- *Corresponding Author:
- Bangjiang Fang
Department of Neurology, National Traditional Chinese Medicine Clinical Research Base, The Affiliated Hospital of Traditional Chinese Medicine, Southwest Medical University, Luzhou, Sichuan 646000, China
E-mail: fangbji@163.com
| This article was originally published in a special issue, “Transformative Discoveries in Biomedical and Pharmaceutical Research” |
| Indian J Pharm Sci 2023:85(4) Spl Issue “59-70” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Fuyuan Xingnao decoction has the characteristics of multi-component, multi-pathway and multi-target. Previous studies have found that it has antioxidant, anti-inflammatory and other biological effects, and is now often used to treat neurological diseases such as intracerebral hemorrhage and cerebral infarction. But the specific key ingredients and mechanism are still unclear. Modern bioinformatics and network pharmacology methods were used to predict molecular network mechanisms between intracerebral hemorrhage and Fuyuan Xingnao decoction. Animal experiments were carried out to verify the effect of Fuyuan Xingnao decoction for the treatment of intracerebral hemorrhage, combined with behavior test and pathological detection. 68 active components in Fuyuan Xingnao decoction are involved and 471 gene targets were identified successfully. There are a total of 359 cross-targets between Fuyuan Xingnao decoction and intracerebral hemorrhage. Subsequently, the “disease-drug-active ingredient-target” network diagram was constructed using Cytoscape software and 97 core targets were screened. In addition, the enrichment of gene ontology and Kyoto Encyclopedia of Genes and Genomes suggests that phosphoinositide 3-kinase/protein kinase B and the apoptosis signaling pathway are most likely to be affected for the treatment of intracerebral hemorrhage. Finally, the use of behavioral tests and hematoxylin and eosin staining in animal experiments confirmed the role of Fuyuan Xingnao decoction in the treatment of intracerebral hemorrhage. Based on network pharmacology and experimental validation results, our in vivo study indicated that Fuyuan Xingnao decoction is effective for the treatment of intracerebral hemorrhage.
Keywords
Fuyuan Xingnao decoction, intracerebral hemorrhage, network pharmacology, hematoxylin and eosin staining
Intracerebral Hemorrhage (ICH) is one of the most common acute and critical conditions in neurology and remains the second leading cause of death worldwide, with a mortality rate of 43 %[1]. Cerebral protection after ICH is a difficult point in clinical treatment. There are two forms of brain injury after ICH, primary brain injury and secondary brain injury. Bleeding from vascular rupture and mass effects are the leading causes of primary brain injury[2]. Hemorrhage-formed hematomas cause compression in brain tissue and induce cerebral edema, leading to more severe secondary brain injury. A large number of studies have proved that after ICH, microglia and macrophages are activated, and most inflammatory factors are released from the cells, triggering a cascade of signal transduction pathways associated with inflammation[3]. During the inflammatory response following ICH, inflammatory cells are activated, the respiratory chain erupts and reactive oxygen species are released, ultimately causing an oxidative stress response[4]. In addition, after ICH, blood releases Red Blood Cells (RBCs) into the brain parenchyma and eventually the red blood cells are lysed into substances such as heme and iron, which exacerbate the inflammatory response and mediate brain damage[5]. Secondary brain injury produces mechanisms related to inflammatory responses, oxidative stress and toxic effects on RBC lysate, all of which ultimately lead to the death of neuronal cells[6,7]. In the current research reports, the death methods of neuronal cells after ICH mainly include apoptosis, autophagy, necrosis and iron death[8]. Mitochondrial dysfunction, inflammatory response, oxidative stress and other factors caused by ICH can cause apoptosis of neuronal cells.
Fuyuan Xingnao decoction is composed of ginseng, Panax notoginseng, leech, Acorus calamus, Tiannanxing, rhubarb and motherwort. Our previous study found that in the clinical treatment of acute ischemic stroke, Fuyuan Xingnao decoction effectively improves the coagulation function of patients, enhances vascular endothelial function, reduces secondary inflammatory responses, improves blood rheological indicators and neurological function[9,10], and inhibits the release of inflammatory transmitters[11,12]. In addition, Fuyuan Xingnao decoction can effectively reduce the degree of cerebral edema in rats with cerebral infarction and reduce the permeability of the blood-brain barrier[13]. This Traditional Chinese Medicine (TCM) compound also has the effect of reducing the volume of infarction in brain tissue[14], which is related to the effect of Fuyuan Xingnao decoction in increasing angiogenesis in ischemic brain tissue and promoting blood vessels to regain blood flow perfusion. The mechanism may be used to promote the upregulation of the expression of Vascular Endothelial Growth Factor (VEGF) protein[15]; upregulation of Insulin-like Growth Factor-1 (IGF-1) expression by inhibition of microRNA-320 (miR-320)[16]; upregulates the expression of Cluster of Differentiation 31 (CD31), a neonatal marker in vascular endothelial cells[17]; up-regulation of Cyclin E1 (CCNE1) and Cell Division Cycle 25 A (CDC25A) reduces the expression level of microRNA-503, which inhibits angiogenesis[18]. Furthermore, anti-apoptosis and anti-inflammatory properties have also been reported[19]. These studies highlight the potential function of Fuyuan Xingnao decoction in the prevention and treatment of cerebral infarction. However, the preventive or protective properties of Fuyuan Xingnao decoction for ICH and its underlying molecular mechanism have not been clarified.
