- *Corresponding Author:
- Xia Jin
Department of Anesthesiology, Huanggang Central Hospital, Huanggang, Hubei Province 438000, China
E-mail: cy13409739622@163.com
| Date of Received | 18 February 2023 |
| Date of Revision | 23 September 2023 |
| Date of Acceptance | 09 May 2024 |
| Indian J Pharm Sci 2024;86(3):925-931 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To explore the effect and possible mechanism of iridoid glucosides from Boschniakia rossica on ovarian cancer cell proliferation and metastasis. Human ovarian cancer cells (OVCAR3) were treated with different concentrations of iridoid glucosides from Boschniakia rossica. Also, human ovarian cancer cells were transfected with plasmid cloning deoxyribonucleic acid, plasmid cloning deoxyribonucleic acid circular-homeodomain-interacting protein kinase 3, microRNA-NC+plasmid cloning deoxyribonucleic acid-circular-homeodomain-interacting protein kinase 3 and microRNA-495-3p mimics+plasmid cloning deoxyribonucleic acid-circular-homeodomain-interacting protein kinase 3, followed by treated with high-dose of iridoid glucosides from Boschniakia rossica. Cell functions were tested by colony formation, transwell and 3-(4, 5-dimethylthiazol-2-yl)-2, 5 diphenyl tetrazolium bromide assays. Gene levels were examined using quantitative reverse transcription polymerase chain reaction and Western blot method. Ribonuclic acid interaction was verified using dual-luciferase reporter assay. Iridoid glucosides from Boschniakia rossica could reduce OVCAR3 cell proliferation and metastasis. Moreover, iridoid glucosides from Boschniakia rossica decreased circular-homeodomain-interacting protein kinase 3 expression and increased microRNA-495-3p expression. Plasmid cloning deoxyribonucleic acid-circular-homeodomain-interacting protein kinase 3 reversed iridoid glucosides from Boschniakia rossica-mediated the inhibition on OVCAR3 cell functions. Circular-homeodomain-interacting protein kinase 3 targeted microRNA-495-3p. Also, microRNA-495-3p mimics abolished the effects of plasmid cloning deoxyribonucleic acid-circular-homeodomain-interacting protein kinase 3 on iridoid glucosides from Boschniakia rossica-mediated cell functions. Iridoid glucosides from Boschniakia rossica inhibited ovarian cancer cell proliferation and metastasis by circular-homeodomain-interacting protein kinase 3/microRNA-495-3p.
Keywords
Iridoid glucosides from Boschniakia rossica, circular-homeodomain-interacting protein kinase 3, microRNA-495-3p, ovarian cancer
Ovarian Cancer (OC) incidence and mortality are increasing year by year, which has seriously threatened human life[1,2]. Generally, patients with advanced OC have a high recurrence rate after postoperative treatment and chemotherapy, and the 5 y survival rate is not satisfactory[3,4]. Targeted therapy has become an ideal choice for OC treatment[5,6], so the search for potential therapeutic targets has become the research focus of inhibiting OC malignant progression.
Traditional Chinese Medicine (TCM) plays a vital function in OC treatment, which can mediate tumorigenesis by regulating gene expression and signaling pathway[7,8]. Boschniakia rossica (B. rossica) is a TCM with antioxidant, antiinflammatory and anti-cancer effects[9,10]. Iridoid Glucosides from B. rossica (IGBR), the main active ingredients of B. rossica, has been proven to have protective effects against acute liver injury in rats[11]. Studies had shown that IGBR played an antilung cancer effect by promoting cell apoptosis[12]. However, IGBR roles in OC progression remains unclear.
Circular Ribonuclic Acid (circRNA) is rich in the adsorption sites of microRNA (miRNA) to play a sponge function, and then regulates OC cell biological behaviors[13,14]. Circular-Homeodomain- Interacting Protein Kinase 3 (Circ-HIPK3) has been found to be upregulated in OC tissues and serves as oncogene in OC[15,16]. MiR-495-3p is considered to be a tumor suppresser in OC[17]. In this study, star base software predicts that circ-HIPK3 can bind with miR-495-3p, but whether circ-HIPK3 regulates OC progression by targeting miR-495- 3p has not been studied. Some studies have shown that the extracts of TCM can regulate circRNArelated axis to regulate cancer progression[18]. Here, we found that IGBR can suppress circ-HIPK3 expression and promote miR-495-3p expression, but whether IGBR mediates OC progression by circ-HIPK3/miR-495-3p pathway has not been investigated.
