- *Corresponding Author:
- Harleen Makar
Department of Pharmaceutics, Delhi Institute of Pharmaceutical Sciences and Research, Pushp Vihar, Sector III, New Delhi-110 017, India
E-mail: harleenmakar@gmail.com
| Date of Submission | 26 July 2010 |
| Date of Revision | 22 February 2013 |
| Date of Acceptance | 03 March 2013 |
| Indian J Pharm Sci 2013;75(2):205-210 |
Abstract
Asymmetric membrane capsules are a type of osmotic drug delivery systems. They are nondisintegrating capsules, which utilize osmotic pressure to drive the drug outwards for controlled delivery. Preceded by systems such as elementary osmotic pump, controlled porosity osmotic pump, single composition osmotic tablet this system has the advantage of simple and easy fabrication as it obviates the necessity of drilling an orifice into the drug delivery system. Moreover; it seems to be a low-cost alternative. The cellulose acetate capsule shell, on coming in contact with the aqueous medium shows in situ pore formation due to leaching of pore formers, which have been incorporated into the shell forming solution. Until date, a number of osmotic agents to the likes of sodium chloride, mannitol has been used to build up osmotic pressure inside the cell. The system is endowed with high water flux, which is a plus point for delivery of poorly soluble drugs like cephalexin in terms of increasing release rates. Studies envisaged in this research include the effect of different concentrations of different pore formers on in vitro drug release as well as the effect of modification of inner contents of the capsule. The system was successful in producing a gradual release of drug for 12 h.
Keywords
Asymmetric membrane capsules, cephalexin, glycerol, osmotically controlled, potassium hydrogen tartrate
Asymmetric membrane capsules (AMCs) are osmotic drug delivery systems (ODDS), which is a type of controlled drug delivery systems [1]. Asymmetric Membranes are special membranes, which by virtue of in situ creation of pores, function to control the osmosis based release of drugs across them. Their main advantage lies in the fact that drug release from AMCs or tablets is independent of physiological factors of the gastrointestinal tract [2]. Cellulose acetate (CA) based films are semipermeable and allow water to pass across. Degree of acetylation of CA is one factor, which affects the water permeability of the membrane so formed. Water passes through the outer coating and moves into the central core of the capsule where it solubilizes the drug. The drug solution generates pressure, which forces the drug out of the capsule through any available opening, be it an orifice or in situ formed pores. The release kinetics from osmotic systems can be expressed as: dM/dt = (A.Pw.dπ.Cd)/L, where dM/dt is the quantity of drug released over time, A is the surface area of the coating, Pw is the permeability of the rate‑controlling membrane to water, dπ is the difference in osmotic pressure between the core and the surrounding fluid, Cd is the concentration of drug in the solution pumped out of the device and L is the thickness of the coating [3]. On similar lines the release duration from AMCs depends on the rate of water influx into the capsule core. This is further affected by the permeability of the capsule membrane and the osmotic pressure generated in the core of the capsule. In AMCs, the drug core is expected to be the controlling factor toward attaining zero-order release, whereas the membrane’s expected contribution is to control the release rate.
First to be developed in the string of ODDS was the elementary osmotic pump; then came controlled porosity osmotic pump, which solved the problem of localized delivery of drugs, which was followed by push‑pull osmotic pump having the exceptional ability to deliver poorly soluble drugs in a controlled manner. An innovation yet to come was the push stick osmotic pump devised to deliver methylphenidate in a two phase, immediate and sustained drug release manner [4]. ODDS lend themselves to a wide range of modifications like addition of crystal‑habit modifying agents for drugs, like polymers [5], surfactants (sodium lauryl sulfate) [6], pH‑modifying agents (acid or basic agent) [7-9], wicking agents in an effort to enhance the contact surface area of the drug [10]. This makes it possible and easier to get the desired release profile for a drug.