Materials and Methods
Screening of active ingredients and potential targets of Fuyuan Xingnao decoction:
In the Traditional Chinese Medicine Systems Pharmacology (TCMSP) database, the active ingredients of ginseng, Panax notoginseng, Acorus calamus, motherwort, Arisaematis rhizoma and rhubarb were screened out according to the conditions of Oral Bioavailability (OB)≥30 % and Drug-Likeness (DL)≥0.18. The active ingredient of leeches was selected by setting the score cut off≥20 and p-value≤0.05 through the Bioinformatics Analysis Tool for Molecular Mechanism of Traditional Chinese Medicine (BATMAN-TCM) database. These targets corresponding to these active ingredients are imported into the Universal Protein Resource (UniProt) database and normalized, and the corresponding human gene names are obtained.
Acquisition of targets related to ICH and screening of intersection targets of Fuyuan Xingnao Decoction and ICH:
Using “intracerebral hemorrhage” as the keyword, the target of ICH disease was searched in the GeneCards database. The target of the active ingredient of the standardized name of the Fuyuan Xingnao decoction was intersected with the target of ICH. Finally, the target of the Fuyuan Xingnao decoction acting on ICH was obtained.
Construction of the network diagram of Fuyuan Xingnao Decoction-Ingredients-Target-ICH:
The potential active ingredients contained in the Fuyuan Xingnao decoction and the drug-disease intersection targets were uploaded to the software Cytoscape 3.8.0 software to construct a relationship network of Fuyuan Xingnao decoction-ingredients-target-ICH and visually adjusted.
Construction of Fuyuan Xingnao decoction-ICH Protein-Protein Interaction (PPI) network and screening of core targets:
The intersection target of Fuyuan Xingnao decoction and ICH was imported into the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) database and the species was selected “Homo sapiens”, constructing a PPI network for Fuyuan Xingnao decoction-ICH. Then, a Tab-Separated Values (TSV)-formatted file will be obtained in the STRING database and the file will be uploaded to the Cytoscape 3.8.0 software for network topology analysis and the core targets will be screened out under the condition that it is greater than the average value of degree.
Gene Ontology (GO) bioprocess analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis:
Enter the above intersecting targets into the Database for Annotation, Visualization and Integrated Discovery (DAVID) database, and the species is selected as “Homo sapiens”, performing GO bioprocesses and KEGG pathway enrichment analysis. Based on p≤0.05, the top 20 biological processes and KEGG pathways were selected.
Animals:
Healthy male Sprague Dawley (SD) adult rats with a mass of 250±20 g were purchased from the Laboratory Animal Center of Southwest Medical University (production and use license: SCXK (Chuan) 2018-17; SYXK (Chuan) 2018-065). In this experiment, all experimental rats had free access to feed and drinking water. All rats are housed in a clean environment controlled by temperature, humidity and light. The study was approved by the Animal Ethics Committee of the Science and Technology Department of Southwest Medical University (Approval number: 2020904 date: December 30, 2020).
Experimental drugs:
The composition of Fuyuan Xingnao decoction: Fuyuan Xingnao decoction is composed of ginseng 10 g, Panax notoginseng 10 g, Acorus calamus 12 g, leech 10 g, motherwort 30 g, Tiannanxing 15 g, rhubarb 10 g. All drugs are specially processed and prepared, in which ginseng and leeches are extracted using alcohol and Acorus calamus is extracted from volatile oil. Then together with the rest of the medicinal materials, it is prepared into a 2 g/ml concentrate, which is sterilized and placed in a cool and dry place.
Animal grouping and dosing regimen:
Referring to the table of drug dose conversion coefficients for human and animal kilograms of body weight and using the standard adult weight of 60 kg, the formula was obtained. Rat equivalent dose=human dose administered per kg of body weight×6.25. According to the above formula, the low dose group of Fuyuan Xingnao decoction was 5 g/kg/d, the middle dose group of Fuyuan Xingnao decoction was 10 g/kg/d and the high dose group of Fuyuan Xingnao decoction was 20 g/kg/d. Rats in the low, middle and high dose groups of Fuyuan Xingnao decoction were gavaged with corresponding doses of drugs and rats in the sham surgery group and model group were gavaged with the same amount of normal saline for three consecutive days. After the end of gavage, rat brain tissue is removed for subsequent experiments.
Zea Longa score:
Refer to the Zea Longa 5-point method to develop neurological function, scoring standards are 0 points: No symptoms of neurological deficit; 1 point: The left forelimb cannot be fully extended when the tail is upside down; 2 points: Rear-end when walking in a circle to the hemiplegic side and the forelimb on the hemiplegic side drags or curls up; 3 points: Tipping to the side of hemiplegia when walking; 4 points: Impairment of consciousness. Rats with scores in the 1-3 score range are successful rats with a model of ICH. Rats were scored for neurological deficits 6 h after surgery and rats that met the scoring criteria were included in the experiment. Each group of rats were scored again 72 h postoperatively.
Hematoxylin and Eosin (HE) staining:
Brain tissue was fixed with 4 % paraformaldehyde for more than 24 h for paraffin embedding and then sliced. Brain tissue slices were routinely dewaxed and rehydrated, and then stained with hematoxylin for 10 min according to the HE staining kit instructions. Next, the slices were rinsed with tap water for 3 min. Slices were differentiated in 1 % hydrochloric acid, alcohol for 10 s and rinsed with tap water for 1 min. Slices were returned to blue with ammonia water for 15 s and rinsed with tap water for 1 min. The slices were hydrated in 85 % alcohol for 5 min and eosin staining solution was counterstained for 3 min to stain the cytoplasm. Finally, the slices are rinsed with tap water and the gradient alcohol is dehydrated and transparent. Neutral gum seal, after drying, the pathological changes of brain tissue around the hematoma were observed, photographed and saved.
