- *Corresponding Author:
- I. Ahmad
Institute of Pharmaceutical Sciences, Baqai Medical University, 51, Deh Tor, Toll Plaza, Super Highway, Gadap Road, Karachi-74600, Pakistan
E-mail: ali_sheraz80@hotmail.com
| Date of Submission | November 22, 2010 |
| Date of Revision | March 20, 2012 |
| Date of Acceptance | March 27, 2012 |
| Indian J Pharm Sci, 2012, 74 (2): 133-140 |
Abstract
The effect of pH, media, phosphate concentration and ionic strength on the kinetics of thermal degradation of betamethasone valerate and betamethasone dipropionate has been investigated. A validated HPLC method has been used to determine the parent compounds and their major thermal degradation products identified in the reaction. Betamethasone-17-valerate gave rise to two major products, namely, betamethasone-21-valerate and betamethasone alcohol, and betamethasone dipropionate degraded into three major products, namely, betamethasone-17-propionate, betamethasone-21-propionate and betamethasone alcohol, in different media. Betamethasone valerate showed maximum stability at pH 4-5 while betamethasone dipropionate was maximally stable at pH 3.5-4.5. The degradation of betamethasone valerate and betamethasone dipropionate was found to follow first-order kinetics and the apparent first-order rate constants (kobs ) for thermal degradation in different media range from 0.399-9.07×10 -3 h -1 and 0.239-1.87×10 -3 h -1 , respectively. The values of the rate constants decrease with increasing solvent polarity, phosphate concentration and ionic strength. The second-order rate constants ( k?) for the phosphate ion inhibited reactions lie in the range of 3.02-1.30×10 -6 M -1 s -1 .
Keywords
Betamethasone valerate, betamethasone dipropionate, kinetics, thermal degradation
Betamethasone valerate and betamethasone dipropionate are synthetic glucocorticoids used widely for the treatment of dermatoses and other skin diseases in the dosage forms like cream, gel, ointment, solution and lotion [1,2]. These esters are sensitive to heat [3,4] and undergo degradation to a number of compounds having lower therapeutic activity as compared to that of the parent compounds [5]. The degradation of betamethasone and related corticosteroids has been the subject of many investigations in the form of solutions [6−9], solid state [6,10] and in various dosage forms [11−13]. The major degradation reactions encountered with these esters are oxidation [14−19] and ester group migration followed by hydrolysis [6−9,11−13,20−27]. These reactions are influenced by pH, solvent polarity, oxygen content and temperature [14−17,20−25].
The present study involves the identification of the thermal degradation products of these esters and evaluation of the kinetics of thermal degradation of betamethasone esters in phosphate buffer, organic solvents, and cream and gel formulations using a validated HPLC method. The influence of pH and solvent polarity on the degradation reactions has been studied. The effect of phosphate concentration and ionic strength on the rate of thermal degradation has also been evaluated. These aspects have been studied in detail in various media and the rates correlated with different kinetic parameters.
Materials and Methods
Betamethasone−17−valerate, betamethasone−21−valerate and betamethasone alcohol were kindly donated by GSK Pakistan (Pvt) Ltd., Karachi, Pakistan. Betamethasone dipropionate, Betamethasone−17 −propionate and betamethasone−21−propionate were gifted by Crystal Pharma, Malaysia. AR grade chemicals like disodium hydrogen phosphate, potassium chloride and citric acid were obtained from Merck, Germany. Formulation ingredients carbomer 940, cetostcaryl alcohol, hydroxyethyl cellulose, propylene glycol and isopropyl alcohol were purchased from North Chemicals, Columbia; Croda, Japan; Spectrum, USA; Dow Chemicals, Germany and Bio M, Malaysia, respectively. All the solvents used were of spectroscopic grade from Tedia, Japan. The HPLC grade water used in the mobile phase was purified through a Milli−Q system, Millipore, USA.
Preparation of cream and gel formulations
The cream formulation was prepared by soaking carbomer−940 overnight in water. Betamethasone esters dissolved in a small quantity of isopropyl alcohol was mixed with a mixture of propylene glycol, cetostearyl alcohol and water and then mixed with the carbomer suspension in a silver sun mixer. The pH of the cream was adjusted to 4.0 with 1 M sodium hydroxide solution under gentle mixing. In the case of gel formulation the betamethasone esters solution in isopropyl alcohol and carbomer suspension in water were mixed thoroughly with a mixture of propylene glycol and water in a silver sun mixer. Diisopropanolamine was then added to the mixture under vigorous mixing. The pH of the gel was adjusted to 4.0 with 4 M hydrochloric acid solution.
pH measurements
All pH measurements were carried out with a WTW pH meter (Model 702, Sensitivity ±0.01 pH units, Germany). The electrode was standardized with buffer solutions (pH 2.0, 4.0 and 7.0) at 25º. For the determination of pH of the formulated products (cream/gel), 2 g sample was mixed thoroughly with 30 ml of double distilled water in a beaker and pH of the mixture was determined.