For asymmetric membrane‑coated capsules with an in situ formed delivery system, the osmotic effect is the main driving force for drug release. Now, drug solubility is expected to be the determining factor for achieving a desirable release rate. It is likely that a drug with low solubility would not create enough osmotic pressure to activate drug release. Owing to this, ample scope exists for examining the influence of core formulation variables on the drug release mechanism from an AMC. Drug modifications tried so far, include making salt forms of poorly soluble drugs [11] to give them a better solubility profile or even cyclodextrin complexation [12‑14]. For a drug, which is poorly soluble in water, suspension would be another plausible form in which the drug can be retained inside an osmotic system for a slow release. In this respect, hydroxypropyl methylcellulose (HPMC) and polyethylene oxide find wide presence in ODDS [15,16]. It is explicit that higher the viscosity of drug solution in the core of the formulation more will be the stability of the poorly water soluble drug against precipitation. So, if we increase the viscosity building agent better results are expected, but at the same time this will lead to a compromise on the amount of drug that can be incorporated and also too high a viscosity may affect the release profile negatively. Therefore, we can say that an optimum concentration of the suspending agent must be found out. It was shown in a research performed on exploration of the release mechanism of drugs from AMCs that as the viscosity of HPMC increased, the amount of nifedipine (a poorly soluble drug) released also increased [17]. The reason proposed for this behavior was said to be the formation of suspension inside the capsule, which increased the solubility of the drug by exposing a higher surface area hence aiding the solubilization.
Cephalexin is a member of the first generation of cephalosporins that possesses antibacterial activity against both Gram‑positive and Gram‑negative bacteria. This drug is widely applied for treatment of bacterial infections both in humans and animals. It is also effective in the treatment of strep throat [18]. It has low solubility in water and hence is suitable to evaluate the AMCs in terms of release behavior that can be expected for drugs with low water solubility in general. It also turns out to be an apt candidate for examining the effect of altering core variables on release pattern of drugs.
Materials and Methods
Cephalexin was obtained as a gift samples from Blue Cross Laboratories Ltd., Nashik, India. Sodium chloride (NaCl), sodium hydroxide (NaOH), acetone and isopropyl alcohol were procured from RFCL Ltd., Delhi, India. Ethyl alcohol and potassium dihydrogen orthophosphate (136.09) were procured from S. D. Fine Chemicals Ltd., Mumbai, India. Potassium hydrogen tartrate (KHT) was procured from Central Drug House (P) Ltd., New Delhi, India. Solvents of reagent grade and distilled water were used in all the experiments.
Fabrication of AMC shells
Capsules with asymmetric membrane were produced using a dip‑coating process. The stainless steel mold pins were dipped into polymer solutions consisting of CA dissolved in the mixture composed of acetone, isopropyl alcohol, glycerine and KHT in various ratios (Table 1) followed by air drying for 45 s. This was accompanied by spinning so as to attain uniform coating over the stainless steel mold pin. They were then dipped for quenching in an aqueous solution (10%, w/v, glycerine) for 7 min. After quenching, the pins were withdrawn from the solution and allowed to air dry. After air drying, the capsules were stripped off the pins, their edges were trimmed and they were preserved in desiccators until use.
| Formulation composition | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 |
|---|---|---|---|---|---|---|---|---|---|
| Cellulose acetate (mg) | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 |
| Acetone (ml) | 76 | 76 | 76 | 76 | 76 | 76 | 76 | 76 | 76 |
| Isopropyl alcohol (ml) | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Glycerol (mg) | 1.4 | 2.8 | 5.6 | - | - | - | 2.8 | 3.5 | 4.2 |
| Potassium hydrogen tartrate (mg) | - | - | - | 1.4 | 2.8 | 5.6 | 2.8 | 3.5 | 4.2 |
Table 1: Composition Of Capsule Shells
Filling and sealing of AMCs
The capsules were filled manually with various drug–excipient mixtures (Table 2). The AMCs were then capped and sealed using a syringe with a colored sealing solution, which contained 16% w/v CA in a mixture of acetone and alcohol (4:1). The dye added was FD and C Blue 1.
