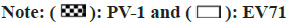- *Corresponding Author:
- Lei Zhang
Department of Preventive Drug Research, Guangdong Provincial Institute of Biological Products and Materia Medica, Guangzhou 510440, China
E-mail: swzl100@sina.com
| This article was originally published in a special issue, “Drug Discovery and Repositioning Studies in Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(4) Spl Issue “84-90” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The model virus is crucial for evaluating virucidal activity of disinfectants. However, the utilization of poliovirus is only temporary because of the global polio eradication program. Enterovirus 71 has the advantages of high virus titer, convenient treatment and little harm, and can be used as a potential model virus for evaluating virus inactivation activity. To investigate resistance of enterovirus 71 to environmental (dry surfaces and hard water) and 10 hand disinfectants, compared with poliovirus-I virus. On dry surface, two viruses had shown reduction in activity with the increment of treating time and the activity of <4 log10TCID50 (log) at 4 h-treated time. However, neither poliovirus-I or enterovirus 71 in virus activity had maintained >4 log in hard water after treatment for 14 d. Six of 10 disinfectants reach the 4-log reduction requirement. Enterovirus 71 compared with poliovirus-I, exhibited the similar resistance to dry surface, hard water and disinfectants. Enterovirus 71 can be considered a suitable and important alternative model virus in the replacement of poliovirus-I to support the claims of virucidal activity. The model virus is crucial to evaluate the virucidal activity of disinfectants. However, the use of poliovirus is only temporary due to the global polio eradication program. Enterovirus 71 has the advantages of high viral titer, convenient treatment and little damage, and can be used as a potential model virus to evaluate virus inactivation activity. To investigate the resistance of enterovirus 71 to the environment (dry surfaces and hard water) and 10 hand sanitizers, compared to the poliovirus-I virus. On dry surfaces, two viruses showed a reduction in activity with increasing treatment time and an activity of <4 log10TCID50 (log) at 4 h of treatment time. However, neither poliovirus-I nor enterovirus 71 virus activity remained >4 log in hard water after treatment for 14 d. 6 out of 10 disinfectants meet the 4 log reduction requirement. Enterovirus 71, compared to poliovirus-I, showed similar resistance to dry surfaces, hard water and disinfectants. Enterovirus 71 may be considered a suitable and important alternative model virus replacing poliovirus-I to support claims of virucidal activity.
Keywords
Enterovirus 71, environmental factors, poliovirus-I, disinfectants, virucidal activity
As a result of the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) pandemic, the interest and demand for virucidal disinfectants have increased. Therefore, the evaluation of virucidal activity of these chemical disinfectants has been received high attention[1]. An appropriate model virus is essential for assessing the virucidal activity, which has a high titer in culture and high resistance to chemical disinfectants and environmental factors[2].
Poliovirus (PV) is a non-enveloped Ribonucleic Acid (RNA) virus classified into human Enterovirus 71 (EV71) and is neurotropic, causing severe neurological diseases in humans[3]. PV is a causative agent of poliomyelitis, resulting in flaccid paralysis[4]. Getting benefit from successful global vaccination efforts of the past few decades, poliomyelitis has been nearly eradicated from the world[5]. PV-I, especially, have many characteristic which are as follows; the virus exhibits simple virus propagation, safe operation and a high level of resistance to disinfectants and environmental conditions[2]. Therefore, PV-I, is used as a model virus by technical standards for disinfection and EN 14476 (phase 2/step 1)[6,7]. In 1988, The World Health Organization (WHO) launched the global polio eradication program, bringing on poliomyelitis significantly decreased. Nevertheless, the use of PV-I will require a higher level of biosafety, so the use of PV-I is only temporary[8]. On the other hand, vaccine derived PV-I cases were sometimes reported[9]. Therefore, it is recommended to substitute PV-I with an alternative model virus. For this reason, human EV71 such as the EV71 can be used. EV71 is ideal because this positive-sense RNA virus are cultivable on continuously growing cells with high titer and operability that it can be easily managed in a standard laboratory setting. Meanwhile, it poses little potential risk to employees performing the tests, because of vaccination[10]. Thus, EV71 can be chosen as a potential model virus for evaluating virucidal activity.
