- *Corresponding Author:
- F. Ari
Uludag University, Science and Arts Faculty, Department of Biology, 16059, Bursa, Turkey
E-mail: ferdaoz@uludag.edu.tr
| Date of Submission | 28 July 2017 |
| Date of Revision | 02 April 2018 |
| Date of Acceptance | 05 October 2018 |
| Indian J Pharm Sci 2018;80(6):1078-1085 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Lichens are complex organisms living in a symbiotic relationship with fungi and algae have recently received special interest in cancer research. The cytotoxic activities of Usnea filipendula Stirt. lichen extract was investigated on colon cancer cell lines, HCT-15 and HT-29. Sulforhodamine B and ATP cell viability tests were used to monitor cytotoxic activity. The mode of cell death (apoptosis/necrosis) was determined using caspase-cleaved cytokeratin 18 (M30), caspase-3/7 activity and fluorescence staining techniques that included, Annexin-V, Hoechst 33342 and propidium iodide. Usnea filipendula showed dose and time-dependent antiproliferative effect in HCT-15 and HT-29 cells. The IC50 values in HCT-15 and HT-29 cells were 17.92 and 41.87 µg/ml, respectively. The extract induced apoptosis in both cell lines especially in HCT-15 cells in which caspase-3/7 activity was increased. Usnea filipendula was cytotoxic to colon cancer HCT-15 and HT-29 cell lines by inducing early or late apoptosis as evidenced by translocation of phosphatidylserine, pyknotic nuclei and nuclear condensation. Further studies would help to understand the full potential of Usnea filipendula as a novel anticancer therapy.
Keywords
Usnea filipendula, apoptosis, colon cancer, cancer cell death
Cancer is the second leading cause of noncommunicable disease deaths in 2012 worldwide and new cases of cancer is expected to rise by 70 percent in next two decades [1]. Natural products are playing a rapidly increasing role in the lead-finding of candidates for the development of chemotherapeutic drugs [2,3]. They offer a valuable source of compounds with a variety of chemical structures with biological activities and provide important prototypes for the development of novel drugs [4,5]. Some drugs administered to patients today are derived from plants and other natural products such as vinblastine, vincristine, etoposide, paclitaxel, topotecan and irinotecan [6]. Natural products are the most important anticancer agents. Three-quarters of antitumor compounds used in medicine are natural products or related to them. Of the 140 anticancer agents approved since 1940 and available for use, over 60 % can be traced to a natural product [7].
Recently, lichens which are complex organisms living in symbiotic relationship with fungi, algae and/or cyanobacteria received special interest [8]. Lichens possess several biological properties, which include antimycobacterial, antitumor, antioxidant, cytotoxic, antipyretic and antiproliferative [8,9-11]. In folk medicine, decoction of lichens has been used to treat several ailments, among them are splenomegaly (enlarged spleen), mild inflammation of the oral and pharyngeal mucosa, dyspepsia, colds and fever [12].
Among the interesting medicinal lichen genera is Usnea, of which some species are used in Traditional Chinese Medicine as antimicrobial agent [13]. The genus Usnea is edible and is utilized in the preparation of traditional foods and medicines in both Eastern and Western countries [14,15]. Usnea filipendula Stirt. was used in the former Soviet Union for cuts and wounds and in Central Java, Indonesia as one of the components of a unique herbal topical medicament to treat headache, fever, dizziness, hazy vision and eyestrain [16,17]. In this study, the cytotoxicity and cell death mechanism of U. filipendula against human colon cancer (HCT-15 and HT-29) cell lines was evaluated.
Materials and Methods
U. filipendula was collected from the trunks of Quercus sp. growing on Mount Uludag, Bursa, Turkey in May 2010 and was identified with an aid of flora book [18].
Extraction of lichen sample
Air-dried lichen samples were carefully cleansed of extraneous materials and ground into powder. Fifteen grams of the ground material was extracted consecutively by adding 150 ml of solvent methanol (Merck, Darmstadt, Germany) in a Soxhlet extractor for 24 h. After that the extraction was performed with water at same conditions. The crude extracts were concentrated using a rotary evaporator at 40°; thereafter, the residues were lyophilized and stored at –20° until used in the tests.
Solutions and cell culture
Stock concentrations of lyophilized U. filipendula samples were prepared in dimethyl sulfoxide (DMSO; Sigma‑Aldrich, St. Louis, MO, USA) at a concentration of 0.05 g/0.5 ml, while the final concentrations were prepared in culture medium. U. filipendula extract (UFE) was used at different concentrations ranging from 1.56 to 100 μg/ml. HCT-15 and HT-29 cell lines were cultured in Roswell Park Memorial Institute (RPMI) medium 1640 (Lonza Bioscience, Verviers, Belgium) supplemented with penicillin G (100 U/ml), streptomycin (100 μg/ml; HyClone, GE Healthcare Life Sciences, Logan, UT, USA), L-glutamine (Gibco®; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 5 % (for HCT-15) 10 % (HT-29) fetal bovine serum (Invitrogen, Paisley, UK), and 1 % non-essential amino acid (Gibco®; Thermo Fisher Scientific, Inc., Waltham, MA, USA; only for HT-29) at 37° in a humidified atmosphere containing 5 % CO2.