| Constituents (mg) formulation no.* | Cephalexin | Sodium chloride | Tris base |
|---|---|---|---|
| F1 CA | 200 | 200 | - |
| F2 CA | 200 | 200 | - |
| F3 CA | 200 | 200 | - |
| F4 CA | 200 | 200 | - |
| F5 CA | 200 | 200 | - |
| F6 CA | 200 | 200 | - |
| F7 CA | 200 | 200 | - |
| F7 CB | 200 | 300 | - |
| F7 CC | 200 | 400 | - |
| F7 CD | 200 | 375 | 125 |
| F7 CE | 200 | 400 | 50 |
| F7 CF | 200 | 350 | 150 |
| F8 CD | 200 | 375 | 125 |
| F9 CD | 200 | 375 | 125 |
Table 2: Ingredients Of Capsular Formulations
Characterisation
The AMCs were characterized according to their appearance, which included glossiness and smoothness. In addition, dimensional characterization was performed using micrometer screw. Asymmetric membranes pieces were excised from capsules and dried at 45° for 12 h and stored in a desiccator before they were to be examined. The asymmetric membrane samples were sputter coated with gold by using fine coat ion sputter and was examined for their morphology and pore structure by EV 50 scanning electron microscope (SEM).
In vitro drug release
All dissolution studies were carried out in USP 27 Basket type apparatus with rotating speed and temperature set at 50 rpm and 37±0.5°, respectively. The dissolution mediums used were 0.1 N HCl as simulated gastric fluid (pH 1.2, 900 ml) for the first 2 h, followed by phosphate buffer (pH 6.8, 900 ml) as simulated intestinal fluid (SIF) for the next 10 h. The change from one medium to another was completed in approximately 5‑7 min. A total 5 ml of the sample was withdrawn with replacement at specified time intervals and suitably diluted and analyzed for the drug at 260 nm. The concentration of drug samples was determined using equations derived from the standard curve. Then percentage cumulative drug release was calculated and plotted against time.
Drug loading study
Influence of drug loading on the osmotic pressure based release of cephalexin from AMCs was examined by incorporating drug in increasing amounts (Table 3). Then in vitro release profile was obtained using USP 27 basket type dissolution apparatus and the profiles were compared for the respective formulations.
| Formulation | Cephalexin (mg) | Sodium chloride (mg) | Tris base (mg) |
|---|---|---|---|
| C1 | 200 | 375 | 125 |
| C2 | 300 | 375 | 125 |
| C3 | 400 | 375 | 125 |
Table 3: composition of capsules for drug Loading study using formulation f7
Osmotic release study
The effects of osmotic pressure were determined by investigating the release of amaranth, a freely water soluble dye from the capsules in two media. For these studies, 50 mg amaranth was filled into the capsule shells and the capsules were sealed. Release of the dye from the AMC is supposed to be influenced by the environment, which exists inside the capsule, which is in turn dependent on the osmotic agent. 50 mg of amaranth along with 100 mg of NaCl inside the capsule and lack of NaCl in the external medium represents osmotic gradient condition. A total of 50 mg of amaranth with 100 mg of NaCl inside the formulation and 10 g/100 ml NaCl solution as external medium represented the hyperosmotic condition.
Intentional defect study
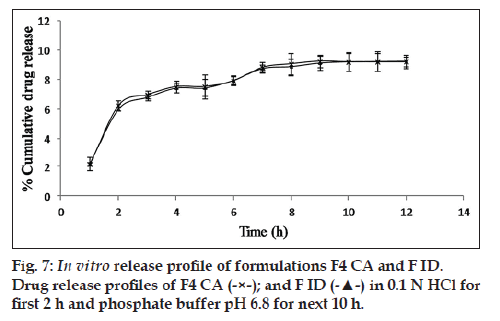
An incision, 0.2 mm in size was made on the capsule shells. Then, the capsule shells were filled with composition same as the formulation F4 CA. This formulation was designated as ID. The dissolution study was carried out in USP 27 Basket type apparatus with rotating speed and temperature set at 50 rpm and 37±0.5°, respectively. The dissolution media were 0.1 N HCl for the first 2 h, followed by phosphate buffer (pH 6.8) for the next 10 h. Five millilitres of the sample was withdrawn at specified time intervals and suitably diluted and analyzed at 260 nm in UV/Vis spectrophotometer. The release profiles of F4 CA and ID were compared.
Results and Discussion
As far as appearance was concerned, formulation F2 produced capsules that were shiny, opaque and showed some wrinkles. Capsules made according to formulation F5 were translucent, not shiny and showed many wrinkles. Best results were shown with formulation F7, wherein the capsules were shiny, opaque and smooth without any wrinkles. Dimensional details are as follows: length of cap was 7.76±0.14 mm, length of body was 13.02±0.13 mm, diameter of cap was 17.87±0.16 mm, diameter of body was 7.06±0.15 mm and total length of sealed capsule was found to be 22.13±0.12 mm.