HPLC apparatus and conditions
The HPLC analysis was carried out on a Shimadzu class−20 A HPLC (Kyoto, Japan) system that consisted of an LC−20AT pump, an SPD−M20A photodiode−array UV/Vis detector and an inbuilt CBM−20A lite communication bus module. Data collection and integration were achieved using Shimadzu LC solution computer software version 1.2 (Kyoto, Japan). All the separations were carried out isocratically on a stainless steel column (250×4.6 mm i.d.) packed with Lichrosorb RP−18 (particle size, 5 µm, Merck) provided with a C−18 guard column (10×4 mm i.d., particle size, 5 µm). A mixture of acetonitrile and water (60:40, v/v) was used as a mobile phase, at a flow rate of 1 ml/min at ambient temperature. The detection was made at 238 nm.
Degradation procedure
In order to study the degradation of the betamethasone esters in solutions [citrate−phosphate buffers (pH 2.5−7.5), phosphate buffer (pH 7.5), acetonitrile and methanol], stock solutions of the compounds were mixed with the desired solvent in pyrex glass bottles to obtain the required initial drug concentration. Zero time samples were taken immediately while the remainder of the reaction mixture was divided into equal aliquots and heated in an oven (Memmert UIM 500, Germany) maintained at 40±1º for pre−determined time intervals. After heating, the samples were removed and the reaction was immediately terminated by adding 1 M HCl or 1 M NaOH solution to adjust the pH of the samples to approximately 4.0 and diluted further for HPLC analysis.
In the case of the cream or gel formulation the samples were spreaded as a 2 mm thick layer in Petri dishes and then degraded for pre−determined time intervals. The effect of pH on degradation was studied by preparing solutions of the compounds in citrate−phosphate buffers at pH 2.5−7.5. The thermal degradation in solution was also carried out in the presence of different concentrations of phosphate buffer.
HPLC analysis
The assay of betamethasone esters was carried out by the HPLC method described under the heading “chromatographic purity” in United State Pharmacopoeia [4]. The method was validated in respect to specificity, linearity, precision and accuracy. The same method was used for the assay of the degradation products of these esters. For the assay of