Sulphorhodamine B (SRB) viability assay
Cancer cells were seeded in 200 μl culture medium in triplicate at a density of 5×103 cells per well of a 96-well plate. Cells were incubated either alone (for controls which include 0.1 % DMSO) or in the presence of UFE for 24, 48, and 72 h. Each experiment was performed as two independent test with three replicated SRB assay relies on the uptake of the negatively charged pink aminoxanthine dye, SRB (Sigma, St. Louis, MO) by basic amino acids in the cells. The greater the number of cells, the greater amount of dye is taken up and, after fixing, when the cells are lysed, the released dye will give a more intense colour and greater absorbance [19]. At the end of the treatment periods (24, 48, and 72 h), cells were fixed by adding 50 μl of 50 % (w/v) ice-cold trichloroacetic acid (TCA; AppliChem, Germany) to each well and incubated for 1 h at 4°. The cells were stained with 50 μl of SRB solution (0.4 % w/v in 1 % aqueous acetic acid) for 30 min at room temperature after the removal of TCA by washing with deionized water five times. The excess dye was washed out with 1 % (v/v) acetic acid and the plate was subsequently air-dried prior to addition of 10 mM Tris base solution (pH 10, 150 μl) to dissolve the protein-bound dye. After solubilisation by shaking for 10 min at 150 rpm, the absorbance was read by using a spectrophotometer (FLASH Scan S12, Analytik Jena) at 564 nm. All the experiment was repeated twice in triplicate. Cell viability of the treated cells was calculated with reference to the untreated control cells using the following Eqn., viability % = 100× [(sample absorbance)/(control absorbance)].
Adenosine triphosphate (ATP) viability assay
ATP assay was performed to confirm the results of the SRB assay. The ATP assay is considered more sensitive than the SRB assay [20]. The seeding and treatment conditions, as well as the viability calculation, were similar to those for the SRB assay (see above). At the end of the treatment period (72 h), the ATP content was determined according to the manufacturer’s recommendations (ATP Bioluminescence Assay, Sigma, St. Louis, MO, USA). Briefly, ATP was extracted from the cells and then a luciferin-luciferase solution was added. Luminescence was determined in a luminometer (Bio-Tek, USA). The results were expressed in U/l.
Caspase-cleaved cytokeratin 18 (M30) detection
Apoptosis was assayed by measuring the level of caspase-cleaved cytokeratin 18 (ccK18, M30) by a commercially available immunoassay kit (M30- Apoptosense ELISA kit, Peviva AB, Sweden) according to the manufacturer’s instructions. This kit measures the level of the CK18-Asp396 neo-epitope (M30), which is a well-known marker of apoptosis. In a 96-well plate, 5×103 cells were seeded per well in 200 μl culture medium in triplicate. Cells were treated for 72 h with 100 μg/ml of lichen extracts. At the end of the treatment period, the cells were lysed with 10 % NP-40 (Sigma‑Aldrich) for 10 min on a shaker. The contents of identical wells were pooled and centrifuged at 2000 rpm for 10 s to remove the debris. All samples were placed into wells coated with a mouse monoclonal antibody as a catcher. After washing, a horseradish peroxidase-conjugated antibody (M30 antibody) was used for detection. The absorbance was determined with an ELISA reader at 450 nm (FLASH Scan S12, Eisfeld, Germany).
Fluorescence imaging for apoptosis using Hoechst Annexin-V and propidium iodide (PI)
Translocation of phosphatidylserine (PS) molecules from the inner side to the outside of the cell membrane is one of the earlier events of apoptosis [21,22]. Annexin-V is able to bind to PS, allowing the apoptotic cells to become visible. However, Annexin-V may also bind to necrotic cells. Therefore, PI is additionally used as a second dye to distinguish apoptotic and necrotic cells from each other. Late apoptotic/necrotic cells are both Annexin-V and PI positive while early apoptotic cells are only Annexin-V positive. Briefly, cancer cells were seeded in a 96-well plate at the density of 5×103 cells per well, and then the cells were treated for 12 and 24 h with UFE at the 100, 50, and 25 μg/ml doses. After the treatment, cells were stained with Annexin-V-FITC and PI using the Annexin-V-Fluos kit (Roche Diagnostics GmbH, Mannheim, Germany), according to the recommendation of the company. In addition to this standard protocol, a nucleus-staining fluorescent dye, Hoechst dye 43332 (200 μg/ml, 1:40, AppliChem GmbH, Darmstadt, Germany) was added to the staining solution to also detect apoptosis on the basis of nuclear morphology as an additional analysis to the examination of cell membrane. The cells were then visualized under fluorescence microscope.