Our research has two objectives, both of which are crucial for evaluating EV71 as a model virus. The first aim was to evaluate EV71 resistance to dry surface and suspension (hard water) with lasting for different duration. The second aim was to evaluate EV71 resistance to different ingredients hand disinfectants, which widely used commercial disinfectants in China.
Materials and Methods
Virus propagation and cell culture:
EV71 and PV-I were obtained from Guangdong Provincial Center for Disease Prevention and Control, China. Hep-2 cells and Vero cells were used to EV71 and PV-I virus propagation, respectively. The viral growth medium for Hep-2 cells and Vero cells was Modified Eagle Medium (MEM, GIBCO), supplemented with 2 % Fetal Calf Serum (FCS, GIBCO), 100 U/ml penicillin and 100 μg/ ml streptomycin (GIBCO). The culture flasks were maintained at 37° in a 5 % Carbon dioxide (CO2) atmosphere and monitored daily until the appearance of cytopathic effects. By repeatedly freezing and thawing infected cells three times, the virus is released through the infected cells. Centrifuge the suspension at 4000 rpm for 30 min to eliminate cell debris, and collect the upper culture medium for storage at -70°.
Infectivity assay:
For titers of EV71 and PV-I by the Tissue Culture Infectious Dose 50 (TCID50) assay, cells were grown to 95 % confluence in flat-bottom 96-well plates (Corning 96-well plates). Thereafter, virus samples were prepared by a 10-fold dilution series with MEM including 2 % FCS. The supernatant from each well was removed and replaced with 150 μl of the appropriately diluted virus sample. Following incubation for 7 d, inverted microscopy was employed to distinguish infected wells from non-infected ones. The highest dilution of the virus suspension that induced a cytopathic effect in 50 % of cell monolayers was determined through microscopic observation. The TCID50 value was calculated using the Reed and Muench method[11].
Resistance test to environment factors (wet and dry):
Resistance test on dry surface: The test was performed according to EN 14476:2013+A2:2019 with a modification (European Committee for Standardization)[12]. The cleaning of the stainless steel discs (20 mm diameter) was performed as already described[2]. The discs were prepared by autoclaving. A total of 50 μl of the virus was added to each pretreated discs surface and dried at room temperature. Then, the discs were transferred into sterile petri dish and placed for 0.5, 1, 2, 4, 12, 24, 48 and 72 h. At the end of the every treating time, the discs were transferred into 950 μl of culture medium. Vials were vortexed for 60 s to collect residual viruses and immediately dilute the eluent 10 times to determine virus infectivity.
Resistance test in hard water: A total of 50 μl of the virus was added into 450 μl of sterile hard water. Then, the mixture was placed at room temperature for 1, 2, 3, 4, 5, 6, 7 and 14 d, respectively. At the end of the every exposure time, the 50 μl mixture were transferred into 950 μl of culture medium. Vials were vortexed for 30 s followed by 10-fold dilution for determining viral infectivity.
Chemical disinfectants:
There are 10 commercially and commonly used chemical disinfectants for testing. Determination of active ingredient according to technical standards for disinfection in China[6]. The disinfectants include A (sodium hypochlorite, 0.037 % effective chlorine), B (75 % ethanol and 0.4 % triclosan), C (70 % ethanol and 0.5 % Polyhexamethylene Biguanide Hydrochloride (PHMB)), D (70 % ethanol and 0.5 % chlorhexidine glucanate), E (0.25 % chlorhexidine glucanate and 0.25 % PHMB), F (70 % ethanol and 0.105 % PHMB), G (60 % ethanol and 10 % isopropanol), H (55 % ethanol and 0.4 % PHMB·HCl), I (70 % ethanol) and J (77 % ethanol and 0.12 % Hydrogen peroxide (H2O2)). Treatment times and concentrations used were according to instructions on these disinfectants.