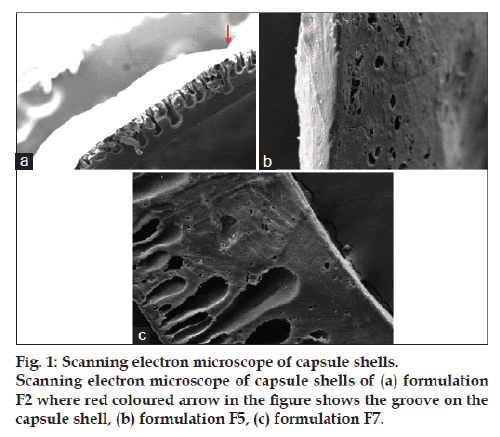
Scanning electron micrograph of formulation F2 shows two layers of asymmetric membrane (fig. 1a). The picture clearly shows the dense thin layer on the outside and a thicker highly porous layer on the inside of the shell. The arrow in the picture shows the groove, which is a wrinkle on the surface of the capsule, reinforcing the fact that the shell is not smooth. Scanning electron micrograph of formulation F5 shows the cross‑sectional view of capsule shell (fig. 1b). The picture shows the appearance of a thick porous layer supporting the outer dense thin layer. The number of pores is less as compared to those in formulation F2. Cross‑sectional view of AMC shells clearly shows that formulation F7 having glycerol and PHT in combination has a clearer asymmetric structure as compared to that produced by formulations F2 and F5 (fig. 1c).
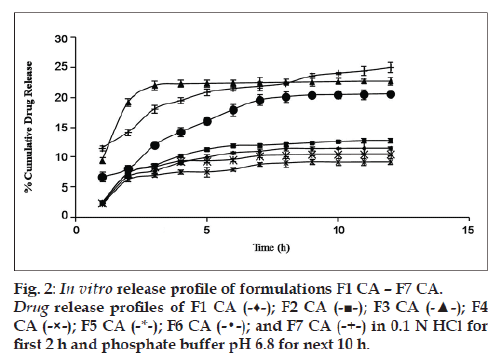
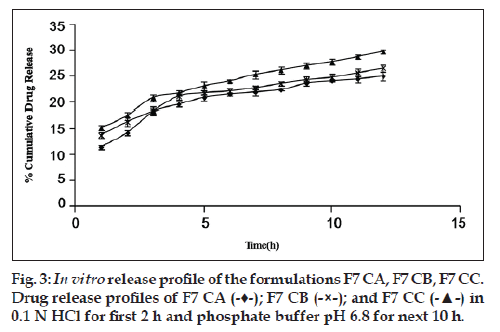
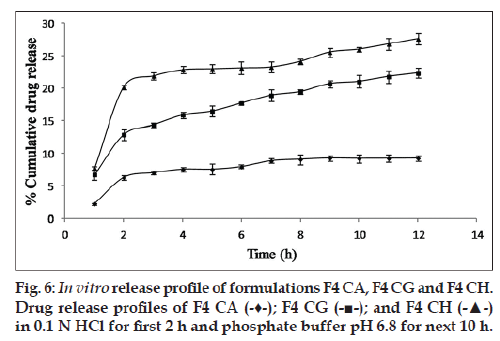
Glycerol and PHT in combination work successfully when incorporated in the CA solution. The highest cumulative percentage drug release of formulation F7 CA (25% approx.) among all other formulations proves the same (fig. 2). In order to rule out the doubt that such an increase in percentage cumulative drug release could be due to doubling of the amount of pore former, formulations F3 and F6 (both having pore formers in quantity 40% of CA used) were made and evaluated. It was found that formulation F7 CA had a higher release of drug, i.e., 24.998±0.857% as compared with F3 CA and F6 CA, 22.758±0.679 and 20.589±0.216, respectively (fig. 2). In relation to osmotic systems, it has been proposed that amount of pore former added in the shell forming solution follows an inverse relation with a lag time in the release profile, but a decrease in lag time is also makes the formulation unable to give a sustained release for over 7‑8 h [19]. Our research supported the same finding. In the next step as the amount of osmogen was increased, investigations were done using three drug:osmogen ratios of 1:1, 1:2, 1:3. On increasing the amount of osmogen, the cumulative percentage drug released also increased (fig. 3).
