- *Corresponding Author:
- C. Vijaya
Department of Pharmaceutics, Centre for PG Studies, Ultra College of Pharmacy, Madurai–625 020, India
E-mail: vijak2@rediffmail.com
| Date of Submission | 24 December 2010 |
| Date of Revision | 08 November 2011 |
| Date of Acceptance | 16 November 2011 |
| Indian J Pharm Sci, 2011, 73 (6): 615-620 |
Abstract
Gelation of pectin caused by divalent cations especially calcium ions has been applied to develop an ophthalmic formulation of azithromycin in the present study. Rapid elimination of drug on instillation into cul de sac would be minimal with in situ gelling ophthalmic solution leading to increased precorneal contact time and prolonged drug delivery. In the formulation development studies pectin was used in different concentrations (1-5% w/v) and different proportions of the hydrocolloids hydroxypropyl methylcellulose and sodium carboxymethyl cellulose of different grades of viscosity were used. The primary criteria for formulation optimization were gelling capacity and rheological behaviour. In addition, formulations were evaluated for pH, and antimicrobial efficacy and drug release. The clarity, pH, gelation in simulated tear fluid and rheological properties of the optimized formulations were satisfactory. The formulations inhibited the growth of Staphylococcus aureus effectively in cup–plate method and were proved to be safe and non irritant on rabbit eyes. The results indicate that pectin based in situ gels can be successfully used to prolong the duration of action of azithromycin.
Keywords
Azithromycin, in situocular gel, ion-induced gelation, pectin
Azithromycin is a semisynthetic macrolide antibiotic used for the treatment of trachoma caused by Chlamydia trachomatis, a gram negative bacteria. According to WHO, there are about 84,000,000 people who had been affected by trachoma. It is reported that the existent oral dosage forms of azithromycin are suitable to treat the ocular infections such as conjunctivitis and others caused by sensitive pathogens [1]. But when azithromycin is taken orally to cure ocular infection, it needs at least 1.0 g azithromycin per dose to ensure the drug content in aqueous humor, tear fluid and conjunctival coat reach the minimal inhibitory concentration (MIC). Thus not only will it waste much but it can bring on side-effects because of high drug level in every tissue. Therefore, a topical ophthalmic formulation of azithromycin with a low dosing frequency would be much more convenient for patients and thus ensure better compliance, thereby reducing the risk of selection of resistant bacteria [2,3]. An US patent on topical treatment of ocular infections relates to a sustained release ophthalmic composition comprised of an aqueous suspension of the azalide antibiotic and a polymer suspending agent [4]. However ocular pharmacokinetic results indicated that only a viscous solution of azithromycin can maintain a precorneal concentration above the MIC90 over 24 h [5]. Azasite®, the viscous azithromycin ophthalmic solution, is based on Durasite® drug delivery technology evolved from cross linked polyacrylic acid [6]. But highly viscous solutions and gels lack accuracy and ease of administration. Lachrymation and hazy vision associated with preformed hydrogels may be avoided by using in situ activated gel forming systems which are viscous liquids that upon exposure to physiological conditions change into a gel or solid phase in the cul de sac upon its instillation into the eye. in situ gelation approach combines advantages of both solutions and gels, such as accuracy and ease of administration of the former and prolonged precorneal retention of the latter [7,8].
Sol–gel phase transition on eye surface can be triggered by a change in temperature, pH, or ionic strength. Carbopol 940 and Polaxamers are used as the gelling agents in pH triggered and temperature sensitive in situgelling systems respectively for sustaining the drug delivery [9,10]. Ion activated solgel transition also known as osmotically induced gelation occurs on ocular surface due to the ions in the tear fluid resulted by a change in electrolyte composition[8]. Gelrite® (gellan gum) in ophthalmic formulations, which gels upon instillation in the eye due to the presence of cation has shown prolonged precorneal contact time in humans [11]. Sodium alginate has been used as the gelling agent in sustained release ocular formulations of ofloxacin and gatifloxacin [12,13]. Pectins also gel in presence of divalent cations and in situ gelling of pectin mediated by calcium ions in lacrimal fluid has been reported in an US patent [14,15]. Also in situ gelling of pectin has been used to sustain drug delivery from formulations (e.g. acetaminophen, theophylline and cimetidine) [16]. However the in situ gelling capacity of pectin has not been exploited in ophthalmic preparation for improved drug availability. So the present work was aimed at the preparation of in situ gelling ophthalmic formulations of azithromycin using pectin as the gelling agent.
