- *Corresponding Author:
- V. N. Upasani
Department of Microbiology, M. G. Science Institute, Ahmedabad, Gujarat 380009, India
E-mail: vnu_halophiles@yahoo.com
| Date of Received | 10 April 2021 |
| Date of Revision | 05 May 2022 |
| Date of Acceptance | 13 December 2022 |
| Indian J Pharm Sci 2022;84(6):1602-1607 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Endophytic fungi are present within all plants and more than one million species are predicted to be present. They are widely studied as they promote plant growth as well as resistance to environmental stress, produce bioactive compounds that have industrial applications and play a vital role in biogeochemical cycles. The medicinal plant Cassia (commonly known as seenas) belongs to the Order Fabales Family Caesalpiniaceae. This plant is a native of tropics which is useful as an ornamental plant, cassia gum, cuisines, etc. It is also a source for the laxative drug known as Sennosides complex, which has been used by Egyptians since ancient times. This drug is mainly obtained from Cassia augustifolia and Cassia fistula. The active constituents of this drug are dianthrone glycosides (Sennosides A, B, C, D, etc.), free anthraquinones (aloe-emodin, chrysophanol, rhein) and anthraquinones glycosides. However, due to urbanization the plant source has become scarce, so an attempt has been made to isolate endophytic fungi from this medicinal plant and screen for biosynthesis of Sennosides complex. Plants were collected from six (6) locations/sites at Ahmedabad, Anand (Boriavi), Bavla, Bhuj (Madhapur and Saiyadpur), Dantiwada in Gujarat; and Jodhpur in Rajasthan. 22 isolates were identified based on colony, microscopic/morphological characteristics; and identity of thirteen isolates was also done by phylogenetic analysis. The endophytes belonged to the genera Alterneria, Aspergillus, Cladosporium, Curvularia, Fusarium, Penicillium, Phellinus, etc. The isolates were obtained to screen for production of Sennosides (extracellular). A further study on the potential for their application in sustainable development, and alternative for bioprospecting of bioactive compounds is envisaged.
Keywords
Alterneria, Cassia senna, Cladosporium, endophytic fungi, Fusarium, Phellinus
Medicinal plants serve as a natural source of drugs with less side-effects and have been exploited for this purpose for thousands of years in traditional medicine. However, the natural plant resources are diminishing (due to endangered species or difficulty in cultivation), and also the yield of drug is gradually decreasing. Therefore, it has become a necessity to find alternative sources for these drugs by plant cell or tissue culture, chemical synthesis, semi-synthetic route, or from endophytes[1]. Endophytic microorganisms (namely bacteria and fungi) residing within most vascular plants have sought the attention of researchers since it became known that an endophyte Taxomyces andreanae produced Taxol (an anticancer drug) similar to its host Taxus brevifolia[2,3]. The medicinal plant Cassia senna L. is a well-known tropical medicinal plant that produces Sennosides complex, derived from the leaves. It is a major pharmaceutical laxative prescribed worldwide obtained chiefly from Cassia augustifolia and Cassia fistula.
