- *Corresponding Author:
- Fei Gao
School of Chinese Classics, Beijing University of Chinese Medicine, Chaoyang, Beijing 100013, China
E-mail: 18810900206@163.com
| This article was originally published in a special issue, “Clinical Advancements in Life Sciences and Pharmaceutical Research” |
| Indian J Pharm Sci 2024:86(5) Spl Issue “118-122” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This study explores the consequences of microRNA-665 suppression on depression, shedding light on the mechanisms involved through the phosphoinositide 3-kinase/protein kinase B/c-Jun N-terminal kinase signaling pathways. The findings aim to identify new therapeutic targets and offer deeper insights for treating depression. To model depression, mice were subjected to a chronic unpredictable mild stress protocol and subsequently distributed randomly into four groups; control, depression model, microRNA-665 silencing, and microRNA-665 silencing combined with phosphoinositide 3-kinase/protein kinase B inhibitor. MicroRNA-665 expression was silenced using adenoviral vectors. Serum cortisol levels were measured using enzyme-linked immunosorbent assay. In the depression model, silencing microRNA-665 notably decreased immobility duration in both the forced swim test and the tail suspension test. Additionally, it led to a significant increase in the total movement distance observed in the open field test. Enzyme-linked immunosorbent assay results showed a decrease in serum cortisol levels in the microRNA-665 silencing group. Reverse transcription-quantitative polymerase chain reaction analysis indicated that silencing microRNA-665 regulated the expression levels of phosphoinositide 3-kinase, protein kinase B, and c-Jun N-terminal kinase genes towards normal levels. Silencing miR-665 expression significantly improved the behavioral performance of depressed mice and normalized the levels of associated proteins and genes through the phosphoinositide 3-kinase, protein kinase B, and c-Jun N-terminal kinase signaling pathways, as well as regulated physiological indicators associated with depression.
Keywords
Depression, fatigue, microRNA, tumor, apoptosis
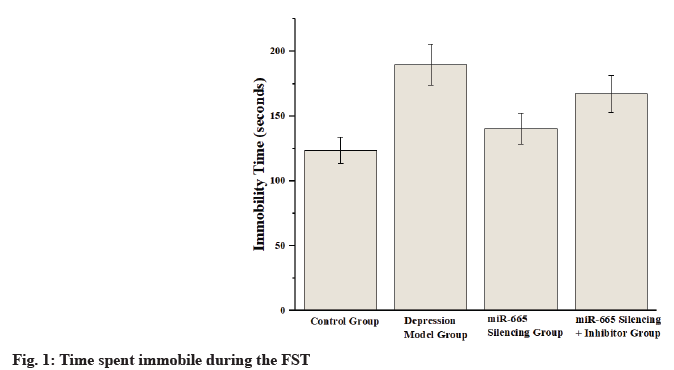
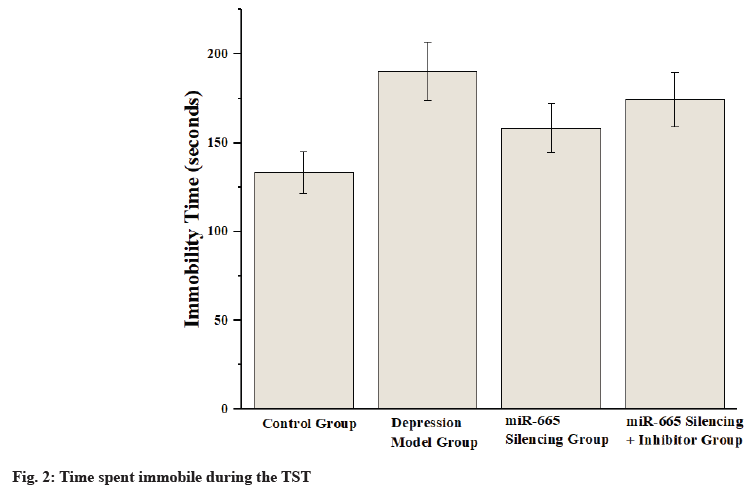
Depression, a prevalent and severe mental illness, profoundly impacts individual’s quality of life and social functionality[1,2]. Key symptoms include a persistent sadness, reduced interest in everyday activities, alterations in sleep and appetite patterns, fatigue, concentration difficulties, and severe suicidal ideation[3]. Primary symptoms involve a prolonged period of low mood, reduced engagement in everyday activities, disturbances in sleep and appetite, persistent fatigue, difficulties in concentration, and intense thoughts of suicide. Although current treatments provide some relief, 30 %-50 % of patients exhibit poor responses to these therapies. Furthermore, antidepressants are frequently linked to negative side effects, such as weight gain and sexual dysfunction, further compromising patient’s quality of life. Thus, comprehending the pathogenesis of depression and identifying new therapeutic targets remain critical areas of psychiatric research[4]. Recent research has increasingly emphasized the significance of microRNAs (miRNAs) in the onset of depression[5]. miRNAs are pivotal in regulating various biological and processes related to diseases, such as cellular growth, differentiation and apoptosis, and metabolic control. In depression research, miRNAs are considered potential biomarkers and therapeutic targets[6]. miR-665 has been shown to exhibit abnormal expression in various diseases, playing key roles in conditions such as tumors and cardiovascular diseases[7,8]. Despite this, research on the role of miR-665 in neurological disorders is limited. Some studies indicate that the Phosphoinositide 3-Kinase (PI3K)/Protein Kinase B (AKT)/c-Jun N-Terminal Kinase (JNK) signaling pathways play a crucial role in regulating cellular growth, differentiation, survival, and metabolism, and it plays a critical role in various psychiatric conditions[9-12]. The PI3K/AKT pathway supports