Neutralization validation:
The neutralization validation was performed according to technical standards for disinfection 6 with a modification by means of the dilutionultracentrifugation method[13]. The mixture were vortexed for 15 s, followed by incubation at 20° for 60 s, and then centrifuged with 85 000 g at 4° for 2.5 h. Discarding the supernatant and suspending the precipitates with 1 ml MEM, following diluting the mixture by serial 10-fold with MEM including 2 % FCS. The virus titers were determined by TCID50.
Virus inactivation test:
Mix the virus suspension (0.2 ml) with 0.8 ml of the test disinfectant. Vortex the virus disinfectant mixture for 10 s and incubate at 20° for 1 min. As mentioned earlier, after the end of the exposure period, neutralize 0.1 ml of the mixture by ultracentrifugation dilution method. The virus titer was determined by TCID50. A reduction of infectivity of ≥4 log10TCID50 (4- log) steps (inactivation 99.99 %) was considered evidence of sufficient antiviral activity against the tested virus[6]. Take the logarithm of the difference between the virus titers of each disinfectant and the virus titers of the virus control as the average, and calculate the average log10 reduction factor attenuation coefficient.
Results and Discussion
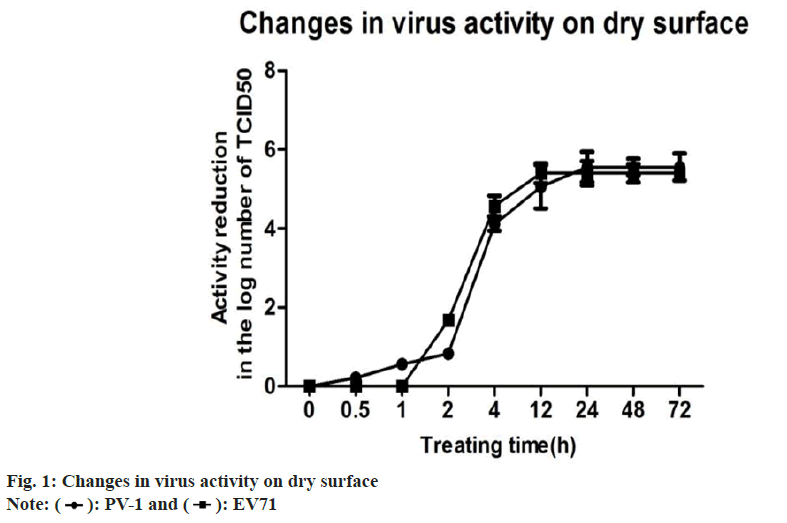
There have been few reports on EV71, which was used as model viruses to evaluate virucidal activity. Two viruses are applied to the stainless steel surface and then dried, which more accurately imitates the actual situation. Surfaces play an important role in viral transmission directly from contaminated surfaces to susceptible individuals[14]. In order to evaluate the relation between virus’s activity and time on the dry surface, the treating time of discs were set as 0.5, 1, 2, 4, 12, 24, 48 and 72 h, respectively. When placing dried viruses on stainless steel carriers with lasting for different consecutive time intervals. The results displayed the relations of vitality decreased over time. It could result in >4-log reduction at 4 h-treated time for both viruses. This observations are also supported by Eggers’ study which exert 4-log reduction of the PV-I[15]. Based on the present study, both of two viruses have displayed a similar resistance, which are sensitive to the drying environment. It should be noted that EV71 may persist on dry surface for approximately 2 h-4 h. However, several virucidal carrier testes (made of various materials, such as plastic, glass and fabrics) may need to confirm this concept and further understand (Table 1 and fig. 1).