Caspase-3/7 activity
After 72 h of treatment with UFE (25, 50 and 100 μg/ml), human colon (HCT-15 and HT-29) cancer cell lines were analysed for the detection of apoptosis and cell death using Muse caspase-3/7 (cat #MCH100108, Merck Millipore) assay according to the manufacturer’s instructions. This kit uses a novel Muse™ caspase-3/7 reagent NucView™ for the detection of caspase-3/7 activity and a cell death dye 7-aminoactinomycin D (7- AAD) that provides information on membrane integrity or cell death. This assay enables to differentiate four populations in each sample by cytofluorimetric separation on a Muse automated cell analyser (Merck, Millipore): non-apoptotic live (lower left panel: 7-AAD negative, caspase-3/7 reagent negative), apoptotic live (lower right panel: 7-AAD negative, caspase-3/7 reagent positive), apoptotic/dead (upper right panel: 7-AAD positive, caspase-3/7 reagent positive) and necrotic (upper left panel: 7-AAD positive, caspase-3/7 reagent negative) cells.
Statistical analysis
All statistical analyses were performed using the Stat 12 statistical software package for Windows (Serial number: 40120559342). Significance was calculated using one-way analysis of variance (ANOVA) and Tukey post hoc test. A value of p<0.05 was considered statistically significant. The results are expressed as the mean±SD (standard deviation).
Results and Discussion
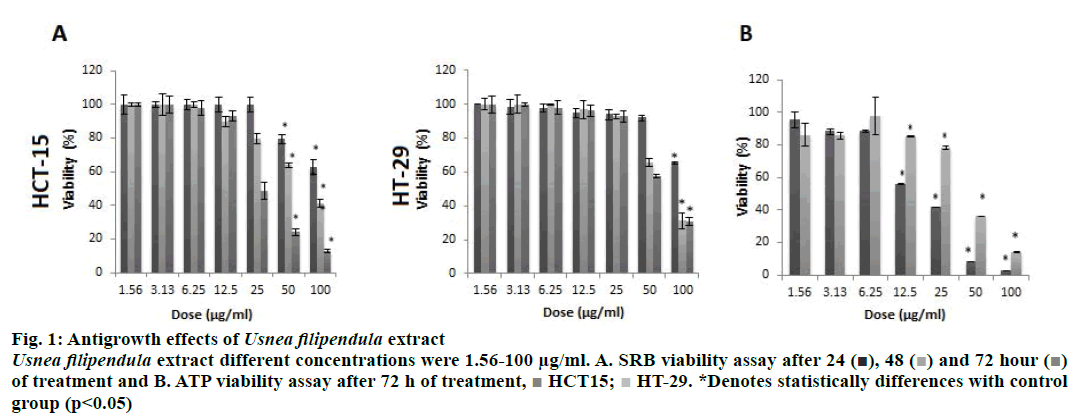
Antigrowth effect was assessed on HCT-15 and HT-29 cells lines by SRB assay after 24, 48, and 72 h. Because UFE is most cytotoxic after 72 h of treatment, the more sensitive ATP assay was performed to confirm SRB results (Figure 1). UFE exhibited a dose-dependent but time-independent cytotoxic effect (p<0.05) in both cell lines. Based on the dose-response curve, HCT-15 cells were more sensitive to UFE than HT-29 cells. The IC50 values (50 % inhibitory concentration) calculated using the ATP assay results on HCT-15 and HT-29 cells were 17.92 μg/ml and 41.87 μg/ml, respectively.
Figure 1: Antigrowth effects of Usnea filipendula extract
Usnea filipendula extract different concentrations were 1.56-100 μg/ml. A. SRB viability assay after 24 ( ), 48 (
), 48 ( ) and 72 hour (
) and 72 hour ( )
of treatment and B. ATP viability assay after 72 h of treatment,
)
of treatment and B. ATP viability assay after 72 h of treatment,  HCT15;
HCT15;  HT-29. *Denotes statistically differences with control
group (p<0.05)
HT-29. *Denotes statistically differences with control
group (p<0.05)
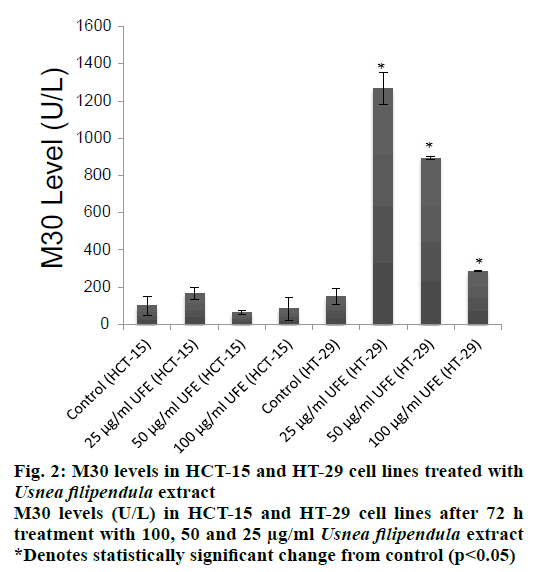
The mode of cell death was investigated by assessing the level of soluble caspase-cleaved cytokeratin 18 (ccCK18, M30) fragments at various concentrations (25, 50 and 100 μg/ml) of UFE after 72 h of treatment. M30 is a well-known marker of apoptosis. HCT-15 cells had statistically insignificant increase of M30 levels compared to the negative control in all doses. However, M30 antigen levels were significantly increased in HT-29 cells treated with 100, 50 and 25 μg/ml UFE (Figure 2).
