [PubMed]
- Nagy IZ, Floyd RA. Hydroxyl free radical reactions with amino acids and proteins studied by electron spin resonance spectroscopy and spin-trapping. Biochim Biophys Acta 1984;790(3):238-50.
[Crossref] [Google Scholar] [PubMed]