cell survival and prevents apoptosis, thereby aiding neuronal growth and development. Conversely, the JNK pathway is involved in stress responses and inflammatory reactions, playing key roles in neuroinflammation and neurodegenerative diseases[13-16]. This study aims to investigate how silencing miR-665 expression impacts depression, focusing on the underlying mechanisms involving the PI3K/AKT/JNK signaling pathway. By establishing a Chronic Unpredictable Mild Stress (CUMS) mouse model, behavioral tests (Forced Swim Test (FST), Tail Suspension Test (TST) and Open Field Test (OFT)), molecular biology analyses (Reverse Transcription-quantitative Polymerase Chain Reaction (RT-qPCR), Western blot, Enzyme-Linked Immunosorbent Assay (ELISA)), and systematic evaluations of the impact of miR-665 silencing on behavioral performance, physiological indicators, and related molecular mechanisms in depression model mice are conducted. Eighty Specific Pathogen Free (SPF)-grade C57BL/6 mice (n=80), comprising both males and females weighing between (20-25) g, were utilized in the study. These mice were housed under strictly controlled conditions, including 22°±2°, a humidity level of 55 %±5 %, and a 12 h light/dark cycle. A CUMS protocol was employed over 4 w to induce depression in mice. The mice were subjected to various daily stressors, including cold water swims, restraint, vibration, and reversed light-dark cycles. The mice were randomly divided into four groups, with each group containing 20 individuals. Control (no CUMS treatment), depression model (CUMS treatment), miR-665 silencing (CUMS treatment with adenoviral vector-mediated miR-665 silencing), and miR-665 silencing+PI3K/AKT inhibitor (CUMS treatment with miR-665 silencing and PI3K/AKT pathway inhibitor). In FST, the mice were placed in a clear cylindrical tank with dimensions of 20 cm in diameter and 25 cm in height. The immobility time was recorded over a 6 min session, where immobility was defined as the minimum movement required to keep the mouse afloat. In TST, mice were suspended by the tail on a suspension device 30 cm above the ground. The total immobility time (seconds) within 6 min was recorded, with immobility defined as complete stillness. In OFT, the mice were placed in an open field apparatus measuring 50×50×40 cm for observation. The total movement distance (meters) within 10 min was recorded to assess activity levels and anxiety-like behavior. RT-qPCR analysis Ribonucleic Acid (RNA) was isolated using Trizol reagent, followed by the synthesis of complementary Deoxyribonucleic Acid (cDNA) through reverse transcription. SYBR Green qPCR kit was used for real-time quantitative PCR analysis. Genes analyzed included miR-665, PI3K, AKT, and JNK, with relative quantification (2-ΔΔCt method). ELISA for serum cortisol levels serum samples were collected by centrifuging orbital blood at 3000 rpm for 10 min. Serum cortisol levels were measured with an ELISA kit according to the manufacturer’s protocol. During the FST, mice in the depression model group showed a notable increase in immobility time (189.7±15.7) s, reflecting significant depressive-like behavior. In contrast, the miR-665 silencing group (140.4±12.1) s, and the miR-665 silencing combined with PI3K/ AKT inhibitor group (167.2±14.3) s exhibited a marked reduction in immobility time, indicating that miR-665 silencing mitigates depressive-like symptoms as shown in fig. 1. The depression model group exhibited significantly increased immobility time (190.1±16.4) s, further validating depressivelike behavior. The miR-665 silencing group (158.3±13.6) s and the miR-665 silencing+PI3K/ AKT inhibitor group (174.2±15.3) s showed significantly reduced immobility time, further supporting the antidepressant effect of miR-665 silencing. In contrast, groups with silenced miR-665 expression exhibited notably reduced immobility times in comparison with the depression model group (p<0.05) as shown in fig. 2. The OFT results showed that the depression model group exhibited significantly reduced total movement distance (8.5±1.0) m, while the miR-665 silencing group (12.8±1.5) m, and the miR-665 