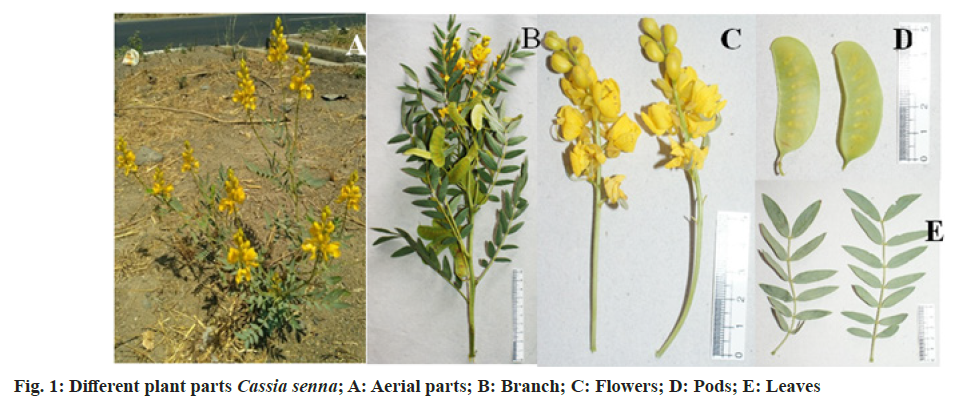
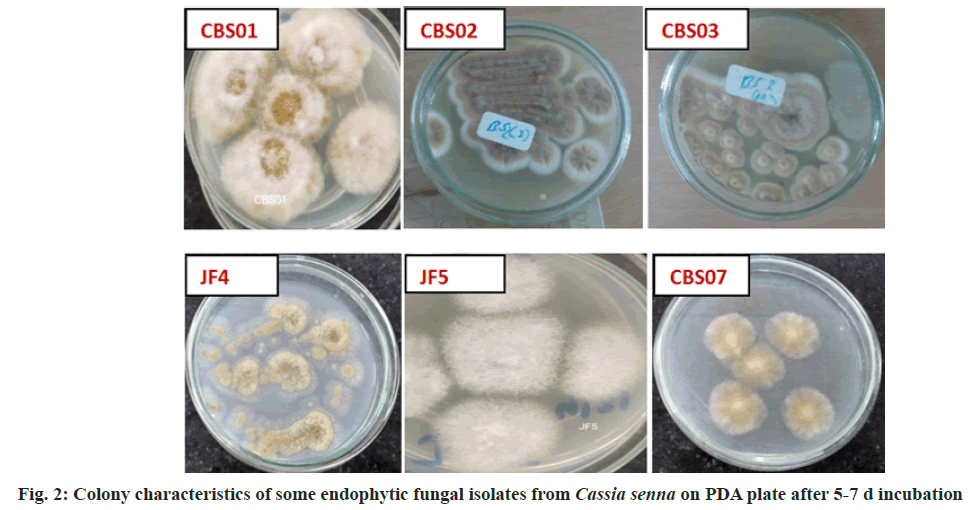
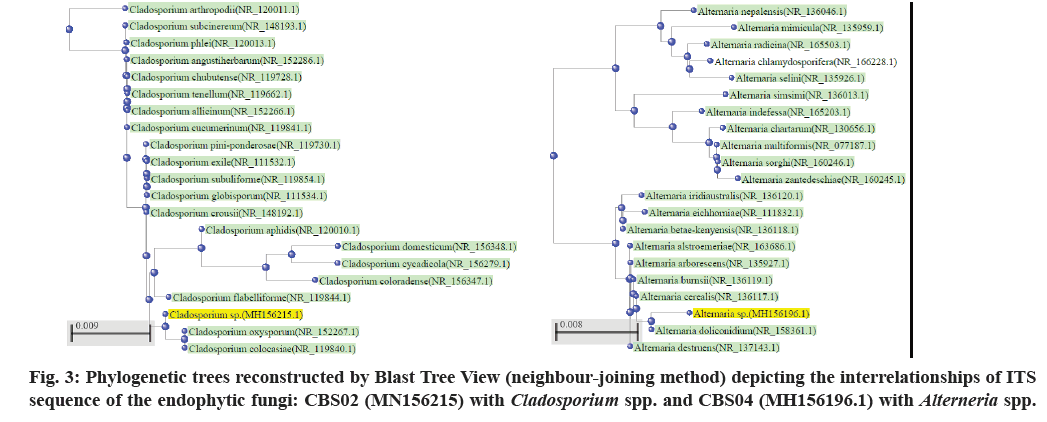
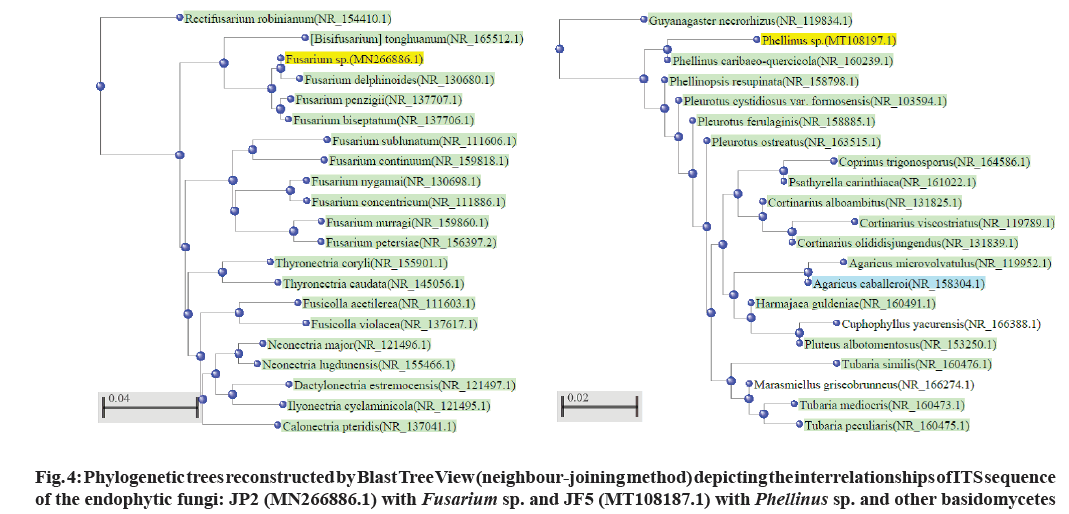
The active constituents of Senna are dianthrone glycosides (Sennosides A, B, C, D, etc.), free anthraquinones (aloe-emodin, chrysophanol, rhein) and anthraquinones glycosides. Other chemical constituents are rhein, emodin, aloe- emodin, naphthalene glycosides, flavonoids, beta-sitosterol, saponins, etc.[4]. The plant mainly flourishes in the arid or semi-arid region[5]. The Cassia plant has become scarce due to urbanization, so an alternative source for sennosides has to be explored. Therefore, an attempt has been made to isolate and identify endophytic fungi from Cassia senna L. plant samples (cultivated as well as wild) collected from Gujarat and Rajasthan; and screen for sennosides complex. The isolated strains could be potential candidates for developing sustainable, cost-effective and eco-friendly alternative for bioprospecting of bioactive compounds (Sennosides complex, antimicrobials, anticancer drugs, etc.). Plants (Cassia senna L) were collected from 6 locations/sites at Ahmedabad, Anand (Boriavi), Bavla, Bhuj (Madhapur and Saiyadpur), Dantiwada in Gujarat and Jodhpur in Rajasthan. The various parts of the plants (flowers, leaves, stem and seeds) were collected in plastic bags and stored in the refrigerator. They were processed for isolation of endophytic fungi within 24-48 h after collection. The plant material was surface sterilized by the modified method of Schulz et al.[6]. The plant material was cut into an appropriate size, rinsed under running tap water for 10 min, shaken for a few seconds in 70 % alcohol and rewashed with sterile distilled water. Next step involved the treatment with HgCl2 (0.1- 1 % w/v)+Teepol 2 drops/100 ml (sterile distilled water) for 3-5 min with shaking and then rinsed in sterile distilled water. Further treatment was done with Commercial bleach (sodium hypochlorite) 7-15 %+Teepol 2 drops/100 ml (sterile distilled water) for 10-30 min with shaking and the material was rinsed several times with sterile distilled water. The treated plant material was inoculated onto Potato Dextrose Agar ((PDA), Hi-media) plates with streptomycin, and incubated in plant growth chamber at 25±3° for isolation of endophytic fungi. Pure fungal cultures were obtained by repeated transfers on PDA plates and preserved on PDA slants at 2° to 8° at refrigeration temperature. The controls included: a) 2 PDA plates exposed at the time of inoculation in the laminar air flow and b) inoculating the sterilized water used in the last rinse (one drop) onto one 1 PDA plate. All the 3 