This study focuses on IGBR roles and mechanisms in OC progression. Based on the above, we hypothesizes that IGBR may affect the biological behavior of OC cells via circ-HIPK3/miR-495-3p axis.
Materials and Methods
Extraction of IGBR:
According to previous studies[11], B. rossica (Qihongtang Pharmaceutical, Bozhou, China) was extracted with 80 % methanol followed by dichloromethane (CH2Cl2) and water (H2O) extraction. The aqueous layer was eluted on Mitsubishi Chemical Corporation (MCI)-gel CHP20P using a gradient methanol solution (10, 30, 50, 70 and 100 %). The eluting components of 50 % methanol were collected, and IGBR (49 % content) was obtained by high performance liquid chromatography. IGBR was diluted with culture medium to 200, 400, and 800 mg/ml.
Cell culture and grouping:
Human OC cells (OVCAR3, Procell, Wuhan, China) were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium containing 20 % Fetal Bovine Serum (FBS), 10 μg/ml insulin and 1 % penicillin-streptomycin. Cells were treated with different concentration of IGBR (200, 400, and 800 mg/ml) for 24 h and recorded as low-dose group, middle-dose group and high-dose group. Non-treated cells were used as control group. Cells were transfected with plasmid cloning Deoxyribonucleic Acid (pcDNA), pcDNA-circ- HIPK3, miR-NC+pcDNA-circ-HIPK3, and miR- 495-3p+pcDNA-circ-HIPK3 using Lipofectamine 3000 (Invitrogen, Carlsbad, CA, United States of America), followed by treated with 800 mg/ ml IGBR for 24 h, and recorded as pcDNA+highdose group, pcDNA-circ-HIPK3+high-dose group, miR-NC+pcDNA-circ-HIPK3+high-dose group, and miR-495-3p+pcDNA-circ-HIPK3+high-dose group.
4, 5-Dimethylthiazol-2-yl)-2, 5 Diphenyl Tetrazolium Bromide (MTT) assay:
OVCAR3 cells in 96 well plates (3×103 cells/well) were treated with MTT solution and formazan solving liquid (Solarbio, Beijing, China). Optical Density (OD) value was detected by a microplate reader at 490 nm.
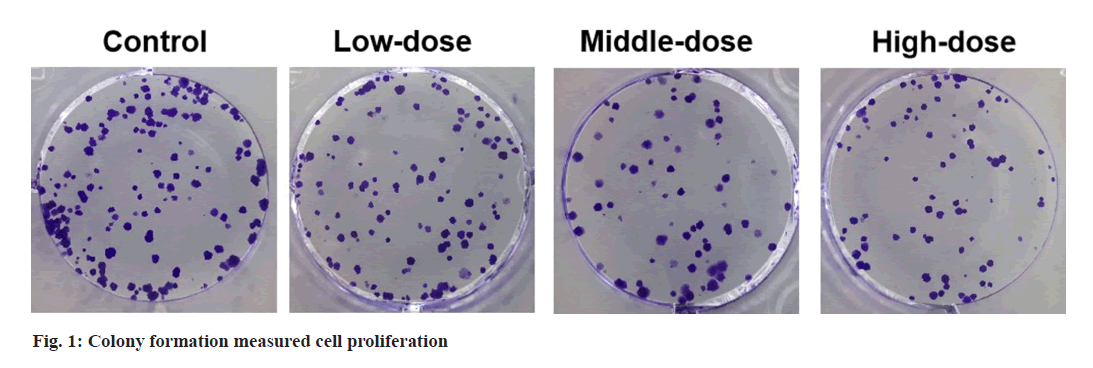
Colony formation assay:
OVCAR3 cells were cultured for 14 d in 6-well plates. The colonies were fixed and stained, followed by counted under a microscope.
Transwell:
OVCAR3 cells were seeded into transwell upper chamber pre-coated with or without Matrigel. After 24 h, migrated and invaded cells were photographed and counted under a microscope.
Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR):
Total RNAs were extracted and synthesized into complementary DNA (cDNA). PCR amplification was performed using SYBR Green (Takara, Tokyo, Japan). Relative circ-HIPK3 and miR-495-3p levels were analyzed by 2−ΔΔCt method.