Materials and Methods
Azithromycin was gifted by Micro Labs Pvt Ltd, Hosur, India. Hydroxypropyl methylcellulose (HPMC E15 LV and HPMC K4M) were obtained from Colorcon Asia, Mumbai, India. Sodium carboxymethyl cellulose (SCMC), medium viscosity grade was purchased from Loba Chemie, Mumbai, India. Pectin, calcium chloride, and nutrient agar were procured from Hi Media Labs, Mumbai, India. Dialysis membrane for diffusion studies (LA387) was the product of Himedia, Mumbai, India. The microbial strain (Staphylococcus aureus NCIM -2079) used was supplied by NCIM, Pune, India. All other chemicals used were of analytical grade.
Preparation of in situ gelling systems:
The solubility of azithromycin was tested in phosphate buffer (pH 6.0, 6.5 and 7.2). Azithromycin was found to be completely dissolved in phosphate buffer (pH 6.0) which was selected as the vehicle for the preparation of in situ gelling systems. Pectin in concentrations of 2-5% w/v and viscosity builders HPMC K4M and SCMC of different proportions and grades were used to prepare totally 10 formulations (Table 1). Pectin was dissolved in required quantity of phosphate buffer pH 6 and stirred well continuously to form a clear solution. The viscosity builder and benzalkonium chloride were dissolved in the sol and allowed to hydrate overnight. The solutions were then stirred with an overhead stirrer. Accurately weighed quantity of azithromycin was dissolved in prepared polymeric solutions and mixed well under constant stirring until uniform solutions were obtained. The developed formulations were filled in sterile glass vials in aseptic condition and closed with butyl rubber closures and sealed with aluminium caps and sterilized by autoclaving at 121° and 15 psi for 20 min.
Tests for clarity and pH of the formulations:
The prepared formulations were evaluated for clarity by visual observation of the solutions against a black and white background in a well-lit cabinet. Also it was observed for formation of turbidity or any unwanted particles dispersed in the solution. The pH of the prepared formulations was determined using Systronics 802 digital pH meter.
Selection of formulations:
Gelling capacity and rheology are the main prerequisites of the in situ gelling systems. Therefore based on these two properties the formulations were selected and evaluated for further studies.
in situ gelling capacity:
The gelling capacity of the formulation was determined by placing the formulation in a vial containing artificial tear fluid in the proportion of 25:7. The composition of the simulated tear fluid (STF) was: NaCl 0.67 g, NaHCO3 0.2 g, CaCl2.2H2O 0.008 g and water upto 100 g [11]. The gel formation was assessed visually and the time was noted for the gelation and also noted the time taken for the gel formed to dissolve.
| Ingredient | P1 | P2 | HLP1 | HLP2 | HLP3 | HKP1 | HKP2 | HKP3 | CP1 | CP2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Pectin (g) | 2 | 3 | 3 | 4 | 5 | 2 | 3 | 3 | 2 | 3 |
| HPMC E15LV (g) | - | - | 1.5 | 1.5 | 2 | - | - | - | - | - |
| HPMC K4M (g) | - | - | - | - | - | 0.5 | 0.5 | 1.0 | - | - |
| SCMC (g) | - | - | - | - | - | - | - | - | 0.5 | 0.5 |
| Azithromycin (g) | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Benzalkonium chloride (g) | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Phosphate buffer pH 6 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| q.s. (ml) | ||||||||||
Table 1: Composition Of in situ Gelling Ophthalmic Formulations
Rheological studies
The developed formulation was poured into small sample adaptor of a Brookfield synchroelectric viscometer DV PRO 2 and the viscosity was determined using spindle no 18 at different angular velocities. The viscosity was recorded from the stabilized readings on the digital display of the viscometer.
Determination of drug content
The drug content of the selected formulations were determined by diluting suitably with 0.2 N NaOH and refluxed for 30 min and the absorbances were observed at 215 nm in UV/Vis double beam spectrophotometer (Systronics 2201).