all these compounds an appropriate volume of the solution sample was initially mixed with the internal standard solution (beclomethasone dipropionate in acetonitrile) and further diluted with the mobile phase to make the final concentration of 50 µg/ ml, filtered through 0.45 µm filter paper and then injected to the liquid chromatograph for analysis. The concentrations of the esters and their degradation products were read from the calibration curves obtained with the reference standards. In the case of cream and gel formulation the whole content of the Petri dish was dissolved in acetonitrile, filtered through 0.45 µm filter paper and then mixed with the mobile phase after the addition of internal standard prior to injection into the liquid chromatograph.
Results and Discussion
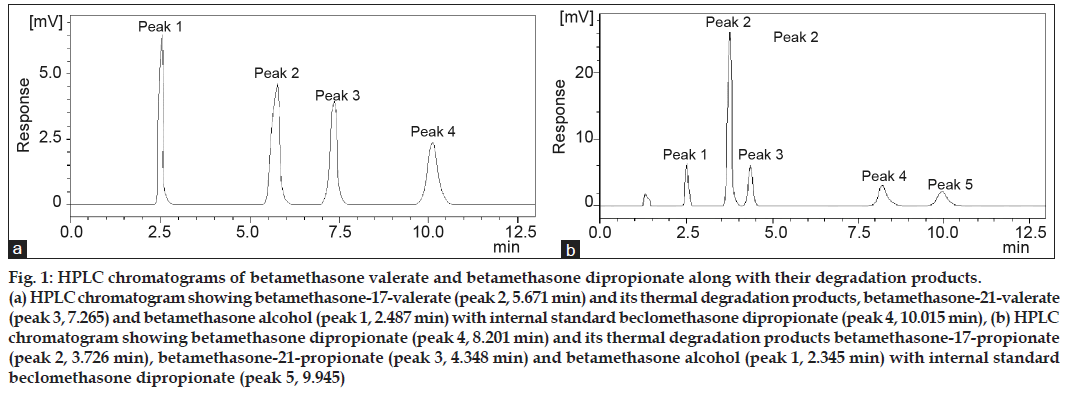
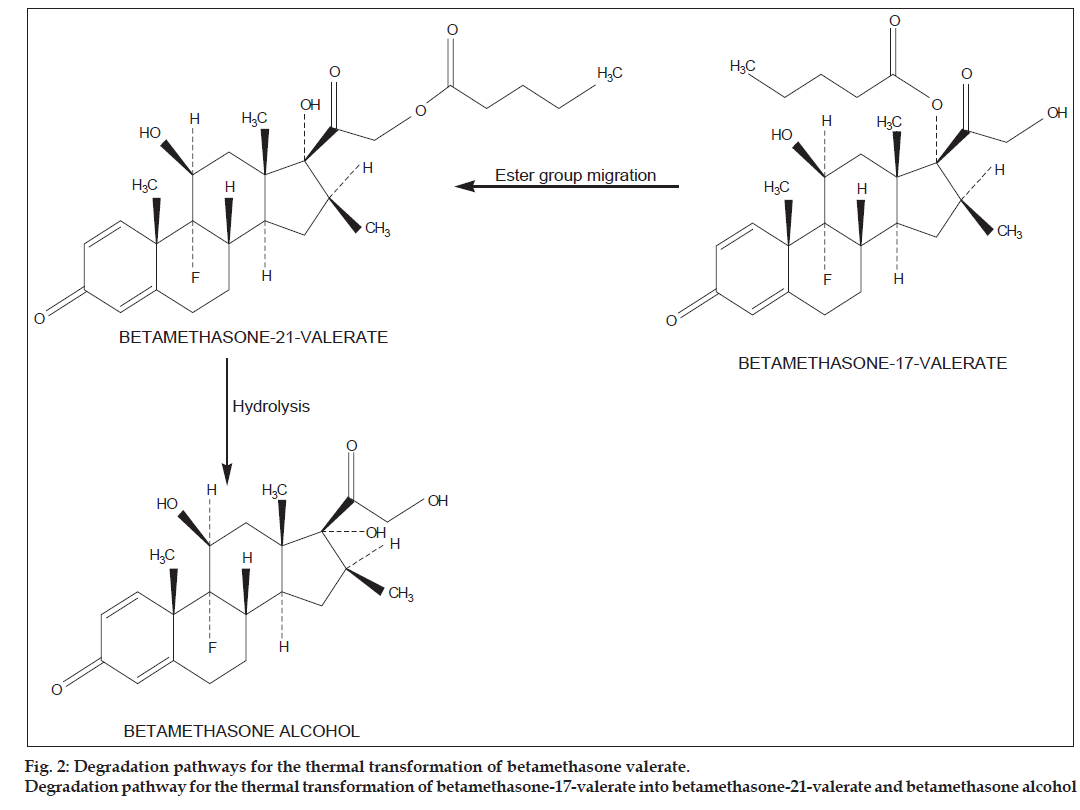
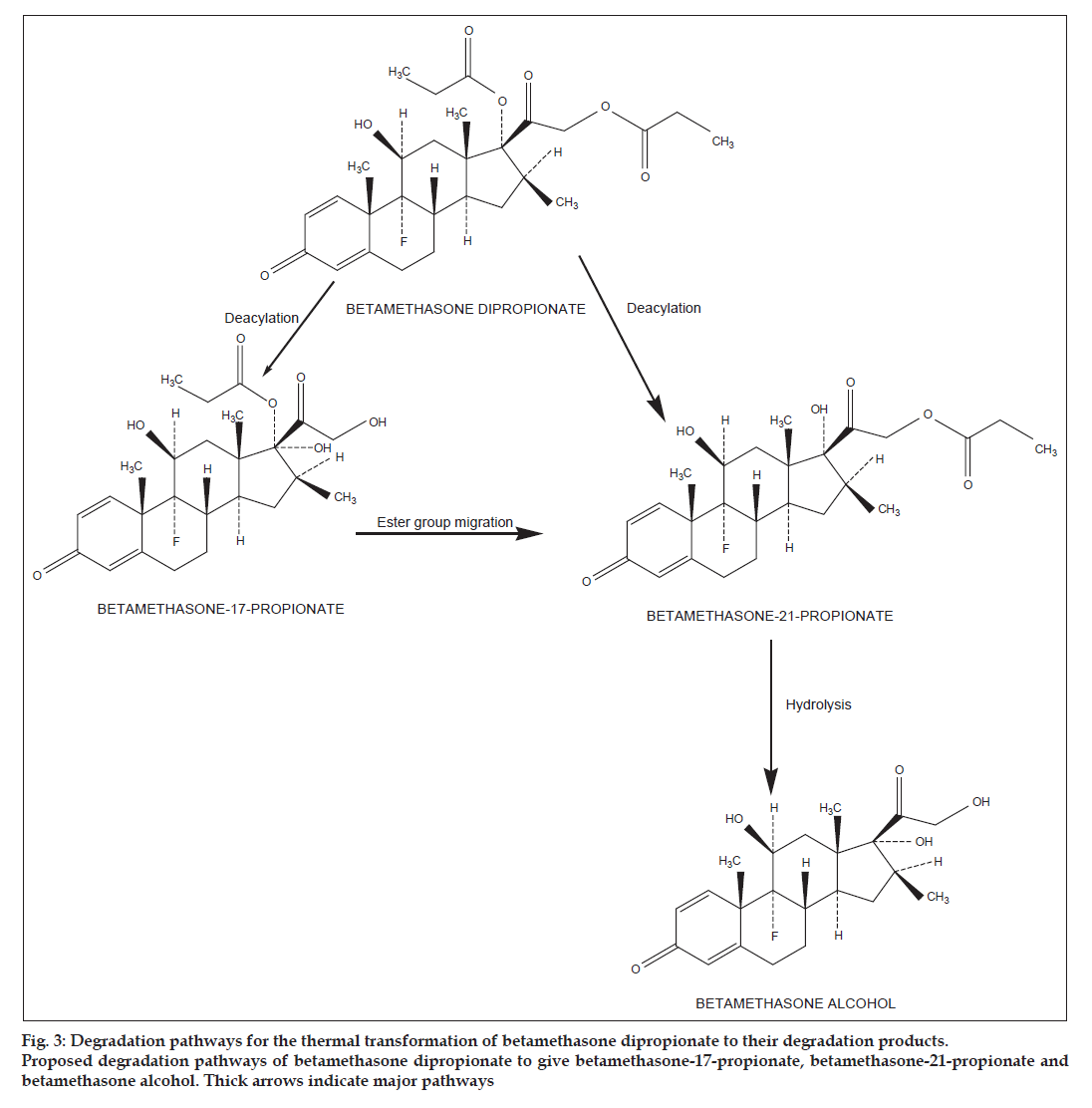
The degradation products of betamethasone esters obtained during the present reactions were identified by comparison of their tR values with those of the reference standards. A typical chromatogram showing betamethasone valerate and its degradation products (betamethasone−21−valerate and betamethasone alcohol) formed in methanol is shown in fig. 1a. In all the media (phosphate buffer, organic solvents, cream and gel) only two thermal degradation products were identified Table 1. These products are formed at a relatively low temperature (40º) and are produced by the ester group migration from C17 to C21, and further hydrolysis as proposed by Yip et al. [12] (fig. 2). In the case of betamethasone dipropionate three degradation products (betamethasone−17−propionate, betamethasone−21−propionate and betamethasone alcohol) were identified by HPLC in all the media studied. A typical chromatogram of betamethasone dipropionate and its degradation products formed in methanol is shown in fig. 1b. The degradation of betamethasone dipropionate with the products formed is shown in fig. 3. The reaction involves deacylation (C17 and C21), interconversion of 17 to 21−propionate and further hydrolysis to betamethasone alcohol. Some minor products were also identified in all the media. The values of the assay data on the degradation of betamethasone valerate and betamethasone dipropionate are given in Table 2 and 3. These data show a gradual decrease in concentrations of the esters in different media and indicate approximately 10−70% loss on degradation.