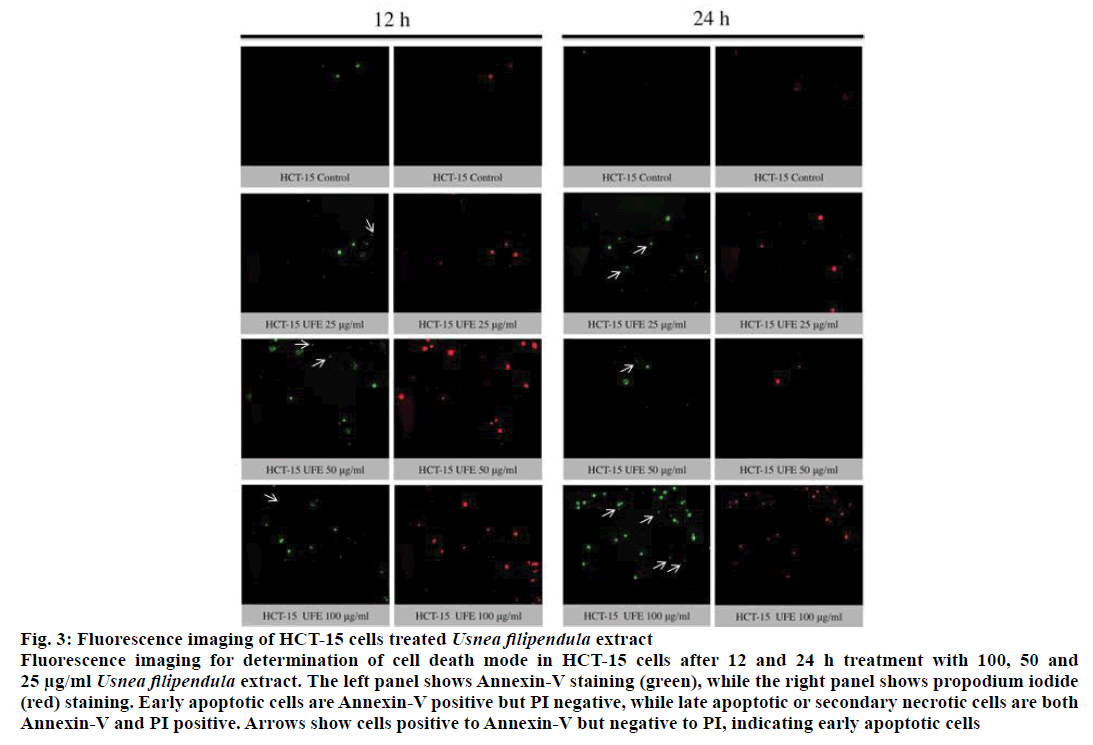
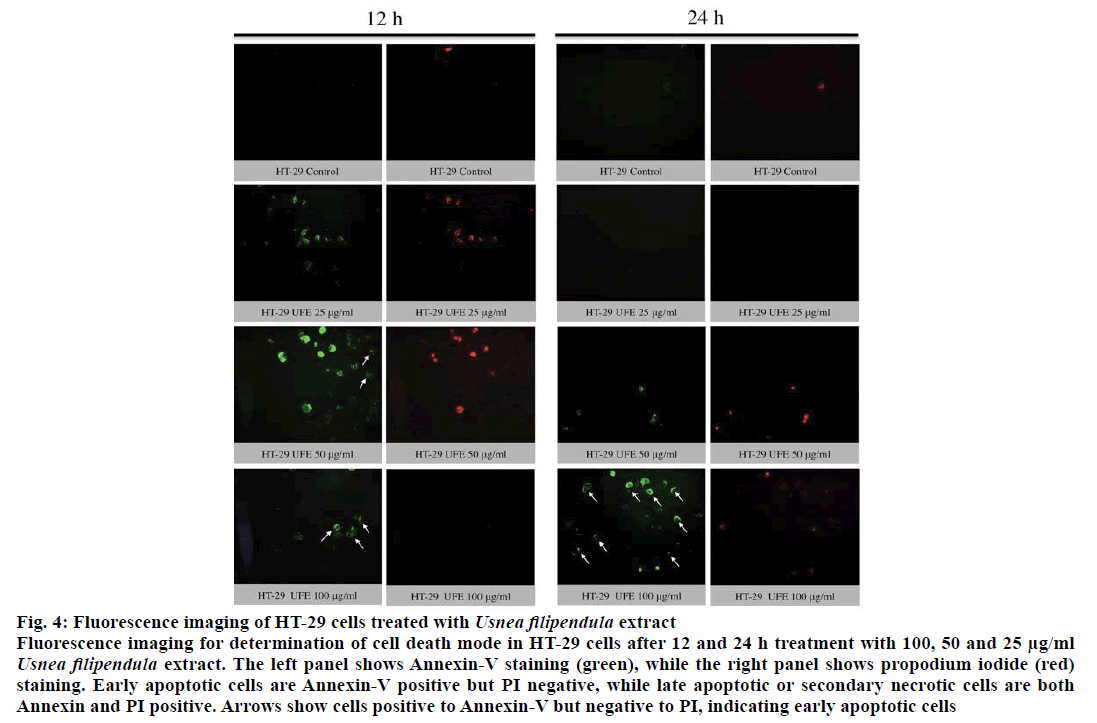
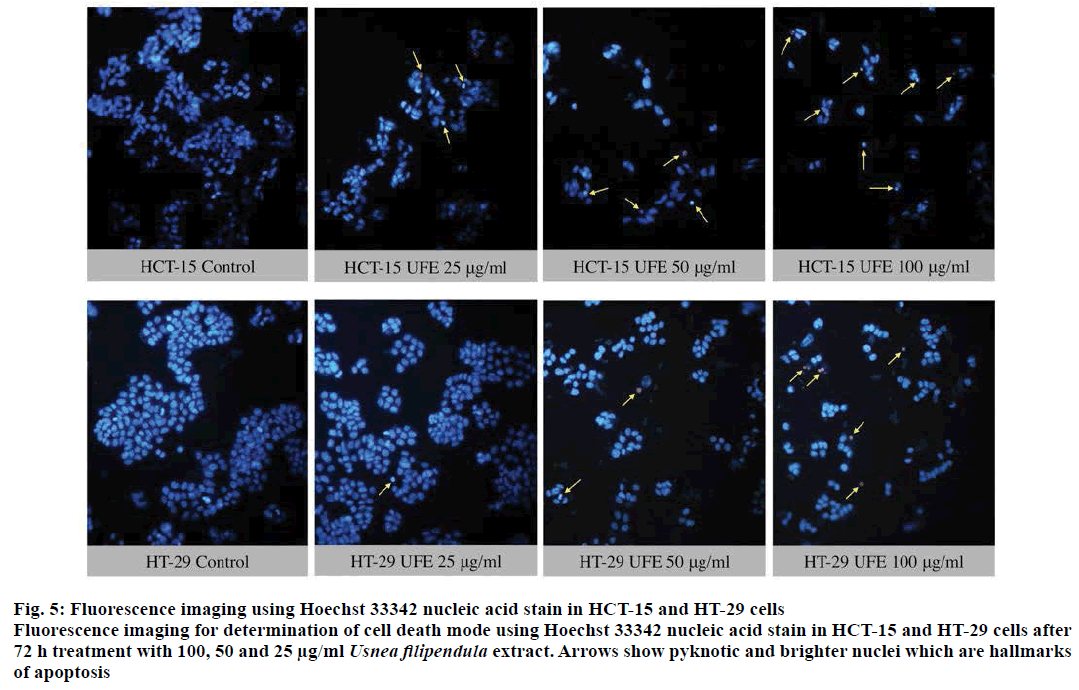
The translocation of PS from cytoplasmic surface to the outer cell membrane occurs in the early stage of apoptosis [22]. The translocation of this membrane protein was assessed using a fluorescent dye Annexin-V, which gives a non-quantitative probe to detect apoptotic cells. Because ATP assay results showed potent cytotoxicity of UFE at 25, 50 and 100 μg/ml against cell lines, the morphology of these cells treated with these concentrations were further evaluated. Late apoptosis or secondary necrosis was observed in HCT-15 cells after 12 and 24 h treatment with UFE (Figure 3). Both early and late apoptosis were observed in HT-29 cells after 12 and 24 h of treatment with UFE (Figure 4). Cell nuclear morphology was also examined using Hoechst 33342 nucleic acid stain. Cells treated with UFE resulted to shrinkage of nucleus and condensation of chromatin, both are hallmarks of apoptosis (Figure 5).