Tests for sterility of the formulations
Ophthalmic preparations must be sterile and sterility testing is a routine quality control test in eye preparations. The test for sterility was carried out under aseptic conditions by membrane filtration method as per IP guidelines [17]. Diluent was used in order to inactivate the antibacterial effect of the active ingredient besides diluting the viscous formulations to enable the filtration.
Antimicrobial efficacy studies
Antimicrobial efficacy was determined by agar diffusion employing ‘cup plate techinique’ on selected formulations [18]. Wells were made with borer in the solidified medium previously seeded with Staphylococcus aureus. The formulations, 25 μl of each were poured into respective wells in an aseptic condition. These plates were kept in incubator for a period of 24 h and then observed for zone of inhibition.
In another plate of specified medium inoculated with Staphylococcus aureus, 7 μl of simulated tear fluid was taken along with 25 μl of the formulations in the wells and observed for the zones of inhibition at 24 h. This test was performed in order to reveal the antimicrobial efficacy of the formulations in the simulated in vivo condition.
Ocular irritation study
This study was performed according to the Draize test [19] on three female albino rabbits each weighing 2-3 kg. The animal experimentation protocol designed as per CPCSEA guidelines was approved by the IAEC, Ultra College of Pharmacy (UCP/ IAEC/2009/052). The sterile formulations were instilled twice a day for a period of 21 days and they were observed periodically for redness, swelling, watering of the eye and other changes.
in vitro drug release studies
A number of approaches have been used by different workers to conduct in vitro drug release from controlled ocular drug delivery systems, including bottle method, diffusion method, modified rotating basket/paddle method and technique involving flow through apparatus [20]. Drug release studies were performed using a diffusion technique reported by Kumar et al. [21] with some modification. A pouch was made by tying the ends of the dialysis tube previously soaked in buffer overnight inside which 1 ml of the formulation was taken along with 0.3 ml of simulated tear fluid. The dialysis bag was carefully placed in the 50 ml of diffusion medium (STF) maintained at 37° and diffusion was carried out for four hours. Aliquots of 5 ml were withdrawn every one hour from the diffusion medium. The samples were diluted with 0.2 N NaOH and refluxed for 30 min and the absorbances were observed at 215 nm in UV/Vis double beam spectrophotometer.
Results and Discussion
The vehicle selected for the ocular formulations was phosphate buffer (pH 6) based on the solubility of azithromycin. A buffered vehicle in ophthalmic preparations is appropriate as the pH plays a significant role with respect to stability of formulations, solubility of drugs, in vivo penetrability and also eye irritation [22]. Viscosity enhancing polymers such as HPMC or SCMC were included in the formulations in order to achieve the desired rheological behaviour. Moreover cellulose polymers possess mucoadhesive property which may help in prolongation of the stay of drug in cul de sac [7].
All the formulations prepared were clear without any turbidity and suspended particles or impurities. The pH of the formulations were between 5.6–6.3 (Table 2) which is an acceptable range for ophthalmic preparations [22].
The gelling capability of the formulations was examined by mixing the solutions with STF in the proportion 25:7 as the application volume of eye drops is 25-50 μl and normal volume of tear fluid in the eye is 7 μl. The solutions on treatment with STF were observed to undergo rapid transition into gel within 5-6 s. The stability of the formed gels with different formulations is given in Table 2. The time taken for the gel to dissolve is denoted as +, ++, or +++ indicating 10–15 min, 30–45 min, 60-90 min of stability, respectively.
The gels formed with pectin alone (P1, P2) were stable only for 10 min but inclusion of viscosity builders was found to enhance the stability of the gel structure for a longer time. Among the rest of the formulations, the gels formed with two formulations HLP3, CP2 were stable for one hour whereas, HKP2 gel remained undissolved for 90 min.