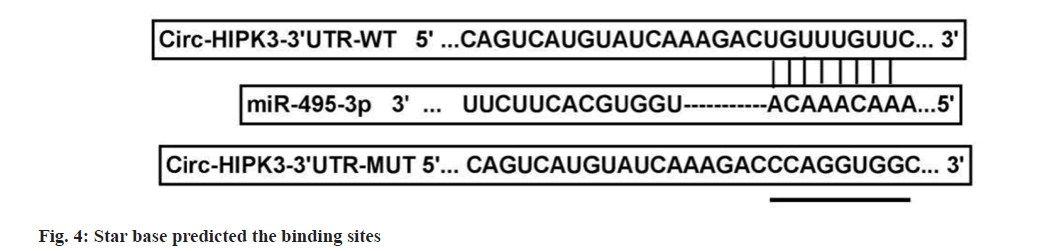
Dual-luciferase reporter assay:
According to the prediction results of star base software, the binding sites and mutant sites of circ-HIPK3 were cloned into pmirGLO vectors. OVCAR3 cells were transfected with vectors and miR-NC/miR-495-3p, and luciferase activity was detected to assess interaction.
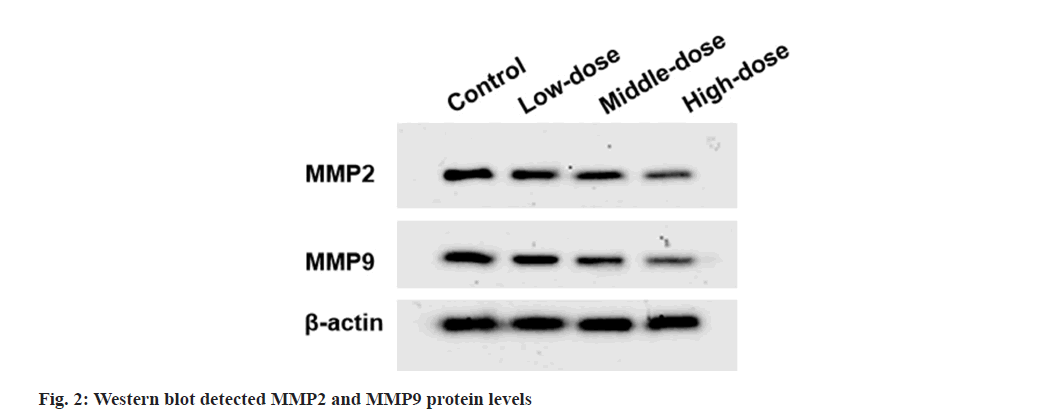
Western blot:
Total proteins were extracted, separated and transferred onto Polyvinylidene Difluoride (PVDF) membranes. After incubated with anti-Matrix Metalloproteinase (MMP)-2 (1:1000, ab97779), anti-MMP-9 (1:1000, ab38898) and anti-β-actin (1:1000, ab8227), membranes were treated with secondary antibody (1:50000, ab205718). Signals were detected using Enhanced Chemiluminescence (ECL) reagents (Beyotime).
Statistical analysis:
Data were analyzed by Statistical Package for the Social Sciences (SPSS) 21.0 software and expressed as x? ±s. Student’s t-test and Analysis of Variance (ANOVA) were used for comparisons. p<0.05 was considered significant difference.
Results and Discussion
Compared with control group, cell viability and colony numbers in low-dose, middle-dose and high-dose IGBR groups were decreased (fig. 1 and Table 1). MMP2 and MMP9 levels, as well as the migrated and invaded cells, were markedly reduced in the different concentrations of IGBR treatment groups (fig. 2 and Table 2). Circ- HIPK3 was downregulated and miR-495-3p was upregulated in IGBR treatment groups in a dosedepended manner (Table 3).