Fig 1: HPLC chromatograms of betamethasone valerate and betamethasone dipropionate along with their degradation products. (a) HPLC chromatogram showing betamethasone-17-valerate (peak 2, 5.671 min) and its thermal degradation products, betamethasone-21-valerate (peak 3, 7.265) and betamethasone alcohol (peak 1, 2.487 min) with internal standard beclomethasone dipropionate (peak 4, 10.015 min), (b) HPLC chromatogram showing betamethasone dipropionate (peak 4, 8.201 min) and its thermal degradation products betamethasone-17-propionate (peak 2, 3.726 min), betamethasone-21-propionate (peak 3, 4.348 min) and betamethasone alcohol (peak 1, 2.345 min) with internal standard beclomethasone dipropionate (peak 5, 9.945)
Fig 3: Degradation pathways for the thermal transformation of betamethasone dipropionate to their degradation products. Proposed degradation pathways of betamethasone dipropionate to give betamethasone-17-propionate, betamethasone-21-propionate and betamethasone alcohol. Thick arrows indicate major pathways
| Compound | Medium | Degradation products | tR value in methanol (min) |
|---|---|---|---|
| Betamethasone valerate | Acetonitrile, methanol, phosphate | Betamethasone 21 valerate | 7.26 |
| (tR=2.48 min) | buffer (pH 7.5) cream, gel | Betamethasone alcohol | 2.48 |
| Betamethasone dipropionate | Acetonitrile, methanol, phosphate | Betamethasone 17 propionate | 3.72 |
| (tR=8.20 min) | buffer (pH 7.5), cream, gel | Betamethasone 21 propionate | 4.34 |
| Betamethasone alcohol | 2.34 |
Table 1: Thermal Degradation Products Of Betamethasone Esters
| Time | Acetonitrile | Methanol | Phosphate | Cream | Gel |
|---|---|---|---|---|---|
| (h) | (M×105) | (M×105) | buffer, pH 7.5 | (M×105) | (M×105) |
| (M×105) | |||||
| 0 | 9.97 | 10.04 | 10.14 | 10.26 | 10.02 |
| 24 | 8.80 | 8.69 | 9.20 | 10.08 | 9.92 |
| 48 | 7.62 | 7.40 | 8.29 | 9.91 | 9.83 |
| 72 | 6.51 | 6.00 | 7.31 | 9.75 | 9.75 |
| 96 | 5.40 | 4.75 | 6.45 | 9.60 | 9.63 |
| 120 | 4.23 | 3.65 | 5.48 | 9.43 | 9.56 |
| 144 | 3.24 | 2.72 | 4.60 | 9.29 | 9.45 |
Table 2: Assay Of Betamethasone-17-Valerate On Degradation In Different Media