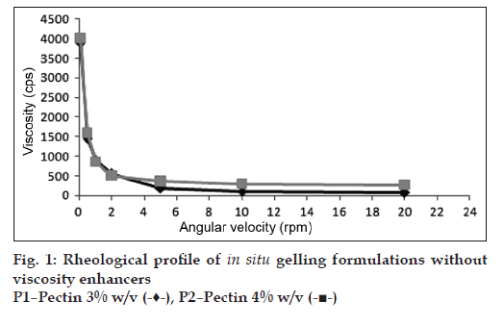
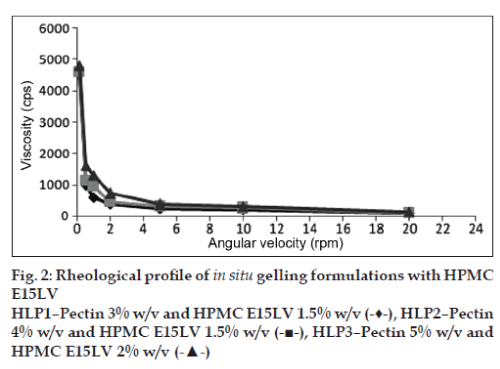
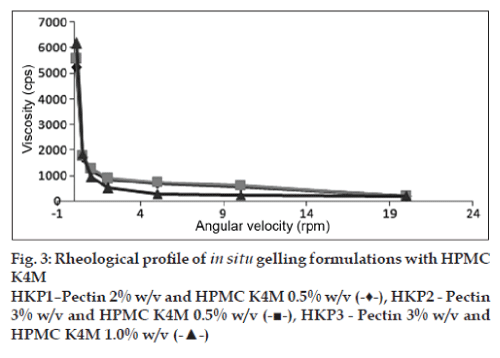
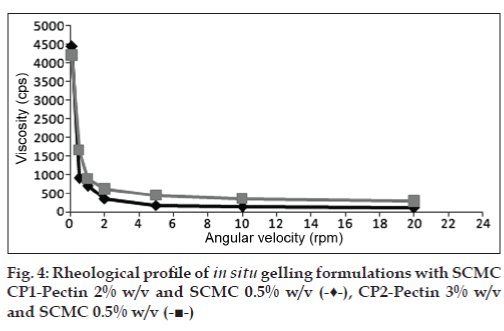
The viscosities of all the formulations at different angular velocities (0.1, 0.5, 1, 2, 5, 10, 20 rpm) as shown in (figs. 1 to 4) reveals the shear thinning nature of the solutions. Pseudoplastic formulations are preferred over viscous Newtonian vehicles in view of ease of withdrawal and instillation of ophthalmic drops. The viscosities at 20 rpm of angular velocity are given in Table 2. The same formulations with greater gel stability (HLP3, HKP3, and CP2) were found to possess higher viscosities at 20 rpm.
Based on the results of rheological studies and gelling behaviour (Table 2) three formulations HLP3, HKP3 and CP2 were selected for further studies. The drug contents of the formulations HLP3, HKP3, and CP2 were found to be 98.25±0.925% w/v, 99.4±1.25% w/v, 99.76±0.98% w/v respectively.
| Parameter | P1 | P2 | HLP1 | HLP2 | HLP3 | HKP1 | HKP2 | HKP3 | CP1 | CP2 |
|---|---|---|---|---|---|---|---|---|---|---|
| pH | 5.6 | 5.8 | 5.8 | 6.2 | 6.3 | 5.8 | 6.2 | 6.2 | 5.8 | 6.1 |
| Gel stability (min) | + | + | + | + | +++ | ++ | ++ | +++ | ++ | +++ |
| Viscosity at 20rpm (cps) | 77 | 256 | 92 | 115 | 134 | 184 | 205 | 210 | 108 | 275 |
Table 2: Evaluation Of in situ Gelling Formulations
The results of the sterility test on the selected formulations indicate that there is no evidence of microbial growth on the media during the incubation period of 14 days confirming the sterility of the formulations as well as the suitability of the sterilization technique. Zone of inhibition below 10 mm specifies that the strain is resistant to the test material, 10-15 mm indicates a moderate activity and above 15 mm of zone of inhibition implies that the microorganism is sensitive to the test substance [19]. The formulation with 3% w/w pectin and 0.5 g of SCMC (CP2) inhibited the growth of Staphylococcus aureus to the greatest extent compared to the other two formulations (HLP3, HKP3, Table 3) demonstrating the higher diffusion of azithromycin. The zones of inhibition observed in agar diffusion study on the formulations along with STF are slightly more than the previous ones (Table 3) which proves the sustained release of the drug from the in situ formed gels.