| Time (h) | PV-I | EV71 | ||
|---|---|---|---|---|
| Virus activity | Reduction activity | Virus activity | Reduction activity | |
| 0 | 5.67 | 0 | 5.5 | 0 |
| 0.5 | 5.33 | 0.22 | 5.5 | 0 |
| 1 | 5 | 0.56 | 5 | 0.5 |
| 2 | 4.67 | 0.83 | 4.33 | 1.17 |
| 4 | 1.33 | 4.34 | 1 | 4.5 |
| 12 | 0.5 | 5.17 | 0 | 5.5 |
| 24 | 0 | 5.67 | 0 | 5.5 |
| 48 | 0 | 5.67 | 0 | 5.5 |
| 72 | 0 | 5.67 | 0 | 5.5 |
Note: (*) the average log10TCID50 of the untreated PV-I control was 5.67±0.28 and the average log10TCID50 of the untreated EV71 control was 5.50±0.55. The virus activity showed the average in log10TCID50. The reduction activity showed the average reduction in log10TCID50 from the controls
Table 1: Changes in Virus Activity on Dry Surface
Both of PV-I and EV71 exhibit the similar timedependent activity pattern. That is, the lower virus activity are shown when the mixture (virus and hard water) have longer exposure time. In present test, both viruses and sterile hard water were kept in contact with each other in a liquid phase with different exposure durations varying from (1-14) d. Although both viruses reduced over time, neither of viruses showed the 4-log reduction requirements. That is, EV71 displayed a very high resistance to be in hard water. Similar results were also observed by previous study which this virus maintain activity in wastewater over several months (Table 2 and fig. 2) [16].
| Time (d) | PV-I | EV71 | ||
|---|---|---|---|---|
| Virus activity | Reduction activity | Virus activity | Reduction activity | |
| 0 | 6.00* | 0 | 6.28* | 0 |
| 1 | 5.56 | 0.44 | 5.61 | 0.67 |
| 2 | 5.44 | 0.56 | 5.61 | 0.67 |
| 3 | 5.44 | 0.56 | 5.61 | 0.67 |
| 4 | 5.28 | 0.72 | 5.5 | 0.78 |
| 5 | 5.16 | 0.84 | 5.44 | 0.84 |
| 6 | 5.05 | 0.95 | 5.28 | 1 |
| 7 | 4.83 | 1.17 | 5.06 | 1.22 |
| 14 | 4.78 | 1.22 | 4.86 | 1.42 |
Note: (*) The average log10TCID50 of the untreated PV-I control was 6.00±0.37 and the average log10TCID50 of the untreated EV71 control was 6.28±0.41. The virus activity showed the average in log10TCID50. The reduction activity showed the average reduction in log10TCID50 from the controls
Table 2: Changes in Virus Activity In Suspension
The main aim of this study was to investigate both viruses resistance to chemical disinfectants. The tested EV71 revealed similar log reductions to that of PV-I. Sodium hypochlorite (NaClO), as a highlevel disinfectants, which is a strong oxidizing agent[17] and recommended by the WHO[18], displayed high virucidal activity against EV71 (Table 3). Conversely, the intermediate-level disinfectants, such as formulations containing chlorhexidine glucanate-PHMB or ethanol and PHMB·HCl can exhibit less activity against EV71. The disinfectant formulation based on alcohol has broad-spectrum antimicrobial activity against bacteria, fungi and enveloped viruses[19]. In this study, ethanol and ethanol-based disinfectants (70 % and 75 %) showed the effective virucidal activity against EV71, which was consistent with previous observations showing ethanol’s effective inactivation abilities against various viruses[20,21].