silencing+PI3K/AKT inhibitor group (10.7±1.2) m exhibited significantly increased total movement distance, indicating that miR-665 silencing improved the activity levels of depressed mice as shown in Table 1. ELISA results showed that the depression model group exhibited significantly elevated serum cortisol levels (120.7±10.6) ng/ml, while the miR-665 silencing group (80.3±6.1) ng/ml and the miR-665 silencing+PI3K/AKT inhibitor group (90.2±8.7) ng/ ml exhibited significantly reduced serum cortisol levels, indicating that miR-665 silencing modulated the stress response in depressed mice. The CUMS model simulates the pathological features of depression by subjecting mice to a series of unpredictable mild stresses, such as cold water swimming, restraint, and vibration, leading to significantly increased cortisol levels, reflecting the physiological response to chronic stress. In contrast, cortisol levels in the miR-665 silencing group were significantly reduced (80.3±6.1) ng/ml, indicating that miR-665 silencing effectively alleviated the stress response and lowered cortisol levels. As a small RNA that regulates gene expression, miR-665 may exert its effects by modulating signaling pathways related to stress and inflammation responses. Additionally, cortisol levels in the miR- 665 silencing combined with PI3K/AKT inhibitor group were also significantly reduced (90.2±8.7) ng/ ml, further supporting the critical role of the PI3K/ AKT signaling pathway in miR-665 regulated stress response as shown in Table 2. RT-qPCR analysis revealed significant alterations in the expression levels of miR-665, PI3K, AKT, and JNK genes in the depression model group. The miR-665 silencing group and the miR-665 silencing+PI3K/AKT inhibitor group exhibited normalized expression levels, indicating the regulatory effect of miR-665 silencing on these genes as shown in Table 3. The primary results of this study reveal that silencing miR-665 expression markedly enhances the behavioral outcomes of depression model mice and normalizes the expression levels of pertinent proteins and genes through the PI3K/AKT/JNK signaling cascade. Behavioral tests showed that depression model mice exhibited significant depressive-like behaviors, characterized by longer immobility durations in the FST and TST, as well as decreased overall movement distance in the OFT. The miR-665 silencing and miR-665 silencing+PI3K/AKT inhibitor groups showed significant improvement in these behaviors, indicating that miR-665 silencing effectively alleviated depressive-like behaviors[17]. The miR-665 silencing and miR-665 silencing+PI3K/ AKT inhibitor groups exhibited normalized expression levels, indicating that miR-665 silencing exerts its effects on depression through regulating the PI3K/AKT/JNK signaling pathway. The PI3K/ AKT signaling pathway is crucial for cell survival and anti-apoptosis, while the JNK pathway plays essential roles in stress responses and inflammatory reactions. Depression patients often exhibit increased neuroinflammation and neuronal apoptosis. This study’s results suggest that miR-665 silencing alleviates depressive symptoms by regulating these signaling pathways. ELISA results showed significantly elevated serum cortisol levels in the depression model group, indicating enhanced stress responses. The miR-665 silencing and miR-665 silencing+PI3K/AKT inhibitor groups exhibited significantly reduced serum cortisol levels, suggesting that miR-665 silencing effectively modulates stress responses[18-20]. Silencing miR-665 increases PI3K/AKT pathway activity and decreases JNK pathway activity, potentially explaining its antidepressant effects. These findings align with previous studies, further confirming the importance of the PI3K/AKT/JNK signaling pathway in depression. Future studies should investigate the interactions between miR-665 and other miRNAs and signaling pathways to fully understand its role in depression. Understanding miR-665 and related signaling pathways in depression will provide new strategies and targets for prevention, diagnosis, and treatment, improving patients’ quality of life, and reducing social and economic burdens.