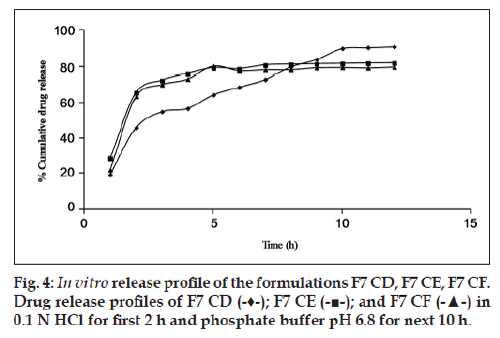
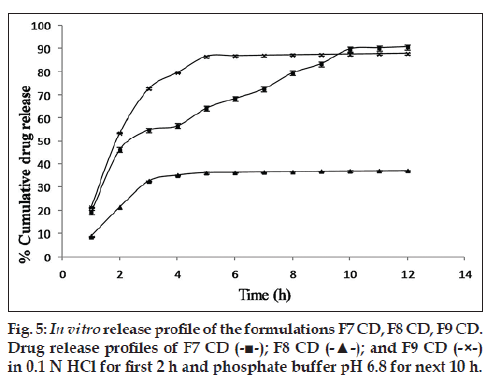
Since the maximum percentage cumulative release seen with the drug:osmogen ratios used was just 30%, hence the next approach was to solubilize the drug. For this purpose, Tris base was used. It provided a pH environment in which cephalexin had high solubility. Moreover, its aqueous solubility lent osmogen properties to it. The addition of Tris base led to a major increase in cumulative percentage drug release. The formulation F7 CD with Tris base along with cephalexin and NaCl has a cumulative percentage drug release of 90.75±0.16% in 12 h, which is much higher than 29.10±0.56% from formulation F7 CC having only cephalexin and NaCl (fig. 4). An optimum concentration of pore formers in combination seems to be required as in F7 CD. On increasing the concentration of pore formers, gradual release of the drug could only be seen for 3‑5 h after which the release became constant as shown (fig. 5).
Influence of drug loading on the release of a drug with low solubility from polymeric capsules with asymmetrical semipermeable membrane by osmotic pressure as examined by incorporating drug in increasing amounts has been shown. An increase of 200% over 9.22±0.48% cumulative release of F4 CA was seen on loading 3 times the drug (fig. 6).
It was seen that a trail of dye started coming out of the capsule after half an hour when it was immersed in a solution, which provided an osmotic gradient condition. No such trail was seen in the capsule which was immersed in hyperosmotic condition i.e., 10% NaCl solution. No appreciable change was seen on release profile on introducing a minor defect in the membrane in the form of an incision (fig. 7).
In summary, this research studies envisaged and proved that PHT acts as a pore former when incorporated in CA capsules. Glycerol and PHT in combination worked successfully as a pore forming mixture when incorporated in the CA solution. Capsules fabricated using a mixture of pore formers such as glycerol and PHT are easier to strip off the mold pins and have a smooth surface as opposed to those fabricated with each of the pore formers alone. With regard to inner contents of the capsule, Tris base helps to increase solubility of cephalexin by providing a suitable pH microenvironment. The prepared AMCs are capable of showing osmotically controlled release. It has been reported that osmotically controlled drug delivery systems have been shown to produce a high degree of in vivo‑in vitro correlation [20]. Hence, there is a need to explore them extensively. By merely controlling the ratio of pore formers in the AMC shell and ratio of osmogen to solubilizes in the capsule core, the drug release profile can be tailored to meet the desired target. This garners support for more detailed researches, which can turn AMCs into successful controlled drug delivery systems.
Acknowledgments
Authors are thankful to Mr. J. C. Koshti, Blue Cross Laboratories Ltd., Nashik, India for providing gift sample of Cephalexin. We acknowledge Dr. B. P. Srinivasan, Director, DIPSAR, New Delhi for providing required set up and instruments and Advanced Instrumentation Research Facility at JNU, New Delhi for providing state of the art SEM equipment for this research.