| Disinfectants | Active ingredients | Treatment | PV-I | EV71 | ||
|---|---|---|---|---|---|---|
| Time (min) | Virus activity | Reduction activity | Virus activity | Reduction activity | ||
| A | (NaClO, 0.037 % effective chlorine) | 1 | 0 | 6.33 | 0 | 6.67 |
| B | Ethanol (75 % v/v) and triclosan (0.4 % w/v) | 1 | 2 | 4.33 | 2.11 | 4.56 |
| C | Ethanol (70 % v/v) and PHMB (0.5 % w/v) | 1 | 1.61 | 4.72 | 1.44 | 5.23 |
| D | Ethanol (70 % v/v) and chlorhexidine glucanate (0.5 % v/v) | 1 | 1.89 | 4.44 | 2.56 | 4.11 |
| E | Chlorhexidine glucanate (0.25 % w/v) and PHMB (0.25 % w/v) | 1 | 4.22 | 2.11 | 4.89 | 1.78 |
| F | Ethanol (70 % v/v) and PHMB (0.105 % w/v) | 1 | 2 | 4.33 | 2.56 | 4.11 |
| G | Ethanol (60 %,v/v) and isopropanol (10 %, v/v) | 1 | 4.56 | 1.77 | 5.39 | 1.28 |
| H | Ethanol (55 % v/v) and PHMB·HCl (0.4 % w/w) | 1 | 5.22 | 1.11 | 4.89 | 1.78 |
| I | Ethanol (70 % v/v) | 1 | 5.22 | 1.11 | 4.67 | 2 |
| J | Ethanol (77 % v/v) and (H2O2, 0.12 % v/v) | 1 | 2.45 | 3.88 | 2.61 | 4.06 |
Note: (*) the average log10 TCID50 of the untreated PV-I control was 6.33±0.52 and the average log10 TCID50 of the untreated EV71 control was 6.67±0.29. The virus activity showed the average in log10 TCID50. The reduction activity showed the average reduction in log10 TCID50 from the controls
Table 3: Virucidal Activity of Disinfectants Against PV-I and EV71
However, in this study, neither ethanol-only preparations (70 %, v/v) nor ethanol-isopropanol preparations (60 %:10 %, v) can provide high activity against EV71. The result is consistent with the conclusion from previous reports[5]. Conversely, addition of other active ingredients to alcohol preparations can improve significantly virucidal activity against EV71. Resistance to ethanol and other ingredients formulated preparations has also been demonstrated previously in tests against EV71 21, confirming our data (fig. 3).
According to present study, EV71 was similarly resistant to surface (suspension) and commonly used disinfectants with PV-I. Considering its similar properties in EV71, it is well accepted that the effective measures applicable to PV-I also apply to EV71. Moreover, EV71, as a clinically relevant virus, belong to the member of EV71, which have similar characteristics to PV-I (standard strains currently in use). Taken together, these results demonstrate that EV71 can be considered a suitable and important alternative model virus in there placement of PV-I to support the claims of virucidal activity to PV-I. We hope that the results of this study can be used to select the most suitable mode of virus for virucidal activity testing experiments, provide proven efficacy to the end user and play an important role in preventing and controlling virus outbreaks and transmission in medical institutions and communities.
Acknowledgment:
This work was supported by Science and Technology Program of Guangzhou (Grants No: 202102080124), Disinfection Scientific Research Project of Chinese Preventive healthcare Association (XD2022-Z-04), the Key Research and Development Program of Guangdong Province (Grants No: 2019B111103001), the Medical Scientific Research Foundation of Guangdong Province of China (Grant No: B2020139, B2021119 and C2022015) and the Project of Administration of Traditional Chinese Medicine of Guangdong Province of China (Grant No: 20211054 and 20222024).
Author’s contributions:
Xunmin Ji and Wei Xiao have contributed equally to this work.
Conflict of interests:
The authors declared no conflict of interests.
References
- Tarka P, Nitsch-Osuch A. Evaluating the virucidal activity of disinfectants according to European Union standards. Viruses 2021;13(4):534.
[Crossref] [Google Scholar] [PubMed]
- Rabenau HF, Steinmann J, Rapp I, Schwebke I, Eggers M. Evaluation of a virucidal quantitative carrier test for surface disinfectants. PloS One 2014;9(1):e86128.
[Crossref] [Google Scholar] [PubMed]
- Burrill CP, Strings VR, Andino R. Poliovirus: Generation, quantification, propagation, purification and storage. Curr Protoc Microbiol 2013;29(1):15H.
- Sabin AB. Pathogenesis of poliomyelitis reappraisal in the light of new data. Science 1956;123(3209):1151-7.
[Crossref] [Google Scholar] [PubMed]
- World Health Organization. Two out of three wild poliovirus strains eradicated. Geneva: World Health Organization; 2019. p. 10-21.
- China Ministry of Health (2002) Technical Standards for Disinfection.