Results and Discussion
Screening of active components of Fuyuan Xingnao decoction and related targets were explained here. After the active ingredient is retrieved in the database, its duplicate data is removed. The 68 potential active ingredients of 7 flavors of Chinese medicine in the Fuyuan Xingnao decoction were obtained including 17 active ingredients in ginseng, 7 active ingredients in Panax notoginseng, 4 active ingredients in Acorus calamus, 8 active ingredients in motherwort, 6 active ingredients in Arisaematis rhizoma, 7 active ingredients in Rheum and 19 active ingredients in leech (Table 1 and Table 2).
| Drug | Active ingredient | OB (%) | DL |
|---|---|---|---|
| Ginseng | Diop | 43.59 | 0.39 |
| Ginseng | Stigmasterol | 43.83 | 0.76 |
| Ginseng | beta-sitosterol | 36.91 | 0.75 |
| Ginseng | Inermin | 65.83 | 0.54 |
| Ginseng | Kaempferol | 41.88 | 0.24 |
| Ginseng | Aposiopolamine | 66.65 | 0.22 |
| Ginseng | Deoxyharringtonine | 39.27 | 0.81 |
| Ginseng | Dianthramine | 40.45 | 0.2 |
| Ginseng | Arachidonate | 45.57 | 0.2 |
| Ginseng | Frutinone A | 65.9 | 0.34 |
| Ginseng | Ginsenoside Rh2 | 36.32 | 0.56 |
| Ginseng | Ginsenoside-Rh4_qt | 31.11 | 0.78 |
| Ginseng | Girinimbin | 61.22 | 0.31 |
| Ginseng | Panaxadiol | 33.09 | 0.79 |
| Ginseng | Suchilactone | 57.52 | 0.56 |
| Ginseng | Alexandrin_qt | 36.91 | 0.75 |
| Ginseng | Fumarine | 59.26 | 0.83 |
| Panax notoginseng | Mandenol | 42 | 0.19 |
| Panax notoginseng | DFV | 32.76 | 0.18 |
| Panax notoginseng | Diop | 43.59 | 0.39 |
| Panax notoginseng | beta-sitosterol | 36.91 | 0.75 |
| Panax notoginseng | Stigmasterol | 43.83 | 0.76 |
| Panax notoginseng | Ginsenoside Rh2 | 36.32 | 0.56 |
| Panax notoginseng | Quercetin | 46.43 | 0.28 |
| Acorus calamus | (1R,3aS,4R,6aS)-1,4-bis(3,4-dimethoxyphenyl)-1,3,3a,4,6,6a-hexahydrofuro[4,3-c]furan | 52.35 | 0.62 |
| Acorus calamus | 8-Isopentenyl-kaempferol | 38.04 | 0.39 |
| Acorus calamus | Cycloartenol | 38.69 | 0.78 |
| Acorus calamus | Kaempferol | 41.88 | 0.24 |
| Motherwort | arachidonic acid | 45.57 | 0.2 |
| Motherwort | Kaempferol | 41.88 | 0.24 |
| Motherwort | Quercetin | 46.43 | 0.28 |
| Motherwort | Isorhamnetin | 49.6 | 0.31 |
| Motherwort | Iso-preleoheterin | 66.29 | 0.33 |
| Motherwort | Preleoheterin | 85.97 | 0.33 |
| Motherwort | Galeopsin | 61.02 | 0.38 |
| Motherwort | ZINC04073977 | 38 | 0.76 |
| Arisaematis rhizoma | 24-epicampesterol | 37.58 | 0.71 |
| Arisaematis rhizoma | [(2R)-2-[[[(2R)-2-(benzoylamino)-3-phenylpropanoyl]amino]methyl]-3-phenylpropyl] acetate | 38.88 | 0.56 |
| Arisaematis rhizoma | beta-sitosterol | 36.91 | 0.75 |
| Arisaematis rhizoma | Sitosterol | 36.91 | 0.75 |
| Arisaematis rhizoma | Stigmasterol | 43.83 | 0.76 |
| Arisaematis rhizoma | CLR | 37.87 | 0.68 |
| Rheum | Eupatin | 50.8 | 0.41 |
| Rheum | Rhein | 47.07 | 0.28 |
| Rheum | Toralactone | 46.46 | 0.24 |
| Rheum | Daucosterol_qt | 35.89 | 0.7 |
| Rheum | beta-sitosterol | 36.91 | 0.75 |
| Rheum | Aloe-emodin | 83.38 | 0.24 |
| Rheum | (-)-catechin | 49.68 | 0.24 |
Table 1: The active components of Ginseng, Sanqi, Acorus, Tatarinowii, Herba leonuri, Arisaematis rhizoma, Rhubarb.
| Drug | Active ingredient |
|---|---|
| Leech | Indole-3-carboxaldehyde |
| Leech | Adenosine |
| Leech | Adenine |
| Leech | Uracil |
| Leech | Xanthine |
| Leech | 2-piperidone |
| Leech | Cholesterol |
| Leech | Campesterol |
| Leech | Nicotinic acid |
| Leech | Uridine |
| Leech | Hypoxanthine |
| Leech | Inosine |
| Leech | Phenylalanine |
| Leech | Proline |
| Leech | Valine |
| Leech | L-isoleucine |
| Leech | Glycerol |
| Leech | Succinic acid |
| Leech | Palmitic acid |
Table 2: The active components of Leech.