controls PDA plates were incubated to check contamination[7]. Cultural characteristics were noted after 4-7 d of growth on PDA agar plates. The growth was mounted on a slide in lactophenol and microscopic study was carried out using Coslab phase-contrast microscope with photomicrographic attachment. The sequencing of Internal Transcribed Spacer (ITS) region of the fungal cultures was done using the Universal primers (ITS1 TCCGTAGGTGAACCTGCGG and ITS4 TCCTCCGCTTATTGATATGC) at Chromus Biotech or Eurofins Labs. Pvt. Ltd. The ITS sequences were deposited with GenBank, BLASTN was used for similarity search and phylogenetic tree were constructed by megablast (Blast Tree View) by Neighbour-Joining method. The fungal isolates were identified based on the colony, morphological characteristics and phylogenetic analysis. In the present paper we have investigated the diverse endophytic fungal isolates from the medicinal plant Cassia senna L. an established source of the drug sennosides which is described in various indigenous Indian medicinal systems. Senna leaves are widely used in treating constipation, loss of appetite, dysentery, splenomegaly, hepatomegaly, jaundice, malaria, wound healing, and has purgative, laxative, expectorant, and carminative properties[8,9]. We have reported earlier the isolation of endophytic fungi from Mucuna pruriens[7]. The characteristics of the Cassia senna L plant are shown in fig. 1. 22 endophytic fungal isolates were obtained and identified based on the colony, morphological characteristics as well as phylogenetic analysis (fig. 2 and Table 1). Phylogenetic analysis based on ITS revealed the presence of endophytic fungi belonging to Ascomycota (Aspergillus, Alterneria, Cladosporium, and Fusarium) and one isolate JF5 belonging to Basidiomycota (Phellinus sp.). The phylogenetic tree constructed with Blast Tree view (Neighbour-joining method) with 20 related species for some of the isolates is depicted in fig. 3 and fig. 4. Two of the isolates Cladosporium sp. (CBS02) and Alterneria sp. (CBL01) have been deposited with NCMR (NCCS) with the accession nos. MCC 9038 and MCC 9037, respectively. These isolates may have capacity to produce the drug (sennosides) and provide an economical/ sustainable alternative source for the drug. A review on the endophytic fungi as a potential source of antifungal compounds has been recently been published[10]. It has been demonstrated that the alkaloid compounds extracted from Cassia senna L. leaves has more effective antibacterial activity as compared to phenolic and terpenoid compounds[11]. It has been shown that the methanolic extracts from Cassia senna inhibited the growth of dermatophytes Thrichophyton rubrum and Trichophyton mentagaphytes[12]. Studies on 40 endophytic fungi isolated from 10 species of medicinal plants from Palolo, Central Sulawesi has shown antibacterial and antioxidant activity these findings support the view of the pharmaceutical applications of these organisms[13].