| Groups | OD 490 nm | Colony numbers |
|---|---|---|
| Control | 1.025±0.11 | 101±6.27 |
| Low-dose | 0.843±0.06* | 76±3.10* |
| Middle-dose | 0.643±0.05* | 54±3.19* |
| High-dose | 0.513±0.04* | 41±2.02* |
| F | 91.968 | 394.686 |
| p | 0.000 | 0.000 |
Table 1: Effects of IGBR on OVCAR3 Cell Proliferation (X?±S, N=9)
| Groups | MMP2 | MMP9 | Migrated cells | Invaded cells |
|---|---|---|---|---|
| NC | 0.83±0.07 | 0.75±0.07 | 186±11.25 | 137±8.63 |
| Low-dose | 0.71±0.06* | 0.62±0.05* | 161±10.03* | 104±7.16* |
| Middle-dose | 0.51±0.04* | 0.45±0.04* | 121±8.12* | 72±3.69* |
| High-dose | 0.40±0.03* | 0.34±0.03* | 85±4.08* | 61±2.24* |
| F | 122.700 | 119.515 | 229.767 | 292.652 |
| p | 0.000 | 0.000 | 0.000 | 0.000 |
Table 2: Effects of IGBR on OVCAR3 Cell Metastasis (X?±S, N=9)
| Groups | Circ-HIPK3 | MiR-495-3p |
|---|---|---|
| Control | 1.00±0.09 | 1.00±0.10 |
| Low-dose | 0.84±0.07* | 1.62±0.12* |
| Middle-dose | 0.63±0.03* | 2.11±0.16* |
| High-dose | 0.42±0.03* | 2.38±0.17* |
| F | 154.764 | 167.738 |
| p | 0.000 | 0.000 |
Table 3: Effect of IGBR on circ-HIPK3 and MiR-495-3p expression (X?±S, N=9)
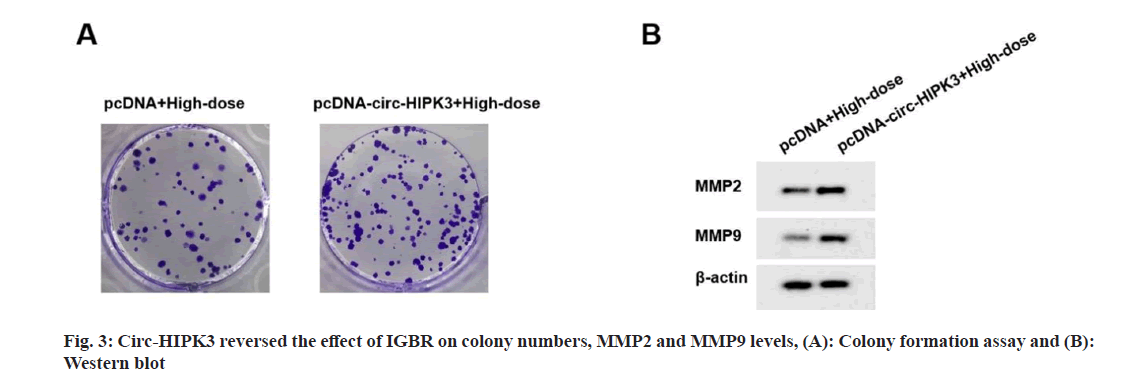
Compared to pcDNA+high-dose IGBR group, cell viability, colony numbers, migrated cells, invaded cells, MMP2 and MMP9 levels were increased in the pcDNA-circ-HIPK3+high-dose IGBR group (fig. 3 and Table 4). Starbase software there had bind sites between circ-HIPK3 and miR-495-3p (fig. 4). MiR-495-3p overexpression inhibited the luciferase activity of Wild-Type (WT)-circ-HIPK3 vector, rather than that of the Mutant (MUT)-circ- HIPK3 vector (Table 5). Besides, miR-495-3p expression could be decreased by pcDNA-circ- HIPK3 (Table 6).