The results of ocular irritation studies indicate that the formulation was non irritant. Excellent ocular tolerance was noted and no ocular damage or abnormal clinical signs were visible. Therefore these formulations can be safely instilled into the eye without any adverse effects.
| in situgel formulation | Zone of inhibition (mm) | |
|---|---|---|
| Without STF | With STF | |
| HLP3 | 15 | 16 |
| HKP3 | 20 | 22 |
| CP2 | 30 | 32 |
Table 3: antimicrobial activity of the in situ Gels against staphylococcus aureus in cup and plate technique.
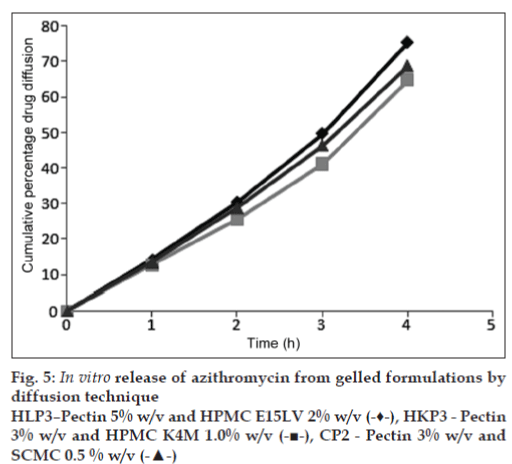
Results of in vitro drug release studies by diffusion through dialysis membrane on the three formulations HLP3, HKP3 and CP2 are given in the (fig. 5). The results clearly assure that a temporally controlled release of azithromycin in the cul de sac will occur for more than 4 h. The pattern of drug diffusion correlates well with the gel stability of the formulations. A more prolonged release was observed with HKP3 which showed the maximum in situ formed gel stability. This indicates that the gels have the ability to retain azithromycin resulting in sustained drug release.
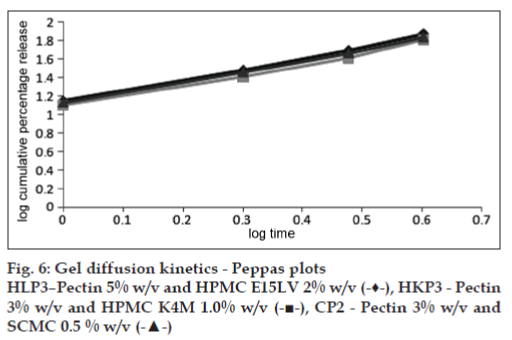
The straight lines obtained in the Peppas plots and the high correlation coefficients (fig. 6, Table 4) confirm the diffusion of the drug through the gels formed in situ with STF. The planar gel diffusion is further proved by the value of the diffusional exponent ‘n’ which is greater than 1 [23].
Among the formulations developed, CP2 containing pectin 3% w/w, and SCMC 0.5% w/v has shown more promising results in prolonging the release and efficacy of azithromycin. Among the cellulose ethers, SCMC exhibits a mucoadhesivecapacity comparable to that of polyacrylic acid [24]. Hence in addition to contributing to the in situ gel formation,the viscosity enhancer SCMC will improve the ocular bioavailability through mucoadhesion also. Hence it can be concluded that pectin based in situ gels can be successfully used to prolong the duration of action of azithromycin.
| in situgel formulation | Diffusional component (n) | R[2] value |
|---|---|---|
| HLP3 | 1.191 | 0.997 |
| HKP3 | 1.153 | 0.991 |
| CP2 | 1.158 | 0.997 |
Table 4: Gel Diffusion Kinetics –Peppas Plot– Diffusional Component
Acknowledgements
The authors wish to thank Prof. K. R. Arumugam, Chairman, Ultra Trust, Madurai and Prof. A. Babu Thandapani, Vice–Chairman, Ultra Trust, for the facilities provided to carry out the present work.
References
- Snyder RW, Glasser DB. Antibiotic therapy for ocular infection. W J Med 1994;161:579-84.
- Huguet P, Bella L, Einterz E, Goldschmidt P, Bensaid P. Trachoma mass treatment with azithromycin 1.5% eye drops in Cameroon: feasibility, tolerance and effectiveness. Br J Ophthalmol 2010;94:157-60.