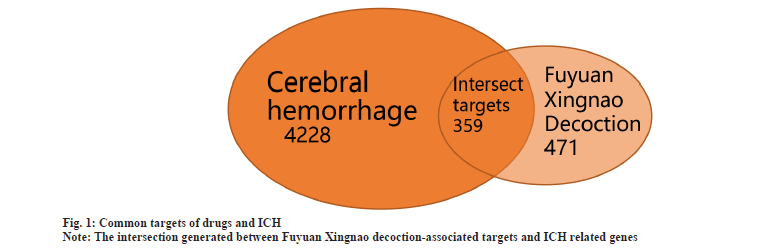
Potential target identification was explained here. The relevant targets of 68 active ingredients were obtained from the database and then the duplicate data were deleted and the gene names were normalized, resulting in a total of 471 relevant targets of the Fuyuan Xingnao decoction. 4228 targets related to ICH were obtained from the GeneCards database. The targets related to Fuyuan Xingnao decoction and the targets related to ICH were intersected and 359 intersection targets were obtained (fig. 1).
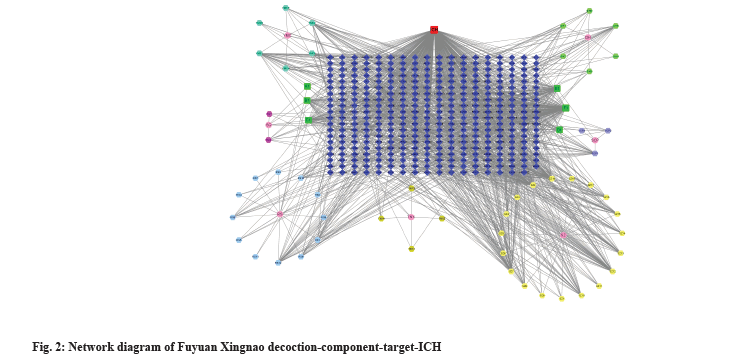
Network diagram of Fuyuan Xingnao decoction-ingredients-target-ICH was shown in fig. 2. The data of the 7 flavors of TCM of the Fuyuan Xingnao decoction, active ingredients and intersection targets were entered into the Cytoscape 3.8.0 software and visualized to obtain the network diagram of Fuyuan Xingnao decoction-ingredients-target-ICH. The red square represents ICH, the hexagons of different colors represent the active ingredients of each Chinese medicine, the green square represents the repeated active ingredients between Chinese medicines, the pink circle represents the constituent drugs in the Fuyuan Xingnao decoction and the dark blue diamond represents the intersection target. In fig. 2, RS stands for ginseng, SQ stands for Panax notoginseng, SCP stands for Acorus calamus, YMC stands for motherwort, TNX stands for Arisaematis rhizoma, DH stands for Rheum and SZ stands for leech. The node size is positively correlated with the degree value and the larger the degree value, the larger the node.
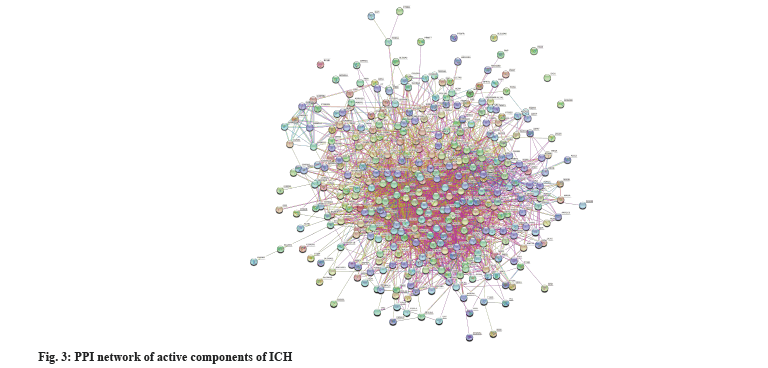
PPI network analysis was shown in fig. 3. 359 intersecting targets were imported into the STRING database and the species was set to “Homo sapiens” to obtain a PPI network diagram. There are 358 nodes and 2729 edges in the figure, the average value of degree is 15.2 and the average local clustering coefficient is 0.344. The TSV format file was uploaded to Cytoscape 3.8.0 software for network topology analysis and the average value of degree was 38, and 97 core targets were screened out under the condition that degree was greater than 38 (Table 3 and fig. 3).
| Target | Degree value | Target | Degree value | Target | Degree value |
|---|---|---|---|---|---|
| AKT1 | 158 | IL2 | 72 | CASP1 | 50 |
| TNF | 147 | PTPRC | 72 | NOS2 | 49 |
| IL6 | 146 | BCL2L1 | 71 | PARP1 | 49 |
| TP53 | 133 | IFNG | 70 | CXCL10 | 48 |
| IL1B | 127 | PECAM1 | 69 | CDK2 | 47 |
| VEGFA | 126 | SERPINE1 | 68 | SELP | 46 |
| JUN | 120 | CRP | 68 | F2 | 46 |
| CASP3 | 119 | MAPK14 | 68 | PRKCD | 45 |
| EGFR | 113 | STAT1 | 68 | CCNB1 | 45 |
| MYC | 111 | MAPK8 | 66 | RUNX2 | 45 |
| PPARG | 110 | CAV1 | 63 | IGFBP3 | 44 |
| ESR1 | 109 | ACE | 63 | SLC2A4 | 43 |
| PTGS2 | 107 | TGFB1 | 63 | RAF1 | 42 |
| HIF1A | 104 | AR | 62 | RB1 | 42 |
| MMP9 | 104 | MPO | 60 | HSPB1 | 41 |
| EGF | 102 | NR3C1 | 59 | CHUK | 41 |
| FOS | 95 | REN | 58 | PLAU | 41 |
| PTEN | 94 | KDR | 57 | CYP3A4 | 40 |
| CXCL8 | 94 | CASP9 | 57 | DPP4 | 40 |
| CCL2 | 93 | PLG | 57 | ESR2 | 40 |
| CCND1 | 92 | CDKN1A | 57 | IGF2 | 40 |
| IL10 | 92 | TNFRSF1A | 56 | PRKCB | 39 |
| ERBB2 | 85 | SPP1 | 55 | F3 | 39 |
| NOS3 | 82 | MMP3 | 55 | CXCL2 | 39 |
| ICAM1 | 77 | IKBKB | 55 | ABCB1 | 38 |
| PPARA | 77 | SELE | 54 | SOD1 | 38 |
| HMOX1 | 76 | IL1A | 54 | GJA1 | 38 |
| MMP2 | 76 | PGR | 54 | NCF1 | 38 |
| VCAM1 | 75 | PRKCA | 53 | IRF1 | 38 |
| MAPK1 | 75 | MMP1 | 52 | CCNA2 | 38 |
| NFKBIA | 75 | GSK3B | 52 | FYN | 38 |
| CASP8 | 75 | NFE2L2 | 52 | ||
| RELA | 73 | CD40LG | 50 |
Table 3: Core Targets Of “Drug-ICH”.