| Groups | Circ-HIPK3 | MMP2 | MMP9 | OD490nm | Colony numbers | Migrated cells | Invaded cells |
|---|---|---|---|---|---|---|---|
| pcDNA+high-dose | 1.00±0.11 | 0.41±0.03 | 0.32±0.02 | 0.510±0.04 | 43±3.11 | 81±3.56 | 63±2.24 |
| pcDNA-circ-HIPK3+high-dose | 2.14±0.14* | 0.88±0.07* | 0.78±0.06* | 0.963±0.08* | 115±8.10* | 201±12.35* | 154±8.12* |
| t | 19.209 | 18.514 | 21.820 | 15.194 | 24.895 | 28.009 | 32.41 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Table 4: Circ-HIPK3 Reversed IGBR-mediated OVCAR3 cell proliferation and metastasis (X?±S, N=9)
| Groups | Luciferase activity | |
|---|---|---|
| WT-circ-HIPK3 | MUT-circ-HIPK3 | |
| miR-NC | 1.00±0.08 | 1.00±0.10 |
| miR-495-3p | 0.43±0.02* | 0.97±0.08 |
| t | 20.737 | 0.703 |
| p | 0.000 | 0.492 |
Table 5: Assessing RNA interaction (X?±S, N=9)
| Groups | Circ-HIPK3 | MiR-495-3p |
|---|---|---|
| pcDNA | 1.00±0.10 | 1.00±0.08 |
| pcDNA-circ-HIPK3 | 2.23±0.16* | 0.39±0.04* |
| t | 19.56 | 20.46 |
| p | 0.000 | 0.000 |
Table 6: qRT-PCR detected MiR-495-3p expression (X?±S, N=9)
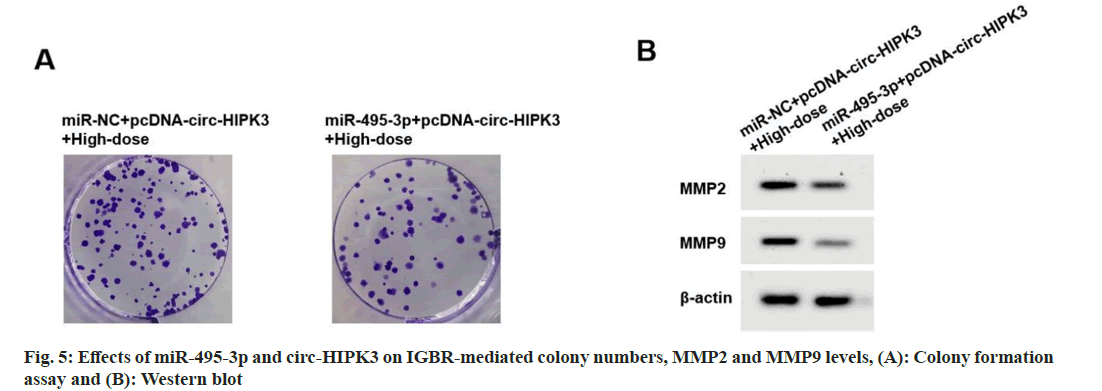
Compared to miR-NC+pcDNA-circ-HIPK3+highdose IGBR group, cell viability, colony numbers, migrated cells, invaded cells, MMP2 and MMP9 levels were reduced in the miR-495-3p+pcDNAcirc- HIPK3+high-dose IGBR group (fig. 5 and Table 7).
| Groups | MiR-495-3p | MMP2 | MMP9 | OD490nm | Colony Numbers | Migrated cells | Invaded cells |
|---|---|---|---|---|---|---|---|
| miR-NC+pcDNA-circ-HIPK3+high-dose | 1.00±0.10 | 0.86±0.06* | 0.77±0.07* | 0.969±0.07* | 112±6.03* | 207±11.19* | 159±5.10* |
| miR-495-3p+pcDNA-circ-HIPK3+high-dose | 1.91±0.13* | 0.39±0.03* | 0.32±0.02* | 0.541±0.03* | 57±2.86* | 91±6.94* | 68±4.91* |
| t | 16.645 | 21.019 | 18.544 | 16.860 | 24.723 | 26.429 | 38.563 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Table 7: Effects of miR-495-3p and Circ-HIPK3 on IGBR-treated OVCAR3 cell proliferation and metastasis (X?±S, N=9)
As an extract of TCM, the anti-cancer effect of IGBR has been confirmed in hepatocellular carcinoma[19] and lung carcinoma cells[12]. However, whether IGBR suppresses OC progression has not been reported. Our study showed that OVCAR3 cell viability and colony numbers were decreased after IGBR treatment, suggesting that IGBR could inhibit OC cell proliferation. In addition, IGBR treatment reduced migrated cells, invaded cells, MMP2, and MMP9 levels, revealing that IGBR restrained OC cell metastasis. These results provide new evidence for the use of IGBR in OC treatment.
Many TCM extracts have been found to mediate cancer progression by regulating the circRNA expression. For example, sinomenine decreased circTRPM7 expression to inhibit cell growth and metastasis in gastric cancer[20]. Matrine restrained proliferation in liver cancer cells via reducing circROBO1 expression[21]. Berberine suppressed endometrial carcinoma cell metastasis through downregulating circ_ZNF608[22]. In this study, we found that IGBR repressed circ-HIPK3 expression. Previous study shows that circ-HIPK3 plays oncogenic role in many cancers, such as breast cancer[23] and hepatocellular carcinoma[24]. More important, the tumor promoter function of circ-HIPK3 in OC has been confirmed in many researches[15,16]. Consistent with these studies, we also confirmed the oncogenic role of circ-HIPK3 in OC, as evidenced by the enhanced proliferation and metastasis of pcDNA-circ-HIPK3 in high-dose IGBR-treated OC cells. This suggested that IGBR indeed suppressed OC progression by repressing proto-oncogene circ-HIPK3 expression.