- Chisholm SA, Neal TJ, Alawattegama AB. Emergence of high-level azithromycin resistance in Neisseria gonorrhoeae in England and Wales. J AntimicrobChemother 2009;64:353-8.
- Dawson CR, Bowman LM. Topical treatment or prevention of ocular infections, United States Patent, US 6569443 B1, 2003.
- Shen Y, Xu SJ, Wang SC, Tu JS. Determination of benzalkonium chloride in viscous ophthalmic drops of azithromycin by HPLC. J Zhejiang UnivSci B 2009;10:877-82.
- Abelson MB, Protzko EE, Shapiro AM. The AzaSite Clinical Study Group. A randomized trial assessing microbial eradication and clinical efficacy of 1% azithromycin ophthalmic solution vs tobramycin in adult subjects with bacterial conjunctivitis. Invest Ophthalmol Vis Sci 2006;47E-Abstract 3589.
- Bourlais CL, Needham T, Leverge R. Ophthalmic drug delivery systems. ProgRetin Eye Res 1998;17:33-8.
- Nanjawade BK, Manvi FV, Manjappa AS. in situ forming hydrogels for sustained ophthalmic drug delivery. J Control Release 2007;122: 119-34.
- Srividya B, Cardoza RM, Amin PD. Sustained ophthalmic delivery of ofloxacin from a pH triggered in situ gelling system. J Control Release 2001;73:205-11.
- Qi H, Chen W, Wu C. Development of polaxamer analogs/Carbopol based in situ gelling and mucoadhesive ophthalmic delivery system for puerarin. Int J Pharm 2007;337:178-87.
- Carlfors J, Edsman K. Rheological evaluation of Gelrite in situ gels for ophthalmic use. Eur J Pharm Sci 1998;6:113-9.
- Liu Z, Nie S, Pan W. Study of an alginate / HPMC based in situ gelling ophthalmic delivery system for gatifloxacin. Int J Pharm 2006;315:12-7.
- Sindhu A, Bharath S, Basavaraj BV. Sustained ophthalmic delivery of ofloxacin from an ion activated in situ gelling system. Pakistan J Pharm Sci 2009;22:175-9.
- Masteikova R, Chalupova Z. Stimuli sensitive hydrogels in controlled and sustained drug delivery. Medicina 2003;39:19-23.
- Ni Y, Yates KM. in situ gel formation of pectin. United States Patent, US 6777000 B2, 2004.
- Coviello T, Matricardi P, Marianecci C, Alhaique F. Polysaccharide hydrogels for modified release formulations. J Control Release 2009;119:5-24.
- Indian Pharmacopoeia, Vol 2, Delhi: Government of India, Ministry of Health and Family Welfare;1996, p. 117-24.
- Cappuccino JG, Sherman N. Microbiology–A Laboratory manual. 4th ed. Delhi: The Benjamin/Cummings Company; 1996, p. 272-3.
- Draize JH, Woodard G, Calvery HO. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J PharmacolTherapeut 1944;82:377-90.
- Sultana Y, Jain R, Rathod R, Asgar Ali, Aqil M. Advances in ophthalmic drug delivery systems–Part II. Pharmainfo.net. 2005, 3 (3). Available on http://www.pharmainfo.net/reviews/advances-ophthalmic-drug-delivery-systems-part-ii [Last cited on 8 Nov 2011].
- Kumar S, Himmelstein KJ. Modification of in situ gelling behavior of carbopol solution by hydroxyl propyl methyl cellulose. J Pharm Sci 1995;84:344-8.
- Allen LV Jr, Nicholas GP, Ansel HC. Pharmaceutical dosage forms and drug delivery systems. 8th ed. New Delhi: Wolters Kluwer; 2005, p. 546-50.
- Colombo P, Santi P, Bettini R, Brazel CS, Peppas NA. Drug release from swelling controlled systems. In: Wise DL, ed. Handbook of Pharmaceutical Controlled release Technology. New York: Marcel Dekker; 2009, p. 187.
- Ludwig A. The use of mucoadhesive polymers in ocular drug delivery. Adv Drug Deliv Rev 2005;57:1595-639.