| Time | Acetonitrile | Methanol | Phosphate | Cream | Gel |
|---|---|---|---|---|---|
| (hours) | (M×105) | (M×105) | buffer, pH 7.5 | (M×105) | (M×105) |
| (M×105) | |||||
| 0 | 10.01 | 9.98 | 9.93 | 10.04 | 10.26 |
| 24 | 9.69 | 9.55 | 9.78 | 9.98 | 10.19 |
| 48 | 9.36 | 9.19 | 9.65 | 9.88 | 10.14 |
| 72 | 9.00 | 8.78 | 9.49 | 9.79 | 10.07 |
| 96 | 8.70 | 8.42 | 9.37 | 9.71 | 10.00 |
| 120 | 8.41 | 8.00 | 9.20 | 9.65 | 9.96 |
| 144 | 8.10 | 7.63 | 9.09 | 9.58 | 9.91 |
Table 3: Assay Of Betamethasone Dipropionate On Degradation In Different Media
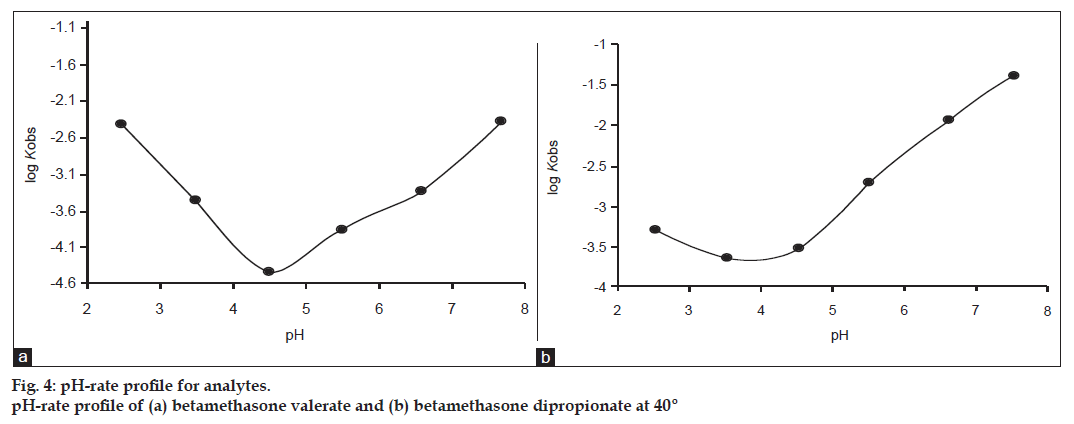
The pH−rate profile for the thermal degradation of betamethasone dipropionate in the range 2.5−7.5 (fig. 4a) represents the breakdown of the ester side chain followed by hydrolysis. The molecule may undergo specific acid−base catalysis resulting in an increase in the rate with a decrease in pH in the acid region and with an increase in the rate in pH in the alkaline region.
The very slow rate around pH 4.5 appears to be due to the solvent catalytic effect, that is, the un−ionized water−catalyzed reaction of the molecule. The rate law for the acid−base catalyzed reaction may be written as: kobs = ko + k1 [H+] + k2 [OH–].
At low pH the term k1 [H+] is greater and specific hydrogen ion catalysis is observed. Similarly, at high pH, the concentration of [OH–] is greater and specific hydroxyl ion catalysis is observed. This explains the V−shaped pH−rate profile for the thermal degradation of betamethasone dipropionate. The pH−rate profile for the thermal degradation of betamethasone valerate (fig. 4b) represents ester hydrolysis over the pH range 2.5−7.5 and probably involves an intermediate in the reaction as observed in the case of the hydrolysis of hydrochlorothiazide [28]. The profile indicates an increase in the rate in the pH range 2.5−3.5 due to H+ ion catalysis. This is followed by a relatively pH independent region extending over the pH range of 3.5−4.5. On increasing the pH there is a gradual increase in the rate above pH 4.5. This appears to be due to the water/hydroxyl ion−catalyzed hydrolysis of the molecule in the neutral and alkaline region. The hydrolysis of betamethasone valerate represents V−shaped curve and is a case of specific acid−base catalyzed degradation.