CircRNA can act as miRNA sponge molecule to regulate cell biological behavior[25]. Through prediction and validation, we suggested that circ-HIPK3 targeted miR-495-3p. A recent study indicated that miR-495-3p hindered papillary thyroid carcinoma cell metastasis[26]. Also, miR-495-3p suppressed colorectal cancer cell proliferation[27]. In OC-related study, low miR- 493-5p level is thought to be associated with the malignant progression of OC[17]. Therefore, miR- 493-5p may play anti-tumor role in OC. Here, IGBR enhanced miR-493-5p expression, and circHIPK3 sponged miR-493-5p to reduce its expression. Further analysis revealed that miR- 493-5p eliminated the promotion effect of circ- HIPK3 on IGBR-treated cell proliferation and metastasis. All results suggest that IGBR may inhibit OC cell progression by circ-HIPK3/miR- 495-3p pathway.
In conclusion, these data showed that IGBR restrained OC cell proliferation and metastasis via circ-HIPK3/miR-495-3p pathway. These findings provide a new direction for developing OC treatment drugs.
Conflict of interests:
The authors declared no conflict of interests.
References
- Roett MA, Evans P. Ovarian cancer: An overview. Am Family Phys 2009;80(6):609-16.
[Google Scholar] [PubMed]
- Webb PM, Jordan SJ. Epidemiology of epithelial ovarian cancer. Best Pract Res Clin Obstetr Gynaecol 2017;41:3-14.
[Crossref] [Google Scholar] [PubMed]
- Moufarrij S, Dandapani M, Arthofer E, Gomez S, Srivastava A, Lopez-Acevedo M, et al. Epigenetic therapy for ovarian cancer: Promise and progress. Clin Epigenetics 2019;11(1):7.
[Crossref] [Google Scholar] [PubMed]
- Nag S, Aggarwal S, Rauthan A, Warrier N. Maintenance therapy for newly diagnosed epithelial ovarian cancer–a review. J Ovarian Res 2022;15(1):88.
[Crossref] [Google Scholar] [PubMed]
- Guan L, Lu Y. New developments in molecular targeted therapy of ovarian cancer. Discov Med 2018;26(144):219-29.
[Google Scholar] [PubMed]
- Lim HJ, Ledger W. Targeted therapy in ovarian cancer. Women’s Health 2016;12(3):363-78.
[Crossref] [Google Scholar] [PubMed]
- Wang Y, Xie L, Liu F, Ding D, Wei W, Han F. Research progress on traditional Chinese medicine-induced apoptosis signaling pathways in ovarian cancer cells. J Ethnopharmacol 2023;319(1):117299.
[Crossref] [Google Scholar] [PubMed]
- Liu Y, Liu X, Wang H, Ding P, Wang C. Agrimonolide inhibits cancer progression and induces ferroptosis and apoptosis by targeting SCD1 in ovarian cancer cells. Phytomedicine 2022;101:154102.
[Crossref] [Google Scholar] [PubMed]
- Yin ZZ, Jin HL, Yin XZ, Li TZ, Quan JS, Jin ZN. Effect of Boschniakia rossica on expression of GST-P, p53 and p21ras proteins in early stage of chemical hepatocarcinogenesis and its anti-inflammatory activities in rats. World J Gastroenterol 2000;6(6):812-8.
[Crossref] [Google Scholar] [PubMed]
- Yao C, Cao X, Fu Z, Tian J, Dong W, Xu J, et al. Boschniakia rossica polysaccharide triggers laryngeal carcinoma cell apoptosis by regulating expression of Bcl-2, Caspase-3, and P53. Med Sci Monit 2017;23:2059-64.
[Crossref] [Google Scholar] [PubMed]
- Quan J, Piao L, Xu H, Li T, Yin X. Protective effect of iridoid glucosides from Boschniakia rossica on acute liver injury induced by carbon tetrachloride in rats. Biosci Biotechnol Biochem 2009;73(4):849-54.