| S No | Isolates | Morphological identification based on Microscopic study | Identification based on ITS Sequence |
GenBank Accession Number |
Location / Place |
|---|---|---|---|---|---|
| 1 | CBS01 | Fusarium sp. | Fusarium sp. | MN128229 | Bhuj 23.2420° N, 69.6669° E |
| 2 | CBS02 | Cladosporium sp. | Cladosporium sp | MH156215 | |
| 3 | CBS03 | Cladosporium sp. | Cladosporium sp | MN156205 | |
| 4 | CBS04 | Alterneria sp. | Alterneria sp. | MH156196 | |
| 5 | CBS07 | Fusarium sp. | Fusarium sp | MN973665 | |
| 6 | CBL01 | Alterneria sp. | Alterneria brassicicola/ tenuissmia | MH156192 | |
| 7 | BL1 | Aspergillus sp | Aspergillus neoellipticus | MN266885 | Boriavi (Anand) |
| 8 | BP3 | Aspergillus sp | Aspergillus terreus | MN133937 | 22°35'54.9"N 72°56'00.4"E |
| 9 | JF4 | Aspergillus sp. | Aspergillus sp | MN128226 | Jodhpur 26°15'49.8"N 72°58'34.7"E |
| 10 | JP2 | Fusarium sp. | Fusarium sp | MN266886 | |
| 11 | JF5 | Fusarium sp. | Phellinus caribaeo- quercicola | MT108197 | |
| 12 | JP3 | Aspergillus sp. | Aspergillus caespitosus | MT107145 | |
| 13 | CBS05 | Cladosporium sp. | Nd | NA | Bhuj 23.2420° N, 69.6669° E |
| 14 | CBS06 | Alterneria sp. | Nd | NA | |
| 15 | CBS08 | Neurospora sp | Nd | NA | |
| 16 | BL2 | Aspergillus sp. | Nd | NA | Boriavi (Anand) |
| 17 | BP1 | Helminthosporium sp. | Nd | NA | 22°35'54.9"N 72°56'00.4"E |
| 18 | BP2 | Fusarium sp. | Nd | NA | |
| 19 | BS01 | Penicillium sp. | Nd | NA | |
| 20 | BS02 | Aspergillus sp. | Nd | NA | |
| 21 | JF1 | Fusarium sp | Nd | NA | Jodhpur 26°15'49.8"N 72°58'34.7"E |
| 22 | JP1 | Penicillium sp. | Nd | NA | |
| 23 | MGS001 | Aspergillus sp. | Aspergillus sp. | NA | Ahmedabad 23.0225° N, 72.5714° E |
Note: Nd: Not done; NI: Not Identified; NA: Not Applicable
Table 1: List of Endophytic Fungi Isolated From Cassia senna L. Plant Samples
25 endophytic fungi have been isolated and identified based on macroscopic and microscopic studies from leaves of 10 medicinal plants from two locations in Surat, India[14]. A more extensive study on the diversity of endophytic fungi from 7 medicinal plants of Western Ghats (Maharashtra, India) has been reported by Nalini et al.[15]. It was interesting to note that out of the 1525 isolated ascomycetes represented only 3 %, whereas majority of the isolates belonged to coelomycetes (65 %). However, this study did not include medicinal plants belonging to the genus Cassia. An endophytic fungus Fusarium tricinctum was isolated from the roots of Lithospermum officinale L, which produced shikonin (a naphthoquinone with bioactive properties)[16]. Thus, the present paper reports the isolation and identification of endophytic fungi from different parts of the medicinal plant Cassia senna L. These isolates may be a useful source for antifungal, antibacterial, anti-cancer, sennosides and other compounds of biotechnological importance. To our knowledge this is the first study on endophytic fungi from this medicinal plant from Gujarat and Rajasthan.