Figure 3: Fluorescence imaging of HCT-15 cells treated Usnea filipendula extract
Fluorescence imaging for determination of cell death mode in HCT-15 cells after 12 and 24 h treatment with 100, 50 and
25 μg/ml Usnea filipendula extract. The left panel shows Annexin-V staining (green), while the right panel shows propodium iodide
(red) staining. Early apoptotic cells are Annexin-V positive but PI negative, while late apoptotic or secondary necrotic cells are both
Annexin-V and PI positive. Arrows show cells positive to Annexin-V but negative to PI, indicating early apoptotic cells
Figure 4: Fluorescence imaging of HT-29 cells treated with Usnea filipendula extract
Fluorescence imaging for determination of cell death mode in HT-29 cells after 12 and 24 h treatment with 100, 50 and 25 μg/ml Usnea filipendula extract. The left panel shows Annexin-V staining (green), while the right panel shows propodium iodide (red)
staining. Early apoptotic cells are Annexin-V positive but PI negative, while late apoptotic or secondary necrotic cells are both
Annexin and PI positive. Arrows show cells positive to Annexin-V but negative to PI, indicating early apoptotic cells
Figure 5: Fluorescence imaging using Hoechst 33342 nucleic acid stain in HCT-15 and HT-29 cells
Fluorescence imaging for determination of cell death mode using Hoechst 33342 nucleic acid stain in HCT-15 and HT-29 cells after
72 h treatment with 100, 50 and 25 μg/ml Usnea filipendula extract. Arrows show pyknotic and brighter nuclei which are hallmarks
of apoptosis
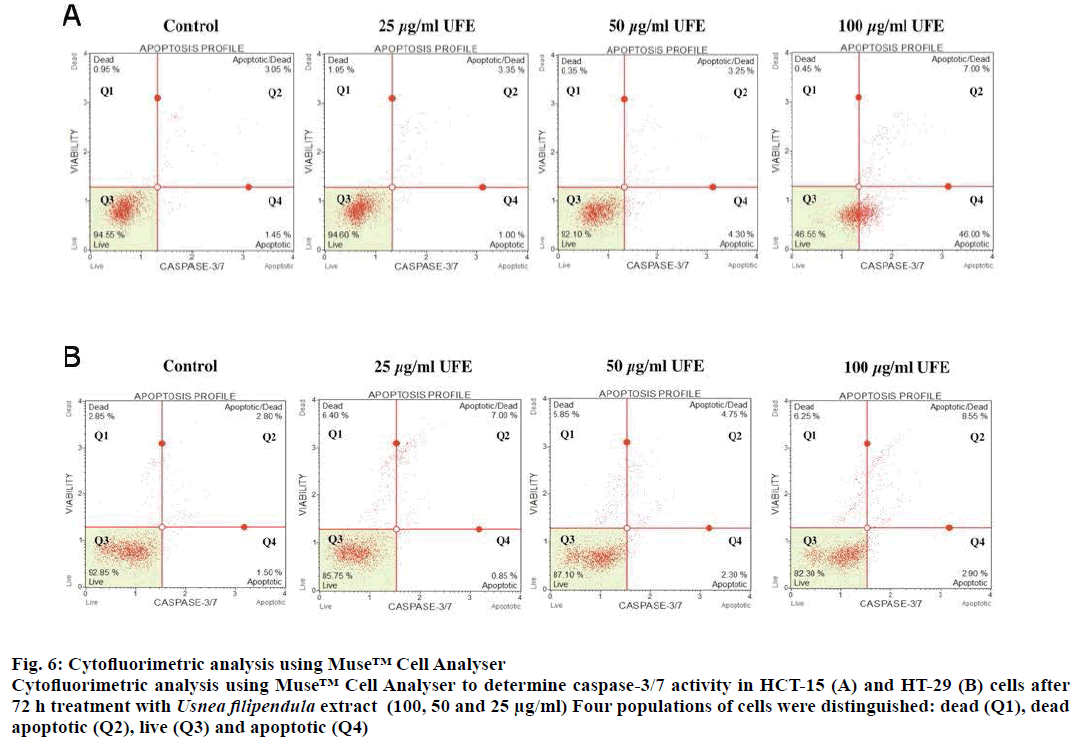
Caspase 3 and 7 are effector caspases that direct cellular breakdown through cleavage of structural proteins. A total of 53 % of HCT-15 cells (Q2+Q4) treated with 100 μg/ml UFE were apoptotic whereas in other doses (50 and 25 μg/ml UFE), only less than 10 % of the cell population was apoptotic (Figure 6A). HT-29 cells did not show significant increase of caspase-3/7 activity in all doses compare to control group (Figure 6B).
Figure 6: Cytofluorimetric analysis using Muse™ Cell Analyser
Cytofluorimetric analysis using Muse™ Cell Analyser to determine caspase-3/7 activity in HCT-15 (A) and HT-29 (B) cells after
72 h treatment with Usnea filipendula extract (100, 50 and 25 μg/ml) Four populations of cells were distinguished: dead (Q1), dead
apoptotic (Q2), live (Q3) and apoptotic (Q4)
The search for novel agents is still a priority goal for cancer therapy due to the rapid development of resistance to chemotherapeutic drugs [23]. The high toxicity usually associated with some chemotherapy drugs and their undesirable side effects demand for new drugs that are both cytotoxic to cancer cells and less toxic to normal cells. Natural products, in this aspect, play an important role and as such, a substantial number of anticancer agents used in the clinic are either natural or derived from natural products from various sources [24,25].
U. filipendula has been reported to have cytotoxic activity by inducing apoptosis-like cell death and DNA damage in human lung cancer (A549, PC3), liver cancer (Hep3B), and rat glioma (C6) cells [26]. Our group has previously reported the cytotoxic effect of U. filipendula in breast cancer (MCF-7 and MDA-MB-231) cells also by inducing apoptosis through cleavage of PARP and induction of caspase-3 [27]. In this study, U. filipendula potential dose-dependent antiproliferative effect in human colon cancer cells was demonstrated. HCT-15 cells were more sensitive to UFE than HT-29 cells as seen by IC50 values and the dose-response curve. The genotypic differences [28] in cells might be the cause of this result. In addition, the HT-29 cells have highly expressed stem cell markers [29,30] so HT-29 cells found to be resistance of UFE than HCT-15.