- HygCen Austria GmbH (2020) Guidelines and Testing Methods for Virucidal Activity, Requirements for Inclusion in the VAH List.
- Eggers M, Terletskaia-Ladwig E, Rabenau HF, Doerr HW, Diedrich S, Enders G, et al. Immunity status of adults and children against poliomyelitis virus type 1 strains CHAT and Sabin (LSc-2ab) in Germany. BMC Infect Dis 2010;10:1-9.
[Crossref] [Google Scholar] [PubMed]
- Wang HB, Zhang LF, Yu WZ, Wen N, Yan DM, Tang JJ, et al. Cross-border collaboration between China and Myanmar for emergency response to imported vaccine derived poliovirus case. BMC Infect Dis 2015;15:1-8.
[Crossref] [Google Scholar] [PubMed]
- Li YP, Liang ZL, Gao Q, Huang LR, Mao QY, Wen SQ, et al. Safety and immunogenicity of a novel human Enterovirus 71 (EV71) vaccine: A randomized, placebo-controlled, double-blind, Phase I clinical trial. Vaccine 2012;30(22):3295-303.
[Crossref] [Google Scholar] [PubMed]
- Lj R. A simple method of estimating fifty per cent endpoints. Am J Hyg 1938;27:493-5.
- European Committee for Standardization (CEN). EN 14476:2013+A2:2019: Chemical disinfectants and antiseptics-quantitative suspension test for the evaluation of Virucidal activity in the medical area-test method and requirements (phase 2/step 1); European Committee for Standardization: Brussels, Belgium; 2019.
- Huang Y, Xiao S, Song D, Yuan Z. Evaluating the virucidal activity of four disinfectants against SARS-CoV-2. Am J Infect Control 2022;50(3):319-24.
[Crossref] [Google Scholar] [PubMed]
- Otter JA, Yezli S, French GL. The role played by contaminated surfaces in the transmission of nosocomial pathogens. Infect Control Hosp Epidemiol 2011;32(7):687-99.
[Crossref] [Google Scholar] [PubMed]
- Eggers M, Schwebke I, Suchomel M, Fotheringham V, Gebel J, Meyer B, et al. The European tiered approach for virucidal efficacy testing–rationale for rapidly selecting disinfectants against emerging and re-emerging viral diseases. Eurosurveillance 2021;26(3):2000708.
[Crossref] [Google Scholar] [PubMed]
- Dong X, Ying J, Chen Y. Molecular epidemiology and evolution of worldwide enterovirus 71 strains isolated from 1970 to 2004. Chin Sci Bull 2007;52(11):1484-90.
[Crossref] [Google Scholar] [PubMed]
- Fukuzaki SA. Mechanisms of actions of sodium hypochlorite in cleaning and disinfection processes. Biocontrol Sci 2006;11(4):147-57.
[Crossref] [Google Scholar] [PubMed]
- World Health Organization. Cleaning and Disinfection of Environmental Surfaces in the Context of COVID-19; 2020.
- Boyce JM, Pittet D. Healthcare infection control practices advisory committee; HICPAC/SHEA/APIC/IDSA hand hygiene task force. Guideline for hand hygiene in health-care settings. Recommendations of the healthcare infection control practices advisory committee and the HICPAC/SHEA/APIC/IDSA hand hygiene task force. Society for healthcare epidemiology of America/association for professionals in infection control/infectious diseases society of America. MMWR Recom Rep 2002;51(16):1-45.
[Google Scholar] [PubMed]
- Singh D, Joshi K, Samuel A, Patra J, Mahindroo N. Alcohol-based hand sanitizers as first line of defence against SARS-CoV-2: A review of biology, chemistry and formulations. Epidemiol Infect 2020;148:e229.
[Crossref] [Google Scholar] [PubMed]
- Su Y, Han J, Li J, Ren Z, Huang L, Xu B, et al. Resistance of poliovirus 1 and enterovirus A71 against alcohol and other disinfectants. J Virol Methods 2021;298:114292.
[Crossref] [Google Scholar] [PubMed]