The product distribution (% ratio) at 10% thermal degradation of betamethasone−17−valerate and betamethasone dipropionate in the pH range 2.5 to 7.5 is given in Table 4. It appears that the thermal degradation of betamethasone−17−valerate increases as a function of pH in the range of 2.5−5.5 leading to the formation of betamethasone−21−valerate (8.33−9.65%) and betamethasone alcohol (0.17−0.9%). The degradation of betamethasone−17−valerate leads to the formation of betamethasone alcohol through betamethasone−21−valerate as an intermediate in the reaction. Some minor unknown products were also detected by HPLC during the degradation reaction. Betamethasone dipropionate leads to the formation of betamethasone−17−propionate, betamethasone−21−propionate and betamethasone alcohol. However, betamethasone−21−propionate is the only product formed at pH 2.5, and betamethasone−21−propionate and betamethasone alcohol are formed at pH 3.5−4.5 and betamethasone−17−propionate and betamethasone− 21−propionate are the only products formed at pH 7.5. Betamethasone−17−propionate, betamethasone−21−propionate and betamethasone alcohol are all formed at pH 5.5 and 6.5. The formation of betamethasone−17−propionate increases with pH whereas the formation of betamethasone−21−propionate and betamethasone alcohol decreases with pH in the pH range 2.5−7.5. It appears that in the pH range 2.5−4.5 any betamethasone−17−propionate formed is unstable and is converted to betamethasone−21−propionate. Since betamethasone alcohol is formed through betamethasone−21−propionate, its decreased formation with pH is in accordance with the decreased formation of betamethasone−21−propionate with pH. It also indicates that betamethasone alcohol is a degradation product of betamethasone−21−propionate.
| Betamethasone valerate | Betamethasone dipropionate | ||||
| pH | Betamethasone−21−valerate | Betamethasone alcohol | Betamethasone-17- propionate | Betamethasone-21- propionate | Betamethasone alcohol |
| 2.5 | 8.33 | 0.17 | − | 10.00 | − |
| 3.5 | 9.10 | 0.90 | − | 9.20 | 0.80 |
| 4.5 | 9.55 | 0.45 | − | 6.80 | 3.20 |
| 5.5 | 9.65 | 0.35 | 0.48 | 8.68 | 0.83 |
| 6.5 | − | − | 3.18 | 6.69 | 0.13 |
| 7.5 | − | − | 5.39 | 4.61 | − |
Table 4: Product Distribution At 10% Degradation Of Betamethasone-17-Valerate And Betamethasone Dipropionate At Different Ph Values
In the present study degradation of betamethasone esters has been carried out in different media, i.e. methanol, acetonitrile, phosphate buffer (pH 7.5), cream and gel formulations. The treatment of the assay data Tables 1 and 2 of degraded betamethasone esters in different media shows that the reaction follows first−order kinetics. The values of the rate constants (kobs) are reported in Table 5. The correlation coefficients for the rate constants lie in the range of 0.998−0.999. It appears that degradation generally decreases in the order of the medium as: Organic solvents > phosphate buffer > cream > gel.
| Medium | Dielectric constant (25°C) | Betamethasone-17-valerte | Betamethasone dipropionate | ||
|---|---|---|---|---|---|
| kobs9(×103h-1) | Correlation coefficient | kobs(×103h-1) | Correlation coefficient | ||
| Methanol | 32.6 | 9.07 | 0.992 | 1.87 | 0.999 |
| Acetonitrile | 40.1 | 7.78 | 0.99 | 1.46 | 0.999 |
| Phosphate buffer (pH 7.5) | 78.5 | 5.48 | 0.994 | 0.59 | 0.997 |
| Cream | − | 0.479 | 0.994 | 0.3 | 0.993 |
| Gel | − | 0.399 | 0.998 | 0.239 | 0.998 |
Table 5: Apparent First-Order Rate Constants (Kobs) For The Degradation Of Betamethasone-17-Valerate And Betamethasone Dipropionate
The rate constants indicate that betamethasone valerate degrades faster than betamethasone dipropionate, suggesting that betamethasone valerate is more susceptible to photodegradation compared to that of the betamethasone dipropionate.