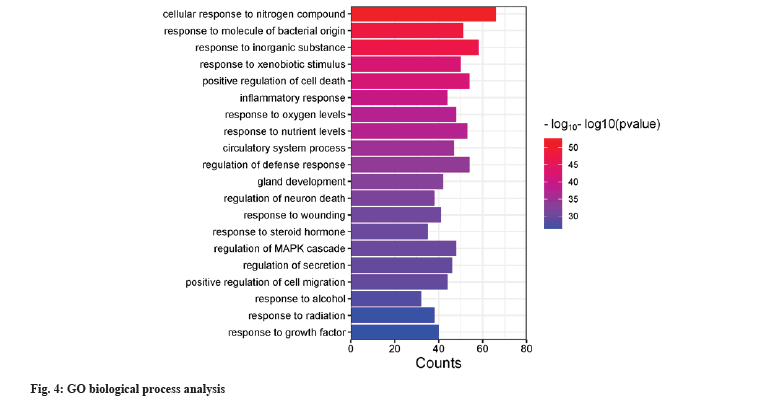
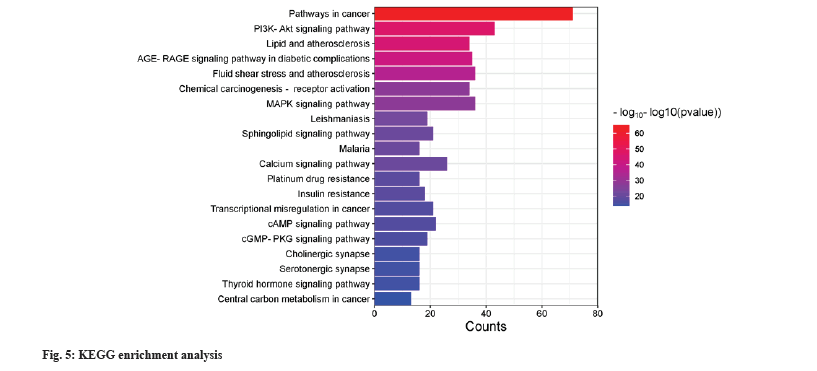
GO bioprocess and KEGG pathway enrichment was shown in fig. 4 and fig. 5. 359 intersecting targets were entered into the DAVID database for GO bioprocess and KEGG pathway enrichment analysis. According to p≤0.05 as the standard and p value size, the first 20 bioprocesses and pathways were selected and displayed by enrichment bar plots. The biological processes mainly involve cell response to nitrogen compounds, regulation of neuronal death, positive regulation of cell death, inflammatory response, response to oxygen levels, circulatory system processes, etc. The pathways involved include cancer pathway, Phosphoinositide 3-kinase/Protein kinase B (PI3K-Akt) signaling pathway, Mitogen-Activated Protein Kinase (MAPK) signaling pathway, calcium signaling pathway, sphingolipid signaling pathway, etc.
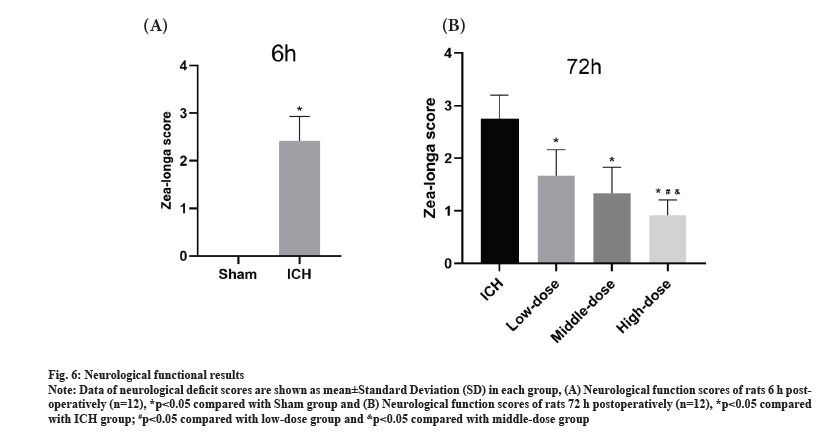
Findings of animal experiment were shown in fig. 6. Neurological function of rats is assessed at 6 h post-modeling and 72 h post-modeling. At 6 h after modeling, rats in the Sham group had no behavioral disorders and the difference was significant compared with the ICH group (p<0.05) (fig. 6A), confirming the successful establishment of an animal model of ICH. At 72 h after modeling, compared with the ICH group, the scores of the low, middle and high dose groups were significantly reduced (p<0.05). In addition, the neurological function score was most significantly reduced between the three dose groups (p<0.05) (fig. 6B). These results showed that the effect of Fuyuan Xingnao decoction improved the symptoms of neurological deficit after ICH and the effect was more pronounced with the increase of the dose.