Exploration of endophytic fungi from Cassia senna L has benefits both in pharmaceutical and ecological fields. In order to assess the endophytic fungal diversity of this medicinal plant, samples were collected from different regions having diverse eco-geographical conditions in Gujarat and Rajasthan. The 22 fungal isolates obtained belonged to the genera Alterneria, Aspergillus, Cladopsorium, Fusarium, Penicillium, Phellinus, etc. Further studies on the bioprospecting of these isolates would provide new bioactive metabolites including sennosides for biotech industry.
Acknowledgements:
The authors gratefully acknowledge the funding from Department of Biotechnology (New Delhi, India) DBT Ref. No. BT/PR9505/NDB/39/374/2013 for the projecton “Bioprospecting of Endophytic Fungi for Sennosides and L-DOPA”. We also thank the authorities and horticulturists who have helped in collection of samples.
Conflict of interests:
The authors declared no conflict of interest.
References
- Venieraki A, Dimou M, Katinakis P. Endophytic fungi residing in medicinal plants have the ability to produce the same or similar pharmacologically active secondary metabolites as their hosts. Hellenic Plant Protect J 2017;10(2):51-66.
- Stierle A, Strobel G, Stierle D. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science. 1993 Apr 9;260(5105):214-6.
[Crossref] [Google Scholar] [PubMed]
- Zhao J, Shan T, Mou Y, Zhou L. Plant-derived bioactive compounds produced by endophytic fungi. Mini Rev Med Chem 2011;11(2):159-68.
[Crossref] [Google Scholar] [PubMed]
- Jalwal P, Middha A. Recent advances on senna as a laxative: A comprehensive review. J Pharmacogn Phytochem 2017;6(2):349-53.
- Tewari VP. Some important fruit trees and shrubs of hot arid regions of Rajasthan state in India, their uses and nutritive values. J Plant Chem Ecophysiol 2016;1(1):1004.
- Schulz B, Wanke U, Draeger S, Aust HJ. Endophytes from herbaceous plants and shrubs: Effectiveness of surface sterilization methods. Mycol Res 1993;97(12):1447-50.
- Vyas DN, Sharma S, Srivastava A, Shrivastava N, Upasani VN. Diversity of endophytic fungi from the medicinal plant Mucuna pruriens. Int J Basic Appl Res 2019;9(5):1119-28.
- Rama Reddy NR, Mehta RH, Soni PH, Makasana J, Gajbhiye NA, Ponnuchamy M, et al. Next generation sequencing and transcriptome analysis predicts biosynthetic pathway of sennosides from Senna (Cassia angustifolia Vahl.), a non-model plant with potent laxative properties. PloS one. 2015;10(6):e0129422.
[Crossref] [Google Scholar] [PubMed]
- Tripathi YC. Cassia angustifolia, a versatile medicinal crop. Int Tree Crops J 1999;10(2):121-9.
- Deshmukh SK, Gupta MK, Prakash V, Saxena S. Endophytic fungi: A source of potential antifungal compounds. J Fungi 2018;4(3):77.
[Crossref] [Google Scholar] [PubMed]
- Hussein H, Al-Khafaji NM, Al-Mamoori AH, Al-Marzoqi AH. Evaluation of in vitro antibacterial properties of the crude phenolic, alkaloid and terpenoid extracts of Cassia senna L. against human gram-negative pathogenic bacteria. Plant Arch 2018;18(1):354-6.
- Adamu HM, Abayeh OJ, Ibok NN, Kafu SE. Antifungal activity of extracts of some Cassia, Detarium, and Ziziphus species against dermatophytes. Nat Prod Rad 2006;5(5):357-360.
- Praptiwi RM, Wulansari D, Fathoni A, Agusta A. Antibacterial and antioxidant activities of endophytic fungi extracts from medicinal plant from Central Sulawesi. J App Pharm Sci 2018;8(8):69-74.
- Jariwala B, Desai B. Isolation and identification of endophytic fungi from various medicinal plants. Bmr Microbiol 2018;4(1):1-7.
- Nalini MS, Sunayana N, Prakash HS. Endophytic fungal diversity in medicinal plants of Western Ghats, India. Int J Biodivers 2014;2014:1-9.
- Mollaei S, Khanehbarndaz O, Gerami-Khashal Z, Ebadi M. Molecular identification and phytochemical screening of endophytic fungi isolated from Lithospermum officinale L. roots: A new source of shikonin. Phytochemistry 2019;168:112116.