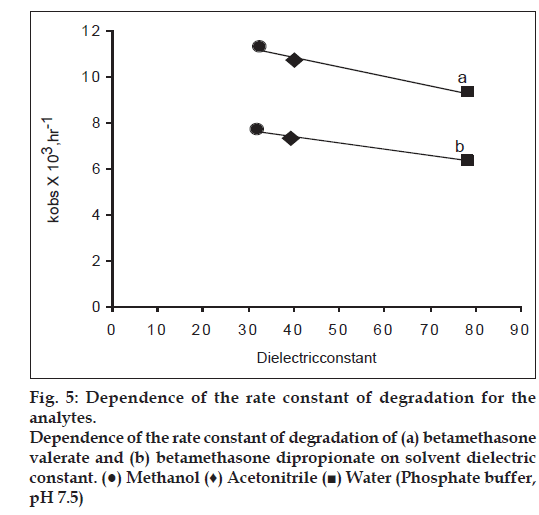
In order to observe the role of solvent on the rate of degradation of betamethasone valerate and betamethasone dipropionate, plots of kobs versus the solvent dielectric constant were prepared (fig. 5). It was found that the rate of degradation is increased with a decrease in the solvent dielectric constant suggesting the presence of a non−polar intermediate in the reaction, which controls the rate of reaction.
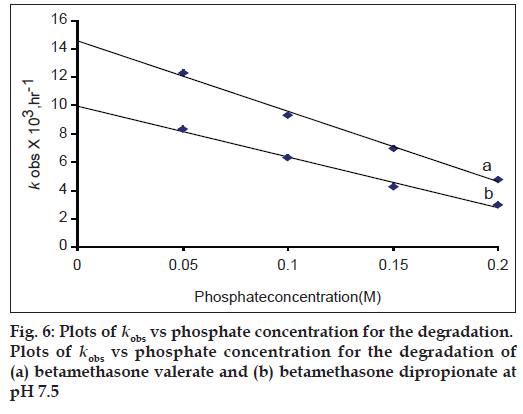
Degradation of betamethasone esters has been carried out in the presence of varying concentration of phosphate buffer. Plots of kobs versus buffer concentration are shown in fig. 6. A decrease in the rate of degradation is observed with an increase in buffer concentration in both cases. Therefore, the buffer appears to cause inhibition in the rate of reaction. This may be due to deactivation of the excited species with an increase in buffer concentration. A decrease in the rate of degradation of such compounds has been observed with an increase in phosphate buffer [8]. It may be concluded that phosphate buffer has a significant effect on the degradation kinetics of betamethasone ester.
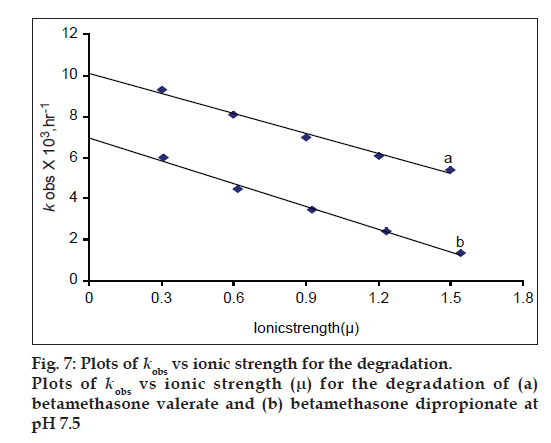
The rate of degradation of both esters decreases with an increase in ionic strength of phosphate buffer (fig. 7). This is also in accordance with the behavior of mometasone furoate on thermal degradation in which the rate of loss is decreased with an increase in the ionic strength [8].
Degradation of betamethasone valerate and betamethasone dipropionate in different media follows first−order kinetics. Betamethasone valerate degrades into betmethasone−21−valerate and betamethasone alcohol whereas betamethasone dipropionate degrades into betamethasone−17−propionate, betamethasone−21−propionate and betamethasone alcohol. The rate of degradation increases with a decrease in the solvent dielectric constant due to the relatively non−polar character of these esters. On the other hand an increase in the concentration and ionic strength of phosphate buffer decreases the rate of degradation probably as a result of the deactivation of activated species. The compounds can be effectively protected against degradation in cream and gel preparations by controlling pH of the formulations.
Acknowledgements
The authors are grateful to M/s. Glaxo SmithKline Pakistan (Pvt) Ltd., Pakistan and M/s. Crystal Pharma, Malaysia for the donation of betamethasone valerate, betamethasone dipropionate and their degradation products. They are also thankful to Dr. Muhammad Ashraf for valuable suggestions and M/s. Nabi Qasim (Pvt) Ltd, Karchi, Pakistan for providing technical facilities to carry out this work.
References
- Sweetman SC, editor. Martindale The Complete Drug Reference, 36th ed. London: Pharmaceutical Press; 2009.