[Crossref] [Google Scholar] [PubMed]
- Yin X, Xu H, Jin A. Effect of iridoid glucosides from Boschniakia rossica on proliferation and apoptosis of lung carcinoma cell. Chin J Public Health 2010;26(7):805-6.
- Wu M, Qiu Q, Zhou Q, Li J, Yang J, Zheng C, et al. CircFBXO7/miR-96-5p/MTSS1 axis is an important regulator in the Wnt signaling pathway in ovarian cancer. Mol Cancer 2022;21(1):137.
[Crossref] [Google Scholar] [PubMed]
- Ma H, Qu S, Zhai Y, Yang X. Circ_0025033 promotes ovarian cancer development via regulating the hsa_miR-370-3p/SLC1A5 axis. Cell Mol Biol Lett 2022;27(1):94.
[Crossref] [Google Scholar] [PubMed]
- Zhou H, Li J, Lai X, Wang K, Zhou W, Wang J. CircHIPK3 modulates VEGF through MiR-7 to affect ovarian cancer cell proliferation and apoptosis. J Buon 2021;26(3):691-7.
[Google Scholar] [PubMed]
- Liu N, Zhang J, Zhang LY, Wang L. CircHIPK3 is upregulated and predicts a poor prognosis in epithelial ovarian cancer. Eur Rev Med Pharmacol Sci 2018;22(12):3713-8.
[Crossref] [Google Scholar] [PubMed]
- Liu S, Xi X. LINC01133 contribute to epithelial ovarian cancer metastasis by regulating miR-495-3p/TPD52 axis. Biochem Biophys Res Commun 2020;533(4):1088-94.
[Crossref] [Google Scholar] [PubMed]
- Du L, Zhang L, Sun F. Puerarin inhibits the progression of bladder cancer by regulating circ_0020394/miR-328-3p/NRBP1 axis. Cancer Biother Radiopharm 2022;37(6):435-50.
[Crossref] [Google Scholar] [PubMed]
- Zhu J. Inhibitory effect of iridoid glucosides from Boschniakia rossica on SK-Hep1 cell EMT and its mechanism. Guangdong Med J 2019;40(22):3103-7.
- Yan J, Yang J, Shen H, Gao R, Lv S. Sinomenine regulates circTRPM7?related pathway to inhibit gastric cancer cell growth and metastasis. Chem Biol Drug Design 2023;102(4):870-81.
[Crossref] [Google Scholar] [PubMed]
- Huang Z, Li H, Li Q, Chen X, Liu R, Chang X. Matrine suppresses liver cancer progression and the Warburg effect by regulating the circROBO1/miR?130a?5p/ROBO1 axis. J Biochem Mol Toxicol 2023;37(10):e23436.
[Crossref] [Google Scholar] [PubMed]
- Liang H, Liu Y, Fu L, Li L, Gong N. Berberine inhibits the development of endometrial cancer through circ_ZNF608/miR-377-3p/COX2 axis. Autoimmunity 2022;55(7):485-95.
[Crossref] [Google Scholar] [PubMed]
- Luo N, Liu S, Li X, Hu Y, Zhang K. Circular RNA circHIPK3 promotes breast cancer progression via sponging MiR-326. Cell Cycle 2021;20(13):1320-33.
[Crossref] [Google Scholar] [PubMed]
- Yu Q, Chen W, Li Y, He J, Wang Y, Yang S, et al. The novel circular RNA HIPK3 accelerates the proliferation and invasion of hepatocellular carcinoma cells by sponging the micro RNA-124 or micro RNA-506/pyruvate dehydrogenase kinase 2 axis. Bioengineered 2022;13(3):4717-29.
[Crossref] [Google Scholar] [PubMed]
- Zhang M, Bai X, Zeng X, Liu J, Liu F, Zhang Z. CircRNA-miRNA-mRNA in breast cancer. Clin Chim Acta 2021;523:120-30.
[Crossref] [Google Scholar] [PubMed]
- Alves LF, Geraldo MV. MiR-495-3p regulates cell migration and invasion in papillary thyroid carcinoma. Front Oncol 2023;13:1039654.
[Crossref] [Google Scholar] [PubMed]
- Zhang JL, Zheng HF, Li K, Zhu YP. MiR-495-3p depresses cell proliferation and migration by downregulating HMGB1 in colorectal cancer. World J Surg Oncol 2022;20(1):101.
[Crossref] [Google Scholar] [PubMed]