The antiproliferative effect of UFE may be due to its secondary metabolites. Usnic acid is the prominent secondary metabolite of Usnea lichen species [31]. Several studies have reported cytotoxicity of usnic acid on various cancer cell lines [32-35]. Usnic acid induces loss of mitochondrial membrane potential along with caspase-3 activation and Annexin V staining in colon cancer HT-29 cells [36]. Increased caspase-3/7 activity and subdiploid nucleus formation were also observed in human hematoma HepG2 cells treated with usnic acid [37].
The mode of cell death was determined biochemically by measuring the levels of caspase-cleaved cytokeratin 18 (M30). It was found that M30 level was significantly increased in HT-29 cells but not in HCT-15 cells. However, morphological examination of HCT-15 and HT-29 cells by fluorescence microscopy has shown both early and late apoptosis as provided by the translocation of PS to the outer leaflet of the lipid bilayer, loss of membrane integrity, pyknotic nuclei and chromatin condensation. There was no increment of M30 level in HCT-15 cells might be due to the lower expression of CK18 in the cells or apoptosis being no cleaved of CK18 occurring in cells. Thus, M30 assay results should be interpreted cautiously as it may give a false negative result for apoptosis [38]. Caspase-dependency of UFE-induced apoptosis was also investigated. Caspase-3 and caspase-7 are major executioner caspases that are both activated universally during apoptosis irrespective of the specific deathinitiating stimulus [39]. This investigation suggested that UFE induced activation of caspase-3 and caspase-7 in HCT-15 cells but not in HT-29 cells at high doses.
The fluorescence staining results indicated greater presence of Annexin V staining in HCT-15 cells at highest doses in time-dependent manner. In HT-29 cells, the caspase-cleaved cytokeratin 18 level was increased, but very low activity of caspase 3 and 7 was observed after cytofluorimetric analysis. It is possible that other caspases orchestrate the apoptotic program in HT-29 cells.
The present study showed that U. filipendula Stirt is cytotoxic to human colon cancer cells and to other cancer cells as previously reported. Because of its cytotoxic effect on several types of cancer cells, advanced in vivo and in vitro studies are required to fully understand the anticancer potential of this species.
Acknowledgements
Authors gratefully the Research Fund of Uludag University for the project that is numbered BUAP(F)-2014/3. The authors would also like to thank Dr Serap Celikler (Uludag University, Bursa, Turkey) for contributing to the extraction and lyophilization of the lichens.
Conflict of interest
There are no conflicts of interest.
References
- World Health Organization. Global status Report on noncommunicable diseases. Available from: https://www.who.int/nmh/publications/ncd-status-report-2014/en/.
- Lee KH. Novel antitumour agents from higher plants. Med Res Rev 1999;19:569-96.
- Schwartsmann G. Marine organisms and other novel natural sources of new cancer drugs. Ann Oncol 2000;11:235-43.
- Cragg GM. Paclitaxel (Taxol): a success story with valuable lessons for natural product drug discovery and development. Med Res Rev 1998;18:315-31.
- Vuorelaa P, Leinonenb M, Saikkuc P, Tammelaa P, Rauhad JP, Wennberge T, et al. Natural products in the process of finding new drug candidates. Curr Med Chem 2004;11:1375-89.
- Demain AL, Viashnav P. Natural products for cancer chemotherapy. Microb Biotechnol 2011;4:687-99.
- Cragg GM, Newman DJ. Plants as a source of anti-cancer agents. J Ethnopharmacol 2005;100:72-9.
- Muller K. Pharmaceutically relevant metabolites from lichens. Appl Microbiol Biotechnol 2001;56:9-16.
- Molnar K, Farkas E. Current results on biological activities of lichen secondary metabolites: a review. Z. Naturforsch C 2010;65:157-73.
- Oksanen I. Ecological and biotechnological aspects of lichens. Appl Microbiol Biotechnol 2006;73:723-34.
- Mitrovic T, Stamenkovic S, Cvetkovic V, Tosic S, Stankovic M, Radojevic I, et al. Antioxidant, antimicrobial and antiproliferative activities of five lichen species. Int J Mol Sci 2011;12:5428-48.
- Zambare VP, Christopher LP. Biopharmaceutical potential of lichens. Pharm Biol 2012;50:778-98.
- Malhotra S, Subban R, Singh A. Lichens-Role in Traditional Medicine and Drug Discovery. Internet J Altern Med 2007;5(2):1-6.
- Upreti DK, Divakar PK, Nayaka S. Commercial and Ethnic use of lichens in India. Econ Bot 2005;59:269-73.
- Prateeksha B, Paliya S, Jadaun V, Kumar J, Kumar S, Upreti DK, et al. The genus Usnea: a potent phytomedicine with multifarious ethnobotany, phytochemistry and pharmacology. RSC Adv 2016;6:21672-96.