- European Pharmacopeia. Council of Europe, 5th ed., France: Strasbourg, Cedex; 2004. p. 1090 2, 1094 5.
- British Pharmacopoeia. London: Her Majesty’s Stationary Office; 2009. p. 251 2, 254 5.
- United States Pharmacopeia, 32nd ed. Rockville, MD: United States Pharmacopeial Convention; 2009. p. 1660 1, 1665 6.
- Hamlin WE, Chulski T, Johnson RH, Wagner JG. A note on the photolytic degradation of antiinflammatory steroids. J Am Pharm Assoc 1960;49:253 5.
- Ferrante MG, Rudy BC. Betamethasone dipropionate. In: Florey K, editor. Analytical profiles of Drug Substances. New York: Academic Press; 1977. p. 57 61.
- Bundgaard H, Hansen J. Studies on the stability of corticosteroids VI. Kinetics of the rearrangement of betamethasone 17 valerate to the 21 valerate ester in aqueous solution. Int J Pharm 1981;7:197 203.
- Teng XW, Cutler DC, Davies NM. Degradation kinetics of mometasonefuroate in aqueous systems. Int J Pharm 2003;259:129 41.
- Würthwein G, Rohdewald P. Activation of beclomethasonedipropionate by hydrolysis to beclomethasone 17 monopropionate. Biopharm Drug Dispos 1990;11:381 94.
- McGinity JW, Patel TR, Naqvi AH, Hill JA. Implications of peroxide formation in lotion and ointment dosage forms containing polyethylene glycols. Drug Dev Commun 1976;2:505 19.
- Jager PD, Kontny MJ, Nagel JH. Stabilized medicinal aerosol solution formulations. US Patent 5676930; 1997.
- Yip YW, Li Wan Po A. The stability of betamethasone 17 valerate in semi solid bases. J Pharm Pharmacol 1979;31:400 2
- Ryatt KS, Feather JW, Mehta A, Dawson JB, Cotterill JA, Swallow R. The stability and blanching efficacy of betamethasone 17 valerate in emulsifying ointment. Br J Dermatol 1982;107:71 6.
- Roth HJ, Eger K, Troschutz R. Pharmaceutical chemistry. Vol. 2. In, Drug Analysis. London: Ellis Horwood; 1979. p. 466 7.
- Guttman DE, Meister PD. The kinetics of base catalyzed degradation of prednisolone. J Am Pharm Assoc 1958;47:773 8.
- Oesterling TO, Guttman DE. Factors influencing stability of prednisolone in aqueous solution. J Pharm Sci 1964;53:1189 92.
- Monder C. Stability of corticosteroids in aqueous solutions. Endocrinology 1968;82:318 26.
- Hansen J, Bongaard H. Studies on the stability of corticosteroids V. The degradation pattern of hydrocortisone in aqueous solution. Int J Pharm 1980;6:307 19.
- McGinity JW, Hill JA, La Via AL. Influence of peroxide impurities in polyethylene glycols on drug stability. J Pharm Sci 1975;64:356 7.
- Yip YW, Li Wan Po A, Irwin WJ. Kinetics of decomposition and formulation of hydrocortisone butyrate in semiaqueous and gel systems. J Pharm Sci 1983;72:776 81.
- Mauger JW, Paruta AN, Gerraughty RJ. Consecutive first order kinetic consideration of hydrocortisone hemisuccinate. J Pharm Sci1969;58:574 8.
- Johnson K, Amidon GL, Pogany S. Solution kinetics of a water soluble hydrocortisone prodrug: hydrocortisone 21 lysinate. J Pharm Sci1985;74:87 9.
- Foe K, Cheung HT, Tattam BN, Brown KF, Seale JP. Degradation products of beclomethasonedipropionate in human plasma. Drug Metab Dispos 1998;26:132 7.
- Barnes AR, Nash S, Watkiss SB. Stability of steroid ointments diluted with compound zinc paste BP. J Clin Pharm Ther 1991;16:103 9.
- Gardi R, Vitali R, Ercoli A: Derivati di condensazionenelle catena laterale di corticosteroidi. Nota III. Preparazione e reazionidei17 monoesteri. Gazz Chim Ital 1963;93:431 50.
- British Pharmaceutical Codex. London: The Pharmaceutical Press; 1973. p. 55.
- Kubota K, Ademola J, Maibach HI. Metabolism and degradation of betamethasone 17 valerate in homogenized living skin equivalent. Dermatology 1994;188:13 7.
- Mollica JA, Rehm CR, Smith JB. Hydrolysis of hydrochlorothiazide. J Pharm Sci 1969;58:635 6.
 Methanol
Methanol  Acetonitrile
Acetonitrile 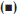 Water (Phosphate buffer, pH 7.5)
Water (Phosphate buffer, pH 7.5)