Fig. 6: Neurological functional results.
Note: Data of neurological deficit scores are shown as mean±Standard Deviation (SD) in each group, (A) Neurological function scores of rats 6 h postoperatively
(n=12), *p<0.05 compared with Sham group and (B) Neurological function scores of rats 72 h postoperatively (n=12), *p<0.05 compared
with ICH group; #p<0.05 compared with low-dose group and &p<0.05 compared with middle-dose group.
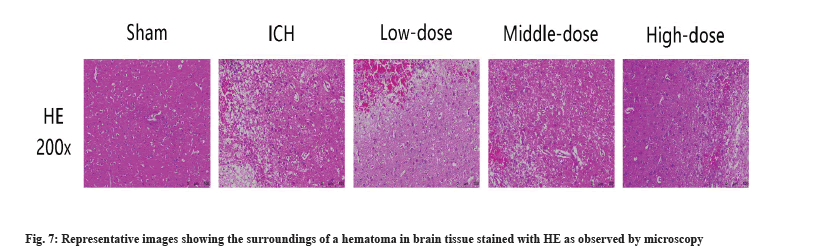
HE staining evaluated the effect of Fuyuan Xingnao decoction on the surrounding tissues of cerebral hematoma. In the ICH group, we observed a decrease in the number of nerve cells, swelling and degeneration. Moreover, nerve cells are poorly structured and arranged out of order. After the treatment of Fuyuan Xingnao decoction, the degree of swelling of nerve cells in brain tissue was reduced and the cells were arranged relatively closely (fig. 7).
TCM is a complementary or alternative medical system used to treat various diseases[20] and is also a potential source of candidate resources for ICH treatment. Fuyuan Xingnao decoction is a commonly used TCM prescription in clinical practice, which has been used to treat diabetes, cerebral infarction, ICH and other diseases[21]. In recent years, through clinical experiments and animal experimental studies, the effect of Fuyuan Xingnao decoction has been extensively studied and it has been proven to have anti-inflammatory[12], antioxidant, anti-apoptotic effects[19]. Fuyuan Xingnao decoction also has the effect of promoting cerebral angiogenesis[17] and neuroprotection[22]. In our previous study, we confirmed the improved effect of this prescription on ICH[23], but the underlying mechanism is unclear. The theory of network pharmacology is basically consistent with the overall principle of TCM, which is very suitable for the analysis of multi-component and multi-target TCM prescriptions[24,25]. Therefore, we used network pharmacology methods, combined with active ingredient screening, drug targeting, bioprocess analysis and pathway analysis, to explore the potential mechanism of Fuyuan Xingnao decoction in the treatment of ICH, and further verified the therapeutic effect of Fuyuan Xingnao decoction in the treatment of ICH in vivo experiments.
We screened a total of 68 active ingredients from Fuyuan Xingnao decoction with 471 corresponding targets and 359 intersecting targets corresponding to ICH. Through the analysis of these intersecting targets, it was found that AKT Serine/Threonine Kinase 1 (AKT1) may be a key target. The analysis results of GO biological processes showed that the regulation of neuronal apoptosis was enriched and our KEGG pathway analysis showed that these targets were significantly enriched in the PI3K-Akt signaling pathway. There are three highly homologous subtypes in the AKT family, AKT1, AKT2 and AKT3. AKT1 participates in the cell survival pathway by inhibiting the apoptosis process, which can prevent apoptosis and promote cell survival. In addition, Akt is an important effector molecule downstream of PI3K. The PI3K/Akt pathway is related to cell proliferation and apoptosis[26]. PI3K is divided into three categories according to its structure and function, and type I PI3K is currently the most widely studied[27]. When PI3K is activated, PI3K acts as a second messenger to transmit the activation signal to Akt, thereby activating Akt phosphorylation and inducing a cascade reaction with downstream molecules. Studies have found that the use of PI3K/Akt pathway inhibitors inhibits the beneficial effect of drugs on the expression of apoptosis markers after ICH. This suggests that the mechanism of action of drugs to improve neuronal apoptosis is related to the activation of the PI3K/Akt pathway to mediate the expression of downstream apoptosis-related proteins[28]. Some researchers have studied the mechanism of action of PI3K/Akt pathway in ICH and used baicalein to intervene in rats with ICH model, and found that baicalein can activate the PI3K/Akt signaling pathway to inhibit the expression of Nuclear Factor kappa B (NF-κB) protein and improve neuronal apoptosis in rats with ICH[29]. The results of our network pharmacological predictions have similarities with previous studies.
Fuyuan Xingnao decoction contains a long list of active compounds effective for stroke. Ginseng is one of the most important drugs in the prescription, whose main active ingredient was ginsenoside and it has anti-inflammatory, antioxidant and anti-apoptosis on cerebral ischemia and hypoxia[30]. It has been shown that ginsenosides Rg3 and Rh2 inhibit neuronal apoptosis and neuroinflammation by upregulating PI3K/Akt and inhibiting extracellular signal-regulating kinases[31]. In addition, the main active ingredient of the important Chinese medicine Panax notoginseng is a saponin, which has been confirmed to reduce cerebral edema, reduce Malondialdehyde (MDA) levels, reduce the expression of inflammatory factors, Interleukin-6 (IL-6), Interleukin-8 (IL-8), Tumor Necrosis Factor-alpha (TNF-α) and improve Superoxide Dismutases (SOD) activity in rats with ICH[32]. In addition, Arisaematis rhizoma may reduce cerebral edema by inhibiting Matrix Metalloproteinase-2 (MMP-2) expression[33]. Acorus calamus can also reduce cerebral edema and brain cell swelling after ICH[34]. Chrysophanol, the active ingredient of Rheum, inhibits apoptosis of nerve cells by regulating the PI3K/Akt/mammalian Target of Rapamycin (mTOR) signaling pathway, reducing the expression of inflammatory factors as well as the level of markers of oxidative stress, thereby improving brain injury in rats with ICH[35]. All these evidences supported the potential of Fuyuan Xingnao decoction in treatment of ICH.