- Al Makmun MT, Widodo SE, Sunarto. Construing traditional Javanese herbal medicine of headache: Translitering, translating, and interpreting Serat Primbon Jampi Jawi. Procedia Soc Behav Sci 2014;134:238-45.
- Timotius KH, Sari İN, Santoso AW. Major Bioactive Compounds of Pilis Plant Materials: A GC-MS Analysis. Pharmacogn Commn 2015;5:190-96.
- Purvis OW, Coppins BJ, Hawskworth DL, James PW, Moore DM. The Lichen Flora of Great Britain and Ireland. 2nd ed. London, UK: The British Lichen Society; 1994.
- Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 1990;82:1107-12.
- Ulukaya E, Ozdikicioglu F, Oral AY, Demirci M. The MTT assay yields a relatively lower result of growth inhibition than the ATP assay depending on the chemotherapeutic drugs tested. Toxicol In Vitro 2008;22:232-9.
- Ulukaya E, Acilan C, Ari F, Ikitimur E, Yilmaz Y. A glance at the methods for detection of apoptosis qualitatively and quantitatively. Turk J Biochem 2011;36:261-9.
- van Engeland M, Nieland LJ, Ramaekers FC, Schutte B, Reutelingsperger CP. Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry 1998;31:1-9.
- Kunjachan S, Rychlik B, Storm G, Kiessling F, Lammers T. Multidrug resistance: Physiological principles and nanomedical solutions. Adv Drug Deliv Rev 2013;65:1852-65.
- Mann J. Natural products in cancer chemotherapy: past, present, and future. Nat Rev Cancer 2002;2(2):143-8.
- Prakash O, Kumar A, Kumar P, Ajeet. Anticancer Potential of Plants and Natural Products-A Review. Am J Pharmacol Sci 2013;1:104-15.
- Ari F, Aztopal N, Oran S, Bozdemir S, Celikler S, Ozturk S, Ulukaya E. Parmelia sulcata Taylor and Usnea filipendula Stirt induce apoptosis-like cell death and DNA damage in cancer cells. Cell Prolif 2014;47,457-64.
- Kasimogullari SC, Oran S, Ari F, Ulukaya E, Aztopal N, Sarimahmut M, et al. Genotoxic, cytotoxic, and apoptotic effects of crude extract of Usnea filipendula Stirt. in vitro. Turk J Biol 2014;38:940-47.
- Kleivi K, Teixeira MR, Eknaes M, Diep CB, Jakobsen KS, Hamelin R, et al. Genome signatures of colon carcinoma cell lines. Cancer Genet Cytogenet 2004;155:119-31.
- Schneider M, Huber J, Hadaschik B, Siegers GM, Fiebig HH, Schüler J. Characterization of colon cancer cells: a functional approach characterizing CD133 as a potentialstem cell marker. BMC Cancer 2012;12:96.
- Guo L, Shi Q, Fang JL, Mei N, Ali AA, Lewis SM, et al. Review of usnic acid and Usnea barbata toxicity. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 2008;26:317-38.
- Nguyen TT, Yoon S, Yang Y, Lee HB, Oh S, Jeong MH, et al. Lichen secondary metabolites in Flavocetraria cucullata exhibit anti-cancer effects on human cancer cells through the induction of apoptosis and suppression of tumorigenic potentials. PloS One 2014;9:e111575.
- Brandão LF, Alcantara GB, Matos MFC, Bogo D, Freitas DS, Oyama NM, et al. Cytotoxic Evaluation of Phenolic Compounds from Lichens against Melanoma Cells. Chem Pharm Bull 2013;61:176-83.
- Bazin MA, Le Lamer AC, Delcros JG, Rouaud I, Uriac P, Boustie J, et al. Synthesis and cytotoxic activities of usnic acid derivatives. Bioorg Med Chem 2008;16:6860-6.
- Mayer M, O’Neill MA, Murray KE, Santos-Magalhaes NS, Carneiro-Leao AM, Thompson AM, et al. Usnic acid: a non-genotoxic compound with anti-cancer properties. Anticancer Drugs 2005;16:805-9.
- Backorova M, Jendzelovsky R, Kello M, Backor M, Mikes J, Fedorocko P. Lichen secondary metabolites are responsible for induction of apoptosis in HT-29 and A2780 human cancer cell lines. Toxicol In Vitro 2012;26:462-68.
- Chen S, Dobrovolsky VN, Liu F, Wu Y, Zhang Z, Mei N, et al. The role of autophagy in usnic-acid induced toxicity in hapatic cells. Toxicol Sci 2014;14:33-4.
- Cevatemre B, Ulukaya E, Sarimahmut M, Oral AY, Frame FM. The M30 assay does not detect apoptosis in epithelial-derived cancer cells expressing low levels of cytokeratin 18. Tumour Biol 2015;36:6857-65.
- Walsh JG, Cullen SP, Sheridan C, Lüthi AU, Gerner C, Martin SJ. Executioner caspase-3 and caspase-7 are functionally distinct proteases. Proc Natl Acad Sci U S A 2008;105:12815-19.