| Group | Total movement distance (meters) |
|---|---|
| Control | 15.2±1.3 |
| Depression model | 8.5±1.0 |
| miR-665 silencing | 12.8±1.5 |
| miR-665 silencing+inhibitor | 10.7±1.2 |
Table 1: Total Movement Distance in the OFT
| Group | Serum cortisol levels (ng/ml) |
|---|---|
| Control | 77.4±5.9 |
| Depression model | 120.7±10.6 |
| miR-665 silencing | 80.3±6.1 |
| miR-665 silencing+inhibitor | 90.2±8.7 |
Table 2: Serum Cortisol Levels Measured by Elisa
| Group | miR-665 | PI3K | AKT | JNK |
|---|---|---|---|---|
| Control | 1.1±0.2 | 1.0±0.2 | 1.3±0.1 | 1.1±0.2 |
| Depression model | 1.5±0.3 | 0.7±0.1 | 0.8±0.1 | 1.3±0.2 |
| miR-665 silencing | 0.5±0.1 | 0.9±0.2 | 1.1±0.3 | 1.0±0.3 |
| miR-665 silencing+inhibitor | 0.7±0.3 | 0.8±0.2 | 1.0±0.1 | 1.2±0.2 |
Table 3: Gene Expression Levels Measured by RT-QPCR
Conflict of interests:
The authors declared no conflict of interests.
References
- Rahgosha P, Hadinezhad P, Hosseini SH. Comparison of brooding and reflection rumination between people with depression and obsessive-compulsive disorder. Iran J Health Sci 2023;11(1):67-74.
- Sahana R, Ramesh K, Kesava PS. Social media disorder and its association with depression and self-esteem among 1st year MBBS students of VIMS Ballari. A cross-sectional analytical study. Indian J Public Health Res Dev; 2024.
- Glanville KP, Coleman JR, Howard DM, Pain O, Hanscombe KB, Jermy B, et al. Multiple measures of depression to enhance validity of major depressive disorder in the UK Biobank. BJPsych Open 2021;7(2):44.
[Crossref] [Google Scholar] [PubMed]
- Sajjad A, Shah S, Abbas G, Aslam A, Randhawa F, Khurram H, et al. Treatment gap and barriers to access mental healthcare among women with postpartum depression symptoms in Pakistan. PeerJ 2024;12:e17711.
[Crossref] [Google Scholar] [PubMed]
- Zheng YB, Sheng XM, Jin X, Guan W. miR-182-5p: A novel biomarker in the treatment of depression in CSDS-induced mice. Int J Neuropsychopharmacol 2024;27(1):64.
[Crossref] [Google Scholar] [PubMed]
- Wei ZX, Xie GJ, Mao X, Zou XP, Liao YJ, Liu QS, et al. Exosomes from patients with major depression cause depressive-like behaviors in mice with involvement of miR-139-5p-regulated neurogenesis. Neuropsychopharmacology 2020;45(6):1050-8.
[Crossref] [Google Scholar] [PubMed]
- Yuan M, Guo YS, Zhang XX, Gao ZK, Shen XY, Han Y, et al. Diagnostic performance of miR-21, miR-124, miR-132, and miR-200b serums in post-stroke cognitive impairment patients. Folia Neuropathol 2022;60(2):228-36.
[Crossref] [Google Scholar] [PubMed]
- Ahmadimanesh M, Etemad L, Rad DM, Ghahremani MH, Mohammadpour AH, Esfehani RJ, et al. Effect of citalopram and sertraline on the expression of miRNA-124, 132, and 16 and their protein targets in patients with depression. Iran J Basic Med Sci 2023;26(7):820.
[Crossref] [Google Scholar] [PubMed]
- Tang M, Ai Y, Zhu S, Song N, Xu X, Liang L, et al. Antidepressant-like effect and mechanisms of essential oils from Citrus reticulata in reserpine-induced depression model mice. Nat Prod Commun 2022.
- Liu C, Yuan D, Zhang C, Tao Y, Meng Y, Jin M, et al. Liquiritin alleviates depression-like behavior in CUMS mice by inhibiting oxidative stress and NLRP3 inflammasome in hippocampus. Evid Based Complementary Altern Med 2022;2022(1):7558825.
[Crossref] [Google Scholar] [PubMed]
- Yi S. Establishment and evaluation of post-stroke depression rat model. J Contemporary Med Pract; 2022.
- Li J, Yang R, Xia K, Wang T, Nie B, Gao K, et al. Effects of stress on behavior and resting-state fMRI in rats and evaluation of telmisartan therapy in a stress-induced depression model. BMC Psychiatry 2018;18(1):1-3.
[Crossref] [Google Scholar] [PubMed]
- de Sousa CN, Medeiros ID, Vasconcelos GS, de Aquino GA, Cysne Filho FM, de Almeida Cysne JC, et al. Involvement of oxidative pathways and BDNF in the antidepressant effect of carvedilol in a depression model induced by chronic unpredictable stress. Psychopharmacology 2022;239(1):297-311.
[Crossref] [Google Scholar] [PubMed]
- Di D, Li M, Gao X, Hao Y, Zhao F. miR-335 over-expression in ventrolateral orbital cortex specifically down-regulates GRM4 to improve depressive behaviors in mice. Int J Clin Exp Med 2020;13(10):7518-26.
[Crossref] [Google Scholar] [PubMed]
- Lian N, Niu Q, Lei Y, Li X, Li Y, Song X. miR-221 is involved in depression by regulating Wnt2/CREB/BDNF axis in hippocampal neurons. Cell Cycle 2018;17(24):2745-55.
[Crossref] [Google Scholar] [PubMed]
- Zheng Y, Xie L, Xu S, Yan W, Zhang H, Meng Y, et al. Effects of miR-202-5p silencing PIK3CA gene expression on proliferation, invasion, and epithelial-mesenchymal transition of cervical cancer SiHa cells through inhibiting PI3K/Akt/mTOR signaling pathway activation. Mol Cell Biochem 2021;476:4031-44.
[Crossref] [Google Scholar] [PubMed]
- Miao Y, Liu J. Tumor-suppressive action of miR-30a-5p in lung adenocarcinoma correlates with ABL2 inhibition and PI3K/AKT pathway inactivation. Clin Transl Oncol 2024;26(2):398-413.
[Crossref] [Google Scholar] [PubMed]
- Hu J, Zhou B, Deng Y, Gao W, Fu A. miR-132 targets PTEN to regulate cognitive impairment and neural plasticity in mice with depression through PI3K/AKT signaling pathway. Int J Clin Exp Med 2020;13(11):8295-307.
- Feng H, Hu P, Chen Y, Sun H, Cai J, He X, et al. Decreased miR-451a in cerebrospinal fluid, a marker for both cognitive impairment and depressive symptoms in Alzheimer's disease. Theranostics 2023;13(9):3021.
[Crossref] [Google Scholar] [PubMed]
- Zhao M, Gao J, Zhang Y, Jiang X, Tian Y, Zheng X, et al. Elevated miR-29a contributes to axonal outgrowth and neurological recovery after intracerebral hemorrhage via targeting PTEN/PI3K/Akt pathway. Cell Mol Neurobiol 2021;41:1759-72.
[Crossref] [Google Scholar] [PubMed]