At present, network pharmacology provides a powerful tool for exploring the compatibility and action mechanism of TCM prescriptions. However, there are some limitations. For example, main active ingredients should be considered in animal medical research of Fuyuan Xingnao decoction. We will verify the efficacy of active ingredients in treating ICH through in vivo experiments. The results show that Fuyuan Xingnao decoction can effectively improve pathological changes and restore damaged nerve function in rats with ICH.
Funding:
This study is supported by Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (ZYYCXTD-C-202207); Sichuan Science and Technology Project (2020YJ0437); Innovation Team of Sichuan Provincial Administration of Traditional Chinese Medicine (2022C007); Luzhou Science and Technology Project (2020-SYF-30); the Project of National Traditional Chinese Medicine Clinical Research Base (XNYDZYY [2020] No. 33) and Innovation Team of the Affiliated Traditional Chinese Medicine Hospital of Southwest Medical University (2022-CXTD-05).
Author’s contributions:
Bangjiang Fang is the corresponding author of the study; Xue Bai is the co-corresponding author of the study; Yanqi Luo and Yanjiao Li are the first authors who contributed equally to this work. Shuangyang Li helped in organizing the information. All authors read and approved the final manuscript.
Conflict of interests:
The authors declared no conflict of interest.
References
- Feigin VL, Stark BA, Johnson CO, Roth GA, Bisignano C, Abady GG, et al. Global, regional and national burden of stroke and its risk factors, 1990-2019: A systematic analysis for the global burden of disease study 2019. Lancet Neurol 2021;20(10):795-820.
[Crossref] [Google Scholar] [PubMed]
- Xue M, Yong VW. Neuroinflammation in intracerebral haemorrhage: Immunotherapies with potential for translation. Lancet Neurol 2020;19(12):1023-32.
[Crossref] [Google Scholar] [PubMed]
- Wan S, Cheng Y, Jin H, Guo D, Hua Y, Keep RF, et al. Microglia activation and polarization after intracerebral hemorrhage in mice: The role of protease-activated receptor-1. Transl Stroke Res 2016;7:478-87.
[Crossref] [Google Scholar] [PubMed]
- Yu YP, Chi XL, Liu LJ. A hypothesis: Hydrogen sulfide might be neuroprotective against subarachnoid hemorrhage induced brain injury. Sci World J 2014:1-9.
[Crossref] [Google Scholar] [PubMed]
- Wagner KR, Sharp FR, Ardizzone TD, Lu A, Clark JF. Heme and iron metabolism: Role in cerebral hemorrhage. J Cereb Blood Flow Metab 2003;23(6):629-52.
[Crossref] [Google Scholar] [PubMed]
- Wang LS, Zhang YQ, Feng Z. Effects of oxidative stress on apoptosis and protein expression of c-myc after intracerebral hemorrhage. Chin J Gen Pract 2017;15(4):608-10.
- Zille M, Karuppagounder SS, Chen Y, Gough PJ, Bertin J, Finger J, et al. Neuronal death after hemorrhagic stroke in vitro and in vivo shares features of ferroptosis and necroptosis. Stroke 2017;48(4):1033-43.
[Crossref] [Google Scholar] [PubMed]
- Sekerdag E, Solaroglu I, Gursoy-Ozdemir Y. Cell death mechanisms in stroke and novel molecular and cellular treatment options. Curr Neuropharmacol 2018;16(9):1396-415.
[Crossref] [Google Scholar] [PubMed]
- Wang AL, Wang X, Zhu TQ. Clinical study on Fuyuan Xingnao decoction assisting the treatment of acute cerebral infarction. New Chin Med 2022;54(9):67-71.
- Zhao L. Effect of Fuyuan Xingnao decoction in the adjuvant treatment of type 2 diabetes mellitus complicated with acute ischemic stroke. J Med Theory Pract 2022;35(23):3999-4001.
- Geng Z, Fang BJ, Ma ZH, Chen Z. Clinical study of Fuyuan Xingnao decoction on acute ischemic stroke. J Emerg Tradit Chin Med 2014;23(11):1970-72.
- Wang LP, Liu CM, Zhang H. Effect of Fuyuan Xingnao decoction combined with Western medicine on lacunar infarction and their impact on the levels of IL-6, TNF-α and NO. Mod J Integr Tradit Chin West Med 2015;24(29):3207-09.
- Huang JY, Wang H, Fang BJ. Experimental study on the effect of Fuyuan Xingnao decoction on the blood-brain barrier intervention in rats with diabetic cerebral infarction. Jiangsu J Tradit Chin Med 2013;45(8):68-70.
- Zhao P, Shen J, Wei JP. Effect of Fuyuan Xingnao decoction on infarct volume in rats with diabetic cerebral infarction. Acad J Shanghai Univ Tradit Chin Med 2013,27(5):66-9.