References
- Feliciano NR, Bouvet AA, Redalieu E, Castellana J, Luders RC, Schwartz DJ, et al. Pharmaco kinetic and pharmaco dynamic comparison of an osmotic release oral metoprolol tablet and the metoprolol conventional tablet. Am Heart J 1990;120:483-9.
- Conley R, Gupta SK, Sathyan G. Clinical spectrum of the osmotic-controlled release oral delivery system (OROS), an advanced oral delivery form. Curr Med Res Opin 2006;22:1879-92.
- Herbig SM, Cardinal JR, Korsmeyer RW, Smith KL. Asymmetric-membrane tablet coatings for osmotic drug delivery. J Control Release 1995;35:127-36.
- Coghill D, Seth S. Osmotic, controlled-release methylphenidate for the treatment of ADHD. Expert Opin Pharmacother 2006;7:2119-38.
- Khanna S, Geigy C. Therapeutic system for sparingly soluble active ingredients. US Patent No. 4992278, 1991.
- Rudnic E, Burnside BA, Flanner HH, Wassink SE, Couch R.A, Pinkett JE. Osmotic drug delivery system. US Patent No. 6110498, 2000.
- Ayer A, Theeuwes F, Wong PS, Alza Corp. Controlled delivery of haloperidol by an osmotic delivery system. US Patent 4610,686, 1986.
- Thombre AG, DeNoto AR, Gibbes DC. Delivery of glipizide from asymmetric membrane capsules using encapsulated excipients. J Control Release 1999;60:333-41.
- McClelland GA, Sutton SC, Engle K, Zentner GM. The solubility-modulated osmotic pump: In vitro/in vivo release of diltiazem hydrochloride. Pharm Res 1991;8:88-92.
- Koparkar AD, Shah SB. Oral osmotic system for slightly soluble active agents. US Patent No. 5284662. 1994.
- Philip A, Pathak K. In situ-formed asymmetric membrane capsule for osmotic release of poorly water-soluble drug. PDA J Pharm SciTechnol 2007;61:24-36.
- Okimoto K, Tokunaga Y, Ibuki R, Irie T, Uekama K, Rajewski RA, et al. Applicability of (SBE) 7m-beta-CD in controlled-porosity osmotic pump tablets (OPTs). Int J Pharm 2004;286:81-8.
- Gan Y, Pan W, Wei M, Zhang R. Cyclodextrin complex osmotic tablet for glipizide delivery. Drug DevInd Pharm 2002;28:1015-21.
- Okimoto K, Rajewski RA, Stella VJ. Release of testosterone from an osmotic pump tablet utilizing (SBE) 7m-beta-cyclodextrin as both a solubilizing and an osmotic pump agent. J Control Release 1999;58:29-38.
- Liu L, Khang G, Rhee JM, Lee HB. Monolithic osmotic tablet system for nifedipine delivery. J Control Release 2000;67:309-22.
- Shokri J, Ahmadi P, Rashidi P, Shahsavari M, Rajabi-Siahboomi A, Nokhodchi A. Swellable elementary osmotic pump (SEOP): An effective device for delivery of poorly water-soluble drugs. Eur J Pharm Biopharm 2008;68:289-97.
- Lin YK, Ho HO. Investigations on the drug releasing mechanism from an asymmetric membrane-coated capsule with an in situ formed delivery orifice. J Control Release 2003;89:57-69.
- Disney FA, Dillon H, Blumer JL, Dudding BA, McLinn SE, Nelson DB, et al. Cephalexin and penicillin in the treatment of group A beta-hemolytic streptococcal throat infections. Am J Dis Child 1992;146:1324-7.
- Makhija SN, Vavia PR. Controlled porosity osmotic pump-based controlled release systems of pseudoephedrine. I. Cellulose acetate as a semipermeable membrane. J Control Release 2003;89:5-18.
- Gohel MC, Parikh RK, Shah NY. Osmotic Drug Delivery – An Update, 2009. Available from: http://www.pharmainfo.net/reviews/ osmotic-drug-delivery-update. [last accessed on 22 February 2013]