- Fang BJ, Zhou S, Chen H, Chen BJ, Guo Q, Zhao P, et al. Effect of Fuyuan Xingnao decoction on vascular endothelial growth factor in diabetic cerebral ischemic rats. Shanghai J Tradit Chin Med 2010;44(5):12-5.
- Shen J, Zhao ZM, Liu C. A study of the effect and molecular mechanism of fuyuan xingnao decoction to infarct volume of rats with diabetic cerebral infarction. Jiangsu J Tradit Chin Med 2018;50(5):78-82.
- Lihua S, Miaoqing Y, Junyi S, Zuo A, Wanying J, Bangjiang F. Experimental study of Fuyuan Xingnao decoction on angiogenesis in ischemic brain tissue of rats with diabetic cerebral infarction. J Emerg Tradit Chin Med 2019;28(2):192-5.
- Sun LH, Ye MQ, Shen J, Zuo Z, Jie WY, Fang BJ. Effects of Fuyuan Xingnao decoction on expression of microRNA-503 and CDC25A, CCNE1 and its experimental study on promoting angiogenesis in diabetic rats with cerebral infarction. J Emerg Tradit Chin Med 2018;27(11):1881-5.
- Li L, Feng J, Li T, Zhang Y. Protective effects of Fu-Yuan-Xing-Nao decoction for focal cerebral ischemia reperfusion injury in rats. J Pharm Pract 2018:34-9.
- Zhou W, Wang J, Wu Z, Huang C, Lu A, Wang Y. Systems pharmacology exploration of botanic drug pairs reveals the mechanism for treating different diseases. Sci Rep 2016;6(1):36985.
[Crossref] [Google Scholar] [PubMed]
- Jiang C, Wang T, Ma Z, Fang BJ. Effectiveness of Fuyuan Xingnao Decoction for patients with diabetes mellitus combined cerebral infarction. Medicine 2019;98(39).
[Crossref] [Google Scholar] [PubMed]
- Lin PL, Yang HT. Effect analysis of Fuyuan Xingnao decoction in treating type 2 diabetes mellitus complicated with cerebral infarction. Strait Pharm J 2021;33(10):95-8.
- Fang BJ, Zhou S, Chen BZ, Huang JY, Geng Z, Ji XQ, et al. The experimental study of Fuyuan Xingnao decoction on blood-brain barrier permeability in hypertensive cerebral hemorrhage rats. Chin Med Mod Distance Educ China 2010;8(18):182-4.
- Wei J, Zhao B, Zhang C, Shen B, Zhang Y, Li C, Chen Y. Jiawei Foshou San induces apoptosis in Ectopic endometrium based on systems pharmacology, molecular docking and experimental evidence. Evid Based Complement Alternat Med 2019:1-12.
[Crossref] [Google Scholar] [PubMed]
- He S, Zhang X, Lu S, Zhu T, Sun G, Sun X. A computational toxicology approach to screen the hepatotoxic ingredients in traditional chinese medicines: Polygonum multiflorum thunb as a case study. Biomolecules 2019;9(10):577.
[Crossref] [Google Scholar] [PubMed]
- Shi X, Wang J, Lei Y, Cong C, Tan D, Zhou X. Research progress on the PI3K/AKT signaling pathway in gynecological cancer. Mol Med Rep 2019;19(6):4529-35.
[Crossref] [Google Scholar] [PubMed]
- Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol 2010;11(5):329-41.
[Crossref] [Google Scholar] [PubMed]
- Chen S, Peng J, Sherchan P, Ma Y, Xiang S, Yan F, et al. TREM2 activation attenuates neuroinflammation and neuronal apoptosis via PI3K/Akt pathway after intracerebral hemorrhage in mice. J Neuroinflammation 2020;17(1):1-6.
[Crossref] [Google Scholar] [PubMed]
- Meng F, Li JY, Yang CB. Baicalin alleviate neuronal apoptosis by regulating PI3K/Akt/NF-κB signaling pathway after intracerebral hemorrhage in rats. Prog Anat Sci 2022;28(5):639-42.
- Sun Y, Yang Y, Liu S, Yang S, Chen C, Lin M, et al. New therapeutic approaches to and mechanisms of ginsenoside Rg1 against neurological diseases. Cells 2022;11(16):2529.
[Crossref] [Google Scholar] [PubMed]
- Hou J, Xue J, Wang Z, Li W. Ginsenoside Rg3 and Rh2 protect trimethyltin‐induced neurotoxicity via prevention on neuronal apoptosis and neuroinflammation. Phytother Res 2018;32(12):2531-40.
[Crossref] [Google Scholar] [PubMed]
- Li X, Zhuang HC. Protective effect of Xuesaitong soft capsules (Panax notoginseng total saponin) on brain tissue of rats with cerebral hemorrhage. Chin J Clin Pharmacol 2021;37(20):2818-21.
- Xiaosong H, Yinli L, Chunyan Y, Lihong T, Yanhui Y. Effects of silkworm Arisaema decoction on the expression of matrix metalloproteinase 2 in rats with acute ischemic brain edema. Mod J Integr Tradit Chin West Med 2016;25(16):1711-13.
- Chen YX, Li GC, Xu LI. Research on each herb of Liangxue Tongyu decoction on cerebral hemorrhage. J Liaoning Univ Tradit Chin Med 2016;18(8):90-2.
- Jadaun KS, Mehan S, Sharma A, Siddiqui EM, Kumar S, Alsuhaymi N. Neuroprotective effect of chrysophanol as a PI3K/AKT/mTOR signaling inhibitor in an experimental model of autologous blood-induced intracerebral hemorrhage. Curr Med Sci 2022;28:1-8.
[Crossref] [